ABSTRACT
A number of new vaccines to prevent childhood diseases have been introduced globally over the last few decades. Only four combination vaccines are currently available in Japan, DTaP/sIPV, DTaP, DT, and MR, leading to complex infant vaccine scheduling. This study aims to investigate mothers' preferences with respect to combination vaccines for their children, should new combination vaccines become available that have not yet been launched in Japan or that will be developed in the future. We conducted a web-based, cross-sectional survey of 1,243 mothers who had at least one child between 2 months and 3 y of age. Mothers were recruited from an online survey panel of registered users. Their preferences were elicited using discrete choice experiments, the analyzed main effects model, and interactions using a mixed logit model. Mothers showed a preference for vaccines that prevented multiple diseases, had fewer injections per doctor visit, lower price, and lower risk of adverse events. Respondents valued a reduced risk of adverse events the most among all attributes in this study. The estimated monetary value of the willingness to pay for a 1% reduction in the risk of adverse events was ¥ 92,557 ($ 907). Therefore, if new combination vaccines are introduced, the risk of adverse events after vaccination is an especially important factor for mothers. While the safety of the vaccines themselves is required, health professionals should also inform mothers about the benefits and risks of vaccination, to allay mothers' concerns about vaccine safety.
KEYWORDS: combination vaccine, discrete choice experiment, japan, preference, willingness to pay, young children
Introduction
A number of new vaccines to prevent childhood diseases have been introduced globally over the last few decades. Many combination vaccines, which combine 2 or more different vaccines into a single injection, have also been developed and introduced. Combination vaccines have several benefits, such as reducing the number of doctor visits and injections because several vaccinations are given at the same time. These benefits could reduce time and money for parents and lessen the trauma of injections for children.1,2 The benefits of combination vaccines to the vaccine coverage rate and timeliness of immunizations are supported by many research studies.3 Accordingly, many combination vaccines that contain the hepatitis B virus vaccine, Haemophilus influenzae type b (Hib) vaccine, and polio virus vaccine, such as DTaP/IPV or DTaP/IPV/Hib, are currently used in many countries.
Only four combination vaccines are currently available in Japan: DTaP/sIPV, DTaP, DT, and MR.4 Recently, some new vaccines and products for use in children have been licensed, such as the Hib vaccine, 7- and 13-valent pneumococcal conjugate vaccines (PCV7 and PCV13), and rotavirus vaccines. Some of these vaccines have been introduced to the National Immunization Program as routine vaccines.4 In the current schedule of routine vaccination, children in Japan receive 10 vaccinations by 1 y of age (standard age of vaccination), or 15–16 vaccinations if voluntary vaccines (excluding influenza vaccines) are included.5 In Japan, rules on vaccination intervals are given in vaccination guidelines: at least 6 d should be allowed after inactivated vaccine and 27 d after a live vaccine before any additional vaccines are given.6,7 In addition, the decision whether to administer successive vaccinations during the same session is left to pediatricians in vaccination guidelines.7-9 Therefore, the vaccination schedule for younger children is very complex. Pediatricians and researchers indicate that there is a need for combination vaccines, not only to benefit recipients and parents, but also to assist medical professionals.4,10 In 2014, the Ministry of Health, Labour and Welfare announced the development of new vaccines, including combination vaccines, in its master plan for vaccination.11 This study aims to investigate mothers' preferences regarding combination vaccines for their children using discrete choice experiments (DCEs), should new combination vaccines become available in Japan that have not yet been launched or are yet to be developed. Specifically, the study assesses which factors regarding vaccination are most valued by Japanese mothers.
Results
Table 1 shows respondent characteristics. The median (range) age of respondents was 33 y old (20–50 years), and most respondents were in their 30 s (70%). The mean number of years of education was 15 y and the most common educational level was bachelor's degree (47%). The majority of mothers were married (98%), homemakers (62%), and had one child (51%). Average annual household income was ¥ 6.2 ± 2.6 million ($ 61,176) and the most common income group was ¥ 5–6 million ($ 49,020–$ 58,824). The average age of respondents' youngest child was 18.2 months (1.5 years) and a quarter of their children attended a daycare center. Sixteen percent of respondents had at least one experience with an adverse event after vaccinating their child.
