Abstract
Opioid analgesic tolerance remains a considerable drawback to chronic pain management. The finding that concomitant administration of delta opioid receptor (DOR) antagonists attenuates the development of tolerance to mu opioid receptor (MOR) agonists has led to interest in producing bifunctional MOR agonist/DOR antagonist ligands. Herein, we present 7-benzylideneoxymorphone (6, UMB 246) displaying MOR partial agonist/DOR antagonist activity, representing a new lead for designing bifunctional MOR/DOR ligands.
Keywords: mu opioid receptor, delta opioid receptor, analgesic tolerance, oxymorphone, benzylideneoxymorphone
Graphical abstract
Chronic severe pain is a considerable burden facing public health, costing the United States an estimated $560-635 billion in health care costs and loss of productivity in 2011.1 Opioid analgesics remain the standard treatment for severe pain, due to their ability to inhibit nociceptive processing and maintain the patient in a state of well-being.2 Despite their benefits and widespread use, long-term use of opioids such as morphine and oxycodone is hindered by the rapid development of analgesic tolerance. Tolerance to intestinal motility does not develop as rapidly as analgesic tolerance; this “differential tolerance” results in severe constipation arising from the increased doses required to compensate for the diminished analgesic effect.3 Analgesics that produce a blunted tolerance effect would be expected to lower healthcare costs and provide beneficial outcomes to patients.
Three opioid receptor (OR) types have been cloned and characterized, namely mu (MOR), delta (DOR), and kappa (KOR), and are members of the G protein-coupled receptor (GPCR) superfamily. All approved opioid analgesics are MOR agonists, and produce analgesic and euphoric effects. Bifunctional opioid receptor ligands possessing dual profiles of OR type agonism and antagonism may also offer certain pharmacologic advantages. For example, buprenorphine (Suboxone; Subutex) is a MOR partial agonist/KOR antagonist that is used as an alternative to methadone for treating opioid dependence.4
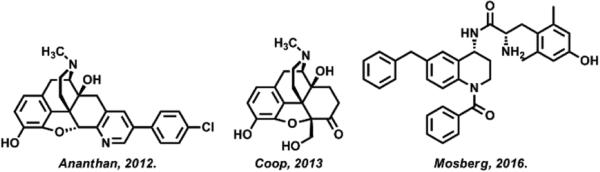
There is considerable evidence that concomitant DOR antagonism attenuates the development of tolerance to MOR agonists. Studies using DOR knockout mice5 and DOR-specific antibodies,6 as well as pharmacologic inhibition of DOR using naltrindole,7 support this hypothesis. These proof-of-concept studies have led to the development of ligands that are bifunctional MOR agonists and DOR antagonists. Building upon the success of DIPP-NH2[Ψ], the first bifunctional, tetrapeptide MOR agonist/DOR antagonist to produce antinociception with a low propensity to produce tolerance and dependence,8 several small-molecule bifunctional MOR/DOR probes have been produced (Figure 1) and demonstrate efficacy in vitro and in vivo.9-12 To overcome limitations of solubility, oral bioavailability, and receptor-type potency, we have set out to identify new chemical leads for bifunctional MOR agonist/DOR antagonist ligands.
Figure 1.
Three examples of small-molecule, bifunctional MOR agonist/DOR antagonist lead molecules.10-12
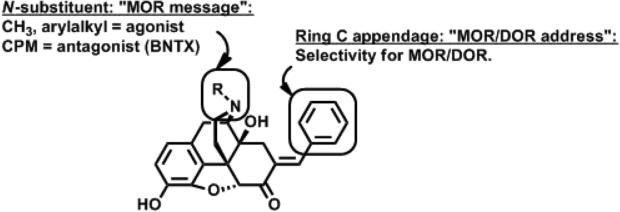
The DOR antagonist benzylidenenaltrexone (BNTX, Figure 2) was first described as a DOR-selective antagonist.13 This compound was rationalized using the “message-address concept,” whereby the naltrexone “message” was selectively targeted to DOR by the 7-benzylidene “address.” Subsequent reports14,15 demonstrate BNTX binds with similar affinity to MOR and antagonizes the effects of MOR agonists with similar potency as DOR agonists.16 Thus, we consider BNTX to be a MOR/DOR antagonist. The N-substituent frequently modulates efficacy in the 4,5-epoxymorphinan series of MOR analgesics. According to a ligand-based, quantitative conformationally-sampled pharmacophore describing DOR ligands,17 N-alkyl substitutions do not alter predicted efficacy in this same series. Applying the message-address concept,18,19 we hypothesize that substitution of the N-cyclopropylmethyl “message” of BNTX with N-alkyl groups satisfying the “message” (Figure 2) would enhance MOR efficacy and maintain low efficacy at DOR, resulting in MOR agonist/DOR antagonist bifunctional ligands.
Figure 2.
Schematic describing the “message-address” rationale for designing bifunctional MOR agonist/DOR antagonist probes based on modification of oxymorphone. CPM = cyclopropylmethyl.
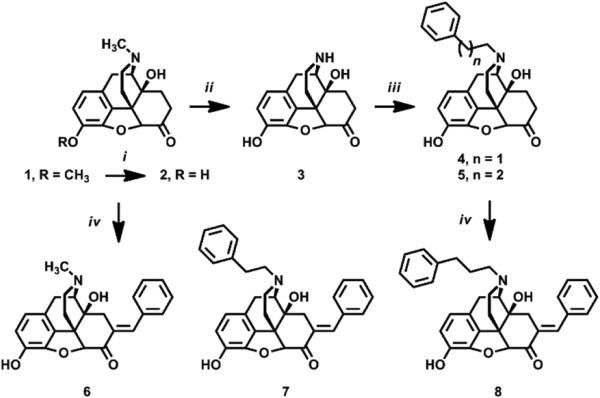
Synthesis of compounds 6 – 8 was achieved from oxycodone as shown in Scheme 1. Oxycodone (1) was converted to oxymorphone (2) using BBr3 in the usual manner.20 To synthesize N-substituted analogues 7 and 8, 2 was N-demethylated to noroxymorphone (3) using α-chloroethyl chloroformate21 and N-alkylated using the appropriate alkyl bromide. Intermediates 2, 4, and 5 were converted to C7-benzylidene analogues 6 – 8 under basic Claisen conditions.13 Purified final products were converted to water-soluble salts (HCl, oxalate) prior to pharmacologic evaluation.22
Scheme 1.
Synthesis of 6-8. Reagents and Conditions: i) BBr3, CHCl3, 0°C, 30 min; NH4OH(aq), 0°C, 30 min. ii) Ac2O, reflux, 24 hr; 1-chloroethyl chloroformate, K2CO3, Cl(CH2)2Cl, reflux, 24 hr; NaOH, MeOH, reflux, 3 hr. iii) R-Br, K2CO3, RT, 24 hr. iv) PhCHO, NaOH, MeOH, 0°C, 18 hr.
OR binding results are shown in Table 1. Compound 6 bound preferentially to MOR and DOR over KOR. Affinity for MOR and DOR generally decreased as a function of N-arylalkyl chain length, whereas KOR affinity was improved for 7 and 8 compared to 6. This caused a net decrease in MOR/DOR preference over KOR, similar to BNTX.14 The N-methyl analogue (6) exhibited the highest affinity for MOR and DOR of the series, approximately 3- and 20-fold greater than N-phenylethyl (7) and N-phenylpropyl (8) analogues for MOR, respectively. This was unexpected, as N-phenylethyl and N-phenylpropyl 4,5-epoxymorphinans are generally higher affinity MOR agonists than their parent N-methyl equivalents.23,24
Table 1.
Ki values of analogues 6 – 8 at MOR, DOR, KOR, and receptor selectivity ratios.
| Ki (nM) ± SEMa |
|||||
|---|---|---|---|---|---|
| Compound | MOR | DOR | KOR | KOR/MOR | MOR/DOR |
| 6 | 17.5 ± 1.10 | 14.4 ± 0.65 | 1067 ± 39 | 61 | 1.2 |
| 7 | 56.0 ± 3.80 | 84.0 ± 2.80 | 602 ± 60 | 10.8 | 0.7 |
| 8 | 313 ± 23 | 168 ± 9.20 | 793 ± 58 | 2.5 | 1.9 |
| BNTXb | 26 | 6.2 | 48 | 2 | 4 |
Competition binding for compounds were performed in CHO cells stably transfected with and overexpressing the human opioid receptor types hMOR, hDOR and hKOR. Assays were performed in triplicate of duplicates and reported as mean Ki values ± SEM. MOR was labeled using 1.3 nM [3H]DAMGO, DOR using 1.2 nM [3H]DPDPE, and KOR using 1.7 nM [3H]U69,593. Ki values for standard compounds: DAMGO (MOR, 1.5 nM), DPDPE (DOR, 2.4 nM), U69,593 (KOR, 1.1 nM).