Table 4.
Vaccination attributes and levels in discrete choice experiments.
| Attributes | Levels |
|---|---|
| Diseases targeted in one vaccine | 2 |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| Number of injections per doctor visit | 1 |
| 2 | |
| 3 | |
| 4 | |
| Payment for one doctor visit (¥)a | 0 |
| 2000 | |
| 4000 | |
| 6000 | |
| 8000 | |
| Risk of adverse events after vaccination | 10% |
| 1% | |
| 0.10% | |
| 0% |
$ 1 = ¥ 102 (as of April 2014)
Table 2 shows the mixed logit model of preference for combination vaccines, the main effects model. The positive coefficient for the number of target diseases in one vaccine suggests that vaccines that target more diseases were preferred to those targeting fewer ones. The negative coefficient for the variables number of injections per doctor visit, payment, and risk of adverse events, suggests that respondents preferred fewer injections, lower prices, and less risk. That is, mothers preferred vaccines that prevent multiple diseases, involve fewer injections per doctor visit, and have a lower price and less risk of adverse events. The value of the coefficient for adverse events was the highest among all attributes. Preferences for combination vaccines differed depending on the characteristics of mothers and their children. Table 2 shows the results for interaction with household income, mothers' employment status, child's age, and number of children, as well as interactions with the 2 main effects. Mothers from lower income households and those who had more children tended to be more sensitive to price. Aversion to adverse events increased with higher price. Preferences for combination vaccines among mothers who were working were not significantly different to mothers who were unemployed.
Table 1.
Respondent characteristics.
| No. |
% |
||
|---|---|---|---|
| Variables | n = 1243 | ||
| Age group, years | |||
| 20–29 | 239 | 19.2 | |
| 30–39 | 866 | 69.7 | |
| 40–49 | 137 | 11.0 | |
| ≥ 50 | 1 | 1.0 | |
| Educational level | |||
| Junior high school | 12 | 1.0 | |
| High school | 227 | 18.3 | |
| Some college | 367 | 29.5 | |
| Bachelor's degree | 579 | 46.6 | |
| Master's degree or higher | 53 | 4.3 | |
| Other | 5 | 0.4 | |
| Marital status | |||
| Married | 1223 | 98.4 | |
| Divorced/Widowed/Single | 20 | 1.6 | |
| Employment status | |||
| Employed | 287 | 23.1 | |
| Under childcare leave | 179 | 14.4 | |
| Housewife | 772 | 62.1 | |
| Other | 5 | 0.4 | |
| Number of children | |||
| 1 | 635 | 51.1 | |
| 2 | 461 | 37.1 | |
| 3 | 127 | 10.2 | |
| 4 | 17 | 1.4 | |
| 5 or more | 3 | 0.2 | |
| Annual household income (¥)a | |||
| Less than 1 million | 12 | 1.0 | |
| 1–2 million | 21 | 1.7 | |
| 2–3 million | 90 | 7.2 | |
| 3–4 million | 149 | 12.0 | |
| 4–5 million | 160 | 12.9 | |
| 5–6 million | 185 | 14.9 | |
| 6–7 million | 177 | 14.2 | |
| 7–8 million | 139 | 11.2 | |
| 8–9 million | 105 | 8.4 | |
| 9–10 million | 77 | 6.2 | |
| More than 10 million | 128 | 10.3 | |
| Age of youngest child, months | 18.2 | 9.9 | |
| Using a daycare center (yes = 1) | 308 | 24.8 | |
| Experience with an adverse event after vaccination (yes = 1) | 199 | 16.2 | |
$ 1 = ¥ 102 (as of April 2014)
The estimated monetary value of changes, as marginal WTP, in the attributes for combination vaccines is shown in Table 3. Respondents were willing to pay ¥ 1045 ($ 10) to increase the number of target diseases in one vaccine. The estimated monetary value of reducing the number of injections per doctor visit was ¥ 1261 ($ 12). The estimated monetary value for a 1% reduction in the risk of adverse events was ¥ 92,557 ($ 907). Respondents most valued a reduction in the risk of adverse events among all the attributes in this study.
Table 2.
Mixed logit model of preferences for combination vaccines.
| Main effects |
Interaction effects |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | p-value | 95% CI | Variable | Coefficient | p-value | 95% CI | ||
| Targeted diseases in one vaccine | 0.362 | 0.000 | 0.2994 | 0.4251 | Targeted diseases in one vaccine | 0.362 | 0.000 | 0.294 | 0.430 |
| Number of injections per doctor visit | −0.437 | 0.000 | −0.4919 | −0.3822 | Number of injections per doctor visit | −0.287 | 0.000 | −0.382 | −0.192 |
| Payment for one doctor visit [Unit: ¥ 1000] | −0.347 | 0.000 | −0.3793 | −0.3137 | Payment for one doctor visit [Unit: ¥ 1000] | −0.248 | 0.000 | −0.340 | −0.157 |
| Risk of adverse events after vaccination | −32.073 | 0.000 | −34.7706 | −29.3748 | Risk of adverse events | −25.695 | 0.000 | −30.395 | −20.996 |
| income*payment | 0.020 | 0.000 | 0.011 | 0.030 | |||||
| adverse event*payment | −0.674 | 0.016 | −1.221 | −0.127 | |||||
| number of children*payment | −0.125 | 0.000 | −0.162 | −0.088 | |||||
| dummy of employment*number of target disease | 0.042 | 0.449 | −0.067 | 0.151 | |||||
| age*adverse event | −0.231 | 0.021 | −0.428 | −0.034 | |||||
| age*number of injctions | −0.008 | 0.001 | −0.012 | −0.003 | |||||
Abbreviation: CI, confidence interval
Discussion
Pediatric researchers in Japan and government have reported the advantages of introducing new combination vaccines,4,11 Currently, combination vaccines for children are limited in the country because few combination vaccines have been officially approved. Therefore, we explored mothers' preferences for the use of new combination vaccines in their children, by using DCEs.
Mothers preferred vaccines that prevent more than one disease, involve fewer injections per doctor visit, and have a lower price and lower risk of adverse events.
The coefficient for adverse events had a negative sign and a relatively large number compared with other attributes. Reduced risk of adverse events is the attribute that mothers valued the most. Vaccine safety is an important factor in mothers' choices regarding vaccine utilization in other countries.12 Research results might also be influenced by historical memories regarding fears about vaccination in Japan.4,13 In 2011, fatalities after successive injection of Hib vaccine and/or PCV7 and/or other vaccines were reported. Based on these reports, the Hib and PCV7 vaccines were temporarily suspended.13 In June 2013, the government suspended its active recommendation of the HPV vaccination program owing to the fear of adverse events, such as complex regional pain syndrome.4,14 In early spring of the same year, media coverage about these adverse events was frequent and the vaccination rate for HPV decreased dramatically.15 Our results might reflect mothers' wishes to avoid risk even if the risk of an adverse event is slight. Therefore, if new combination vaccines are introduced in Japan, the risk of adverse events is an important factor for mothers. For mothers to accept new combination vaccines, the risks of these new vaccines should not be greater than the risks associated with conventional vaccines. The estimated WTP assists in determining perceived value among mothers. The WTP for reduced risk of adverse events was higher than the WTP for more target diseases per vaccine and that for fewer injections.