Data from reference 14
Table 2 shows the results of a [35S]GTPγS assay to determine the relative efficacy of compounds at MOR and DOR. Compounds 6 and 7 were found to be MOR partial agonists with 6 demonstrating approximately 3-fold higher potency than 7. Efficacy data for 8 could not be determined due to low potency. Compounds 6 and 7 possess no significant agonist activity at DOR. Compound 6 was tested further to determine DOR antagonist potency. In the GTPγS functional assay, 6 caused a parallel rightward shift in the SNC80 concentration-response curve with Ke of 138 ± 24 nM (Table 2). Taken together, compound 6 (benzylideneoxymorphone, UMB 246) exhibited a profile of MOR/DOR preferential binding affinity, partial agonist effects at MOR, and antagonist activity at DOR. Compound 6 was therefore selected for further characterization in vivo.
Table 2.
EC50 (nM) and %Emax of oxymorphone analogues 6 – 8 at MOR and DOR.a
| MOR |
DOR |
|||
|---|---|---|---|---|
| Compound | EC50 (nM) | %Emax | EC50 (nM) | %Emax |
| 6 | 72 ± 11 | 39 ± 1.3 | N.D. | N.D.b |
| 7 | 253 ± 70 | 52 ± 3.8 | N.D. | < 10 |
| 8 | N.D. | < 10 | N.D. | N.D. |
Percent maximal stimulation relative to standard agonist (MOR, DAMGO; DOR, DPDPE; KOR, U69,593).
Caused a parallel, rightward shift in SNC80 concentration-response curve using GTPγS assay (Ke = 138 ± 24 nM, n = 3). Tested as freebase.
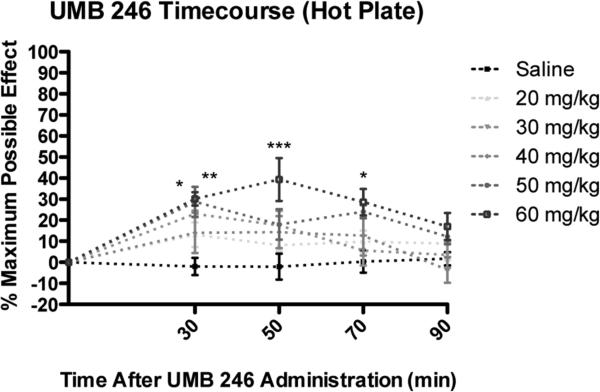
Acute antinociception assays in the mouse were performed following subcutaneous (s.c.) administration of increasing doses of 6.24,25 As shown in Figure 3, 6 produced an increase in Emax to approximately 40 % MPE with Tmax of 50 min in the hot plate nociception test. Peak antinociception effects for UMB2 46 were evident at 50 minutes post-administration for the 60 mg/kg treatment group. Repeated measures ANOVA revealed a significant difference in latency for hot plate nociception testing among treatment groups (p<0.01) and among time points (p<0.001). Bonferroni post hoc analysis revealed that the 50 mg/kg treatment group had significantly greater antinociception, compared to saline control, at 30 (p<0.05) minutes post-administration (Figure 3). Bonferroni post hoc analysis revealed that the 60 mg/kg treatment group had significantly greater antinociception, compared to saline control, at 30 (p<0.01), 50 (p<0.001), and 70 (p<0.05) minutes post-administration.
Figure 3.
Acute dose- and time-response curves for s.c. 6 treatment for the hot plate assay. For details, see 25. Significance relative to saline control: * p<0.05. ** p<0.01. *** p<0.001.