The targeted age of the child might be a factor in new combination vaccines. Mothers with relatively young children were less sensitive about avoiding adverse events compared with mothers who had older children, provided that such adverse events were not severe. Mothers of younger children might perceive greater benefits for combination vaccines because they reduce the burden on children by decreasing the number of injections per doctor visit.16 In Japan, the number of available combination vaccines is currently limited. Moreover, successive vaccination was infrequent several years ago; however, this has recently become more common in the overcrowded vaccine schedule for younger children.9 Therefore, if mothers with younger children were to perceive a combination vaccine as safe, it would be more acceptable to them.
We hypothesized that working mothers would prefer the convenience of targeting multiple diseases in one vaccine because their working schedule could be a barrier to timely vaccine uptake.17 However, their perceived value regarding this factor was not significantly greater than that of mothers who were not employed. On its own, the number of diseases targeted in a combination vaccine might not be sufficient to influence vaccine choice among working mothers. Household income was a factor that correlated with values regarding price for a doctor visit. Mothers from lower income households showed a tendency to be more sensitive to the price of vaccination. Studies have shown that the household economic situation affects vaccine uptake.17,18 Price could be a large barrier to vaccination among children from lower income households.
The risks associated with vaccinations have been a focus of the media.13 In addition, reports of adverse events and other negative information about vaccination have been widely and rapidly spread in recent years by various media sources, including social media.19 While the safety of vaccines themselves is required, communication about risk is also necessary. Health professionals should inform mothers about the risks and benefits of vaccination, to address mothers' concerns regarding the safety of vaccines.20,21 Timing immunization education, including information about adverse events after vaccination, to take place during the perinatal period might be effective.22
This study has a number of limitations. First, the study was conducted using a web-based survey. Respondents were limited to only individuals who could access the Internet, although currently more than 95% of the population in Japan has access to the Internet.23 Nevertheless, we must consider the possibility of selection bias. To keep selection bias to a minimum, we adjusted the regional and economical distribution in advance and adjusted it to a distribution that represented the actual population. According to the survey “Marriage Process and Fertility of Japanese Married Couples” by the National Institute of Population and Social Security Research, 35 y is the average age of mothers whose youngest child is aged 0–2 y and more than 65% do not have paid work (including students). Therefore, we believe that the population of respondents to our web-based survey was not remarkably biased. Second, there were 4 attributes regarding vaccines, each with 4 or 5 levels. These attributes and levels were very limited in the DCEs. In reality, there are more than 4 factors influencing vaccine preferences. Third, we only included mothers as respondents. However, decision-making regarding childhood vaccinations might be influenced by the opinions of fathers and/or other family members.
In conclusion, we identified a preference for new combination vaccines in Japan. Mothers expressed a preference for vaccines that could prevent multiple diseases, resulted in fewer injections per doctor visit, have a lower price, and pose a lower risk of adverse events. Mothers especially valued a reduced risk of adverse events. If new combination vaccines meet these factors, mothers would accept such vaccines for their children. The safety of vaccines themselves is required; however, health professionals should also inform mothers about the benefits and risks of vaccination, to help allay mothers' concerns about vaccine safety. Such information could help in the development and introduction of new combination vaccines in Japan.
Methods
Study population and data collection
We conducted a web-based, cross-sectional survey in April 2014. Respondents were recruited from a survey panel of a private, web-based survey company because nationwide random sampling was unavailable in Japan. First, survey panelists who had at least one child aged between 2 months and 3 y were chosen by the company from baseline data and informed about the survey through e-mail or members' personal website. Next, panelists who were interested in the survey proceeded to the questionnaire on the website. To reduce selection bias, we adjusted the geographic structure and distribution of household incomes of the respondent population to ensure that they were representative of the average nationwide population, using statistics of Japan.24,25 Sample size was decided by referring to the “1000 to 2000,” to produce small confidence intervals even with an inefficient experimental design26 and by taking our budgetary constraints into account. Recruitment was continued until a total of 1,243 respondents had completed the survey.
Questionnaire
The questionnaire comprises 2 parts. The first part contained questions concerning respondents' demographic information, including age, education, marital status, employment, number of children, annual household income, age of respondents' youngest child, daycare center use, and experience with vaccine-associated adverse events and so on.
The second part included questions concerning DCEs. DCEs are used to elicit consumer preferences regarding individual characteristics of goods or services for which markets do not exist.27 In the health sector, it may not be possible to infer consumer preferences or values from data of “what consumers actually do” (revealed preference) because of the characteristics of public goods in health care. Therefore, valuing goods or services in the area of health has been explored as “what consumers say they will do,” the stated preference (SP). DCEs are one of the best-known SP approaches for providing estimates of monetary valuation.28 Discrete choice models are derived under an assumption of utility-maximizing behaviors by the decision maker, who can be an individual, a household, or any other decision-making unit. Such models describe decision makers' choices among alternatives, such as competing goods and services.29
The detailed process of the DCE approach is as follows: Respondents are provided a hypothetical scenario and several choice sets and are then asked to indicate their preferred choice from among several options that are comprised of several characteristics (named attributes) and levels. It is assumed that individuals will consider information about the choice sets based on the provided scenarios and will select the option with the highest utility, that is, utility maximization behavior, as mentioned above.28
In this study, we considered mothers' decision-making regarding combination vaccines for their children. Before beginning the queries with DCEs, respondents were shown an explanation of the hypothetical survey, as follows: “First of all, this is a hypothetical questionnaire. If you vaccinate your child, which vaccination would you choose, Vaccine A and Vaccine B? Each vaccine in this scenario is presumed safe and is officially approved (hypothetical). Both Vaccine A and Vaccine B is made up of 4 characteristics.” Participants were then shown the following 4 attributes (characteristics): the number of injections per one doctor visit, diseases targeted in one vaccine, the payment for one doctor visit, and the risk of adverse events after vaccination. These vaccine attributes were identified from a literature review.2,13,30-34 We then narrowed down the number of factors and defined their levels, as explained below. With respect to effectiveness, we assumed that combination vaccines were not inferior to single vaccinations. Each attribute was defined to ensure respondents' understanding of the term. For example, “the number of diseases targeted in one vaccine” was explained using the example of the DPT combination vaccine because its aim is to prevent diphtheria, pertussis, and tetanus with one injection. “The number of injections per one doctor visit” was explained as injections on the right and left arm during one doctor visit is considered 2 injections. “Payment for one doctor visit” was explained as the amount due at the cashier counter when paying the bill. “Risk of adverse events after vaccination” was explained as the risk of developing a fever (38.0°C or above) within 3 d after vaccination.
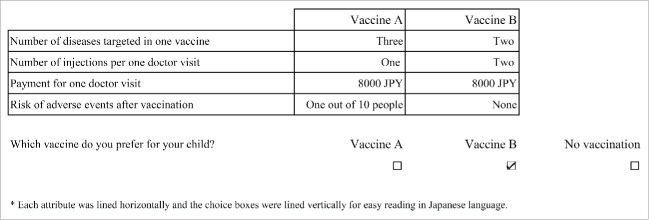
After these explanations, participants were given 3 options (Fig. 1): Vaccine A or Vaccine B, each containing the above 4 attributes and each level, or no vaccination. Each level of vaccination DCE was developed based on a literature review that considered the following: diseases targeted in one vaccine (2, 3, 4, 5, or 6 diseases), number of injections per one doctor visit (1, 2, 3, or 4), payment for of one doctor visit (0, ¥ 2000 ($ 20), ¥ 4000 ($ 39), ¥ 6000 ($ 59), ¥ 8000 ($ 78); $ 1 = ¥ 102 as of April 2014)), and risk of adverse events after vaccination (10%, 1%, 0.1%, or 0%)34-36 (Table 4). We explained that a 10% risk of adverse events from vaccination was “about 10 in 100 children.”
Figure 1.
Example of discrete choice experiment question presented to respondents.
Table 3.