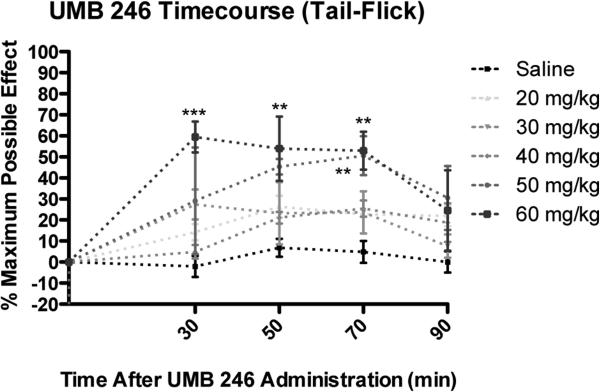
Figure 4 shows the results from the tail-flick nociceptive test. Peak antinociception effects for 6 were evident at 30 minutes post-administration for the 60 mg/kg treatment group. Repeated measures ANOVA revealed a significant difference in latency for tail-flick noticeptive testing among treatment groups (p<0.01) and among time points (p<0.001). Bonferroni post hoc analysis revealed that the 50 mg/kg treatment group had significantly greater antinociception, compared to saline control, at 70 (p<0.01) minutes post-administration (Figure 4). Bonferroni post analysis revealed that the 60 mg/kg treatment group had significantly greater antinociception, compared to saline control at 30 (p<0.001), 50 (p<0.01), and 70 (p<0.01) minutes post-administration.
Figure 4.
Acute dose- and time-response curves for s.c. 6 treatment for the tail-flick assay. For details, see 26. Significance relative to saline control: ** p<0.01. *** p<0.001.
The reduced opioid receptor binding affinity and potency for compounds 7 and 8 was unexpected. Introduction of the C7-benzylidene may introduce a steric effect that requires BOM to adopt an altered binding pose within MOR and DOR active sites, thereby precluding larger N-arylalkyl substituents.
There was some discrepancy between the Ki value (14.4 nM) and Ke value (138 nM) for 6. Ideally, for a completely neutral antagonist these values should be the same. If taken at face value the higher Ke value obtained in more physiologically relevant buffer would suggest that 6 does have some agonist activity, albeit insufficient to show up in the stringent [35S]GTPγS assay. On the other hand the values may be reflective of the different DORs used in the assays (human versus rat) and/or the different environments in the in different cell lines employed (CHO versus C6). In summary, results presented here indicate 6 possesses a profile of MOR partial agonism and DOR antagonism in vitro. 6 also produces antinociception following s.c. administration, which suggests an ability to cross the blood-brain barrier and activate MOR in vivo. This initial structure-activity relationship (SAR) study shows longer-chain N-alkyl substituents do not enhance MOR potency as in the parent oxymorphone series. Further investigation into the structural features that improve MOR efficacy and potency in the 7-benzylideneoxymorphone series are underway and will be reported in due course.
Acknowledgments
The authors thank the National Institute on Drug Abuse, National Institutes of Health (NIDA, NIH) (A.C., DA 13583; C.W.C., DA 021049; J.R.T., DA039997). C.W.C. is grateful to Concordia University Wisconsin School of Pharmacy for financial support. J.R.H. is supported by the National Institutes of Health Postdoctoral training grant no. T32GM008562. CHO cells stably transfected to express each of the human opioid receptor types (hMOR-CHO, hDOR-CHO, hKOR-CHO cells) were generously provided by Dr. Larry Toll (Torrey Pines Institute for Molecular Studies, Port St. Lucie, FL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Institute of Medicine (US) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academy of Sciences; [28 June 2016]. http://www.nationalacademies.org/hmd/Reports/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research.aspx. [Google Scholar]
- 2.Zieglgänsberger W, Tolle RT, Zimprich A, Höllt V, Spanagel R. Pain and the Brain. 1995;22:439. [Google Scholar]