Estimated marginal willingness to pay (WTP) as an attribute of combination vaccines.
| Increase the number of target diseases in one vaccine | 1,045 |
| Reduction the number of injections per doctor visit | 1,261 |
| Reduction the risk of adverse events after vaccination (per one percent) | 92,557 |
| Unit: ¥ |
Theoretically, the attributes and levels yield 52 × 42 = 400 possible combinations. Because it was not feasible to show all combinations to respondents, we used a library of orthogonal arrays to generate 25 profiles. As mentioned above, respondents were shown 3 options; 2 options were randomly selected from the 25 generated options, and there was one fixed option of “No vaccination.” Then respondents were asked to choose one of the 3 options; they were instructed to assume that the options were for their youngest child. These queries were repeated 5 times. In the first query, we provided a choice of “I don't understand what this question means,” in addition to the 3 options. If the respondents chose this option, queries with DCEs were closed.
To confirm respondents' understanding of the questions, attributes, and attribute levels, the questionnaire was pre-tested on 12 persons with at least one child. Using their comments, we made the survey easier to understand for more respondents.
Results derived from the DCEs were analyzed using a mixed logit model, which is a highly flexible model that can approximate any random utility model,29 main effects, and interactions. Potential respondent characteristics associated with attributes, such as employment status, number of children, annual household income, and youngest child's age, were explored as interaction effects.37,38 Marginal willingness to pay (WTP) was elicited by using the coefficients.28
All respondents provided informed consent by clicking “I agree” after reading a description of the survey; they could also choose “I don't agree.” Only those respondents who agreed to the survey proceeded to the questionnaire. The survey protocol was approved by the Ethics Committee of Meiji Pharmaceutical University.
Abbreviations
- DCEs
discrete choice experiments
- DTaP/sIPV
diphtheria, tetanus toxoid, acellular pertussis, and inactivated, Sabin-derived polio vaccine
- DTaP
diphtheria toxoid, tetanus toxoid, and acellular pertussis
- DTaP/IPV/Hib
diphtheria, tetanus, and acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type b
- DT
diphtheria, tetanus
- MR
measles, rubella
- SP
stated preference
- HPV
human papillomavirus
- WTP
willingness to pay
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) KAKENHI (25460817). The funding source did not play a role in any aspect of the study or in our decision to submit the paper for publication.
Authors' contributions
Conception, design, and interpretation of the data: AS, MK; data analysis and writing of the article: AS
References
- [1].Centers for Disease Control and Prevention Multiple Vaccines and the Immune System. http://www.cdc.gov/vaccinesafety/concerns/multiple-vaccines-immunity.html [accessed 18.December.15] [Google Scholar]
- [2].American Academy of Pediatrics. Combination vaccines for childhood immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP) Pediatrics 1999; 103:1064-77. http://www.ncbi.nlm.nih.gov/pubmed/10224194; PMID:10224194; http://dx.doi.org/ 10.1542/peds.103.5.1064 [DOI] [PubMed] [Google Scholar]
- [3].Maman K, Zollner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: From beliefs to evidence. Hum Vaccin Immunother 2015; 11(9):2132-41; PMID:26075806; http://dx.doi.org/ 10.1080/21645515.2015.1044180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saitoh A, Okabe N. Recent progress and concerns regarding the Japanese immunization program: addressing the “vaccine gap”. Vaccine 2014; 32(34):4253-8; http://dx.doi.org/ 10.1016/j.vaccine.2014.06.022 [DOI] [PubMed] [Google Scholar]
- [5].Japan Pediatric Society Vaccination Schedule Recommended by the Japan Pediatric Society. https://www.jpeds.or.jp/modules/en/index.php?content_id = 7 [accessed 10.May.15] [Google Scholar]
- [6].The Review Committee for Vaccination Guidelines Vaccination and Children's Health. 2015 ver. Tokyo: Public Foundation of the Vaccination Research Center; 2015. http://www.yoboseshu-rc.com/publics/download/?file = /files/content_type/type555/196/201507151204248048.pdf [accessed 06.April.16] [Google Scholar]
- [7].Ministry of Health, Labour and Welfare Yobou sessyu zisshi youryou. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000036493.html [accessed 06.April.16]. Japanese [Google Scholar]
- [8].Muta H, Nagai T, Fujioka M, Okafuji T, Ochiai H, Tahara T, et al.. Awareness and practice of vaccination among members of the Society of Ambulatory and General Pediatrics of Japan. JAGP 2014; 17:301-9. Japanese [Google Scholar]
- [9].Katsuta T, Miyachi Y, Nakamura K, Tsuruoka J, Tateyama S, Tokutake T, et al.. Vaccine doji sessyu ni taisuru sessyui no ishiki chosa. J Jpn Pediatr Soc 2013; 117(10):1645-51. Japanese [Google Scholar]
- [10].Suge S. Vaccine no henkou/kongouka to sessyu schedule henkou. Jpn Med J 2014; 4720:31-5. Japanese [Google Scholar]
- [11].Ministry of Health, Labour and Welfare Yobou sessyu ni kansuru kihonteki na keikaku, 2014. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/kihonteki_keikaku/ [accessed 02.December.15]. Japanese [Google Scholar]
- [12].Gust DA, Strine TW, Maurice E, Smith P, Yusuf H, Wilkinson M, et al.. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics 2004; 114(1):e16-22. http://www.ncbi.nlm.nih.gov/pubmed/15231968; PMID:15231968; http://dx.doi.org/ 10.1542/peds.114.1.e16 [DOI] [PubMed] [Google Scholar]
- [13].Saitoh A, Okabe N. Current issues with the immunization program in Japan: Can we fill the “vaccine gap”? Vaccine 2012; 30(32):4752-6; PMID:22521841; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.026 [DOI] [PubMed] [Google Scholar]
- [14].Gilmour S, Kanda M, Kusumi E, Tanimoto T, Kami M, Shibuya K. HPV vaccination programme in Japan. Lancet 2013; 382(9894):768; PMID:23993189; http://dx.doi.org/ 10.1016/S0140-6736(13)61831-0 [DOI] [PubMed] [Google Scholar]
- [15].Morimoto A, Ueda Y, Egawa-Takata T, Yagi A, Terai Y, Ohmichi M, et al.. Effect on HPV vaccination in Japan resulting from news report of adverse events and suspension of governmental recommendation for HPV vaccination. Int J Clin Oncol 2015; 20(3):549-55; PMID:25001869; http://dx.doi.org/ 10.1007/s10147-014-0723-1 [DOI] [PubMed] [Google Scholar]
- [16].Pichichero ME. New combination vaccines. Pediatr Clin North Am 2000; 47(2):407-26. http://www.ncbi.nlm.nih.gov/pubmed/10761511; PMID:10761511; http://dx.doi.org/ 10.1016/S0031-3955(05)70214-5 [DOI] [PubMed] [Google Scholar]
- [17].Ueda M, Kondo N, Takada M, Hashimoto H. Maternal work conditions, socioeconomic and educational status, and vaccination of children: a community-based household survey in Japan. Prev Med 2014; 66:17-21 [DOI] [PubMed] [Google Scholar]
- [18].Wada K, Smith DR. Influenza vaccination uptake among the working age population of Japan: results from a national cross-sectional survey. PLoS One 2013; 8(3):e59272; PMID:23555010; http://dx.doi.org/ 10.1371/journal.pone.0059272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: the global response to Japan's suspension of its HPV vaccine recommendation. Hum Vaccin Immunother 2014; 10(9):2543-50; PMID:25483472; http://dx.doi.org/ 10.4161/21645515.2014.969618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kennedy A, Basket M, Sheedy K. Vaccine attitudes, concerns, and information sources reported by parents of young children: results from the 2009 HealthStyles survey. Pediatrics 2011; 127(Suppl 1):S92-9; PMID:21502253; http://dx.doi.org/ 10.1542/peds.2010-1722N [DOI] [PubMed] [Google Scholar]