- 3.Pappagallo M. Am. J. Surg. 2001;182(Suppl. 5A):11S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 4.Mattick RP, Kimber J, Breen C, Davoli M. Cochrane Database Syst. Rev. 2008;2:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Neuron. 1999;24:243. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 6.Kest B, Lee CE, McLemore GL, Inturrisi CE. Brain Res. Bull. 1996;39:185. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 7.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. J. Pharmacol. Exp. Ther. 1991;258:299. [PubMed] [Google Scholar]
- 8.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM-D, Lemieux C, Chung NN, Coderre TJ. J. Med. Chem. 1999;42:3520. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 9.Ananthan S. AAPS J. 2006;8:E118. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy JR, Bezawada P, Shim J, Jones JW, Kane MA, MacKerell AD, Jr., Coop A, Matsumoto RR. ACS Chem. Neurosci. 2013;4:1256. doi: 10.1021/cn4000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harland AA, Bender AM, Griggs NW, Gao C, Anand JP, Pogozheva ID, Traynor JR, Jutkiewicz EM, Mosberg HI. J. Med. Chem. 2016;59:4985. doi: 10.1021/acs.jmedchem.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, Bilsky EJ, Rothman RB. J. Med. Chem. 2012;55:8350. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portoghese PS, Sultana H, Nagase H, Takemori AE. Eur. J. Pharmacol. 1992;218:195. doi: 10.1016/0014-2999(92)90167-3. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RB, Upthagrove AL, Nelson WL. J. Med. Chem. 1997;40:749. doi: 10.1021/jm960573f. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa S, Portoghese PS. J. Med. Chem. 1998;41:4177. doi: 10.1021/jm980384s. [DOI] [PubMed] [Google Scholar]
- 16.Bhargava HN, Zhao G-M, Bian J-T, Nan Y, Upadhyaya SP, Xu W, Dunn WJ, III, Bauer L. Peptides. 1997;18:695. doi: 10.1016/s0196-9781(97)00121-6. [DOI] [PubMed] [Google Scholar]
- 17.Bernard D, Coop A, Mackerell AD., Jr. J. Med. Chem. 2007;50:1799. doi: 10.1021/jm0612463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwyzer R. Ann. N.Y. Acad. Sci. 1977;297:3. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 19.Portoghese PS. Trends Pharmacol. Sci. 1989;10:230. doi: 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- 20.Rice KC. J. Med. Chem. 1977;20:164. doi: 10.1021/jm00211a036. [DOI] [PubMed] [Google Scholar]
- 21.Ninan A, Sainsbury M. Tetrahedron. 1992;48:6709. [Google Scholar]
- 22.Characterization for 6: 1H NMR (500 MHz, CDCl3) δ 7.65 (s, 1H), 7.26-7.37 (m, 5H), 6.75 (d, J = 8.5 Hz, 1H), 6.66 (d, J = 8.5 Hz, 1H), 4.69 (s, 1H), 3.24 (d, J = 18.5 Hz, 1H), 3.01 (d, J = 15 Hz, 1H), 2.90 (d, J = 6 Hz, 1H), 2.65 (dd, J = 18.5, 6 Hz, 1H), 2.50 (d, J = 8 Hz, 1H), 2.40 (s, 3H), 2.30-2.37 (m, 3H), 1.64 (d, J = 11 Hz, 1H). m/z 390.4 (M+H)+.
- 23.Casy AF, Parfitt RT. Opioid Analgesics. Plenum Press; New York: 1986. [Google Scholar]
- 24.Haddou B, Beni S, Hosztafi S, Malfacini D, Calo G, Schmidhammer H, Spetea M. PloS One. 9:e99231. doi: 10.1371/journal.pone.0099231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Male Swiss-Webster mice were placed within a plastic cylinder (10.8 cm ID) atop a black anodized, aluminum plate (27.9 cm × 26.7 cm × 1.9 cm) uniformly regulated at 53°C (IITC Life Science Inc., Woodland Hills, CA). Mice were treated with saline control or UMB 246 (20-60 mg/kg, s.c.)(n = 3-4 per treatment group). Latencies were recorded 30 min after drug administration and every 20 min thereafter for 90 min.
- 26.Male Swiss-Webster mice tails were placed underneath an overhead halogen light source (IITC Life Science Inc., Woodland Hills, CA) whereby the latency to the first sign of a rapid tail flick was determined and recorded as the behavioral endpoint. Mice were treated with saline control or UMB 246 (20-60 mg/kg, s.c.)(n = 3-4 per treatment group). Latencies were recorded 30 min after drug administration and every 20 min thereafter for 90 min.