- [21].Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers' influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics 2006; 118(5):e1287-92; PMID:17079529; http://dx.doi.org/ 10.1542/peds.2006-0923 [DOI] [PubMed] [Google Scholar]
- [22].Saitoh A, Saitoh A, Sato I, Shinozaki T, Nagata S. Current practices and needs regarding perinatal childhood immunization education for Japanese mothers. Vaccine 2015; 33(45):6128-33; http://dx.doi.org/ 10.1016/j.vaccine.2015.08.069 [DOI] [PubMed] [Google Scholar]
- [23].Ministry of Internal Affairs and Communications White paper: “Information and Communications in Japan”. http://www.soumu.go.jp/johotsusintokei/whitepaper/ja/h26/html/nc253120.html [accessed 18.December.15]. Japanese [Google Scholar]
- [24].Ministry of Internal Affairs and Communications Jinkou Suikei (Heisei 24 nen 10 gatsu 1 nichi genzai) (Population estimates). http://www.stat.go.jp/data/jinsui/2012np/index.htm [accessed 15.April.14]. Japanese [Google Scholar]
- [25].Ministry of Health, Labour and Welfare Heisei 24 nen Kokuminseikatsu Kisochosa no Gaikyo (Comprehensive Survey of Living Conditions). http://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa12/index.html [accessed 15.April.14]. Japanese [Google Scholar]
- [26].Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al.. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013; 16:3-13; http://dx.doi.org/ 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- [27].Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics 2008; 26(8):661-77. http://www.ncbi.nlm.nih.gov/pubmed/18620460; PMID:18620460; http://dx.doi.org/ 10.2165/00019053-200826080-00004 [DOI] [PubMed] [Google Scholar]
- [28].Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Dordrecht: Springer; 2008 [Google Scholar]
- [29].Train KE. Discrete choice methods with simulation. 2nd ed. Cambridge: Cambridge University Press; 2009 [Google Scholar]
- [30].Gidengil C, Lieu TA, Payne K, Rusinak D, Messonnier M, Prosser LA. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012; 30(23):3445-52; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Johns TL, Hutter GE. New combination vaccines: DTaP-IPV (Kinrix) and DTaP-IPV/Hib (Pentacel). Ann Pharmacother 2010; 44(3):515-23; PMID:20197476; http://dx.doi.org/ 10.1345/aph.1M468 [DOI] [PubMed] [Google Scholar]
- [32].Brown KF, Kroll JS, Hudson MJ, Ramsay M, Green J, Long SJ, et al.. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine 2010; 28(26):4235-48; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.052 [DOI] [PubMed] [Google Scholar]
- [33].Dhillon S, Keam SJ. DTaP-IPV/Hib vaccine (Pentacel®). Paediatr Drugs 2008; 10(6):405-16; PMID:18998751; http://dx.doi.org/ 10.2165/0148581-200810060-00008 [DOI] [PubMed] [Google Scholar]
- [34].Nakayama E, Takayama N, Suganuma A, Yanagisawa N. Safety and analysis of thosewho wishes to be DTPa-IPV/Hib vaccines. J Jpn Med Assoc 2013; 142(4):859-66. Japanese [Google Scholar]
- [35].The Review Committee for Vaccination Guidelines Yobosessyu guideline (Vaccination guideline). Tokyo: Public Foundation of Vaccination Research Center; 2013. Japanese [Google Scholar]
- [36].Muta H, Morita J, Tsugawa S. lnfants who receive simultaneous administration of 3 or more vaccines complete immunization schedule earlier. JAGP 2013; 16:12-8. Japanese [Google Scholar]
- [37].Nakayama E, Yanagisawa N, Suganuma A. DTPa–IPV/Hib no anzensei to sesshu kibosha no bunseki. Prog Med 2010; 30:1699-702. Japanese [Google Scholar]
- [38].Shono A, Kondo M. Factors that affect voluntary vaccination of children in Japan. Vaccine 2015; 33(11):1406-11; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.014 [DOI] [PubMed] [Google Scholar]