ABSTRACT
Objectives: To evaluate the persistence of protection from hepatitis B (HB) vaccination among adolescents immunized with a primary series of HB vaccine as infants, and the immune response to booster doses. Methods: Healthy adolescents aged 15–17 y vaccinated with HB vaccine only at birth were enrolled. Baseline serum hepatitis B surface antigen (HBsAg), antibody against hepatitis B surface antigen (anti-HBs) and antibody against hepatitis B core antigen (anti-HBc) were detected by Enzyme-Linked Immunosorbent Assay (ELISA) and anti-HBs level was measured using Chemiluminescent Microparticle Immunoassay (CMIA). The rate of HBV infection was calculated. The seroprotection rate of anti-HBs (≥ 10 mIU/ml) and GMC level were used to evaluate the persistence of immunity from HB vaccination. Those with anti-HBs < 10 mIU/ml were immunized with booster doses of HB vaccine and the anamnestic response was assessed. Results: Of 180 adolescents who received a primary series of HB vaccinations as infants, 3 (1.7%) had HBV infection and 74 (41.1%) had anti-HBs ≥ 10 mIU/ml with a GMC of 145.11 mIU/ml. The remaining 103 (57.2%) with anti-HBs < 10 mIU/ml received a booster dose of 20 μg HB vaccine and achieved the seroprotection rate of 84% (84/100) and a GMC of 875.19 mIU/ml at one month post-booster. An additional dose of 60 μg HB vaccine was administered to the 16 adolescents with anti-HBs < 10 mIU/ml after the first booster. All of them obtained anti-HBs seroprotection with a GMC of 271.02 mIU/ml at 1.5 months after an additional dose. Conclusions: Vaccine-induced immunity persisted for up to 15–17 y in 89.3% (158/177) of participants after a primary HB vaccination in infancy. Administering a booster dose of 20μg HB vaccine elicited an anamnestic immune responses in the majority of individuals with baseline anti-HBs <10 mIU/ml.
KEYWORDS: adolescents, booster vaccination, hepatitis B vaccine, immune memory, long-term immunogenicity
Introduction
Hepatitis B virus (HBV) is a leading cause of acute and chronic hepatitis, including its long-term sequelae, which include liver cirrhosis and hepatocellular carcinoma (HCC). The World Health Organization (WHO) estimates that 2 billion people worldwide have been infected with HBV, and 240 million have chronic HBV infections. Around 650,000 die each year from the complications of chronic hepatitis B. Fortunately, the serious and potentially fatal infection can be prevented by vaccination, and a substantial reduction of new HBV infections and carrier rates have been observed in countries where vaccination has been implemented.1 The Chinese government started to implement the policy of universal hepatitis B (HB) vaccination of all infants in 19922 and integrated HB vaccine into the routine immunization program in 2002.3 After more than decades of efforts, the coverage rate of full 3-dose immunization and the rate of timely birth dose in infants increased from 30% and 22% (1992) to 99% and 95% (2014), respectively4 and the HB surface antigen (HBsAg) prevalence in the general population dropped from 9.75% in 1992, to 7.18% in 2006, and 2.64% in 1–29 y old in 2014.5
Universal vaccination could induce protective antibody against HB surface antigen (anti-HBs) (≥10 mIU/ml) in approximately > 95% healthy children after a primary series of HB vaccine.6,7 However, the duration of protection after the primary series of either plasma-derived or recombinant HB vaccines was not well investigated. Some studies suggested that vaccine-induced immunity may persist for up to 15–20 y or even longer after a complete series,8,9 and it is followed by a gradual decline in titer of anti-HBs year by year. The anti-HBs positive rate in the children who developed protective anti-HBs level after the primary HB vaccination series dropped from 99% (1 y after vaccination) to 83% (5 years), 71% (7 years), and 37% (15–17 years).10,11 If the anti-HBs level fell below 10 mIU/ml, the individual was vulnerable to be infected with HBV. Thus, the necessity for booster doses decades after HB vaccination needs to be assessed. In 2000, the European Consensus Group on Hepatitis B Immunity issued a statement recommending against the use of boosters of HB vaccine in immune-competent individuals 15 y after primary immunization,12 and in the position paper published in 2009, WHO suggested that there was “no compelling evidence for recommending administering a booster dose of HB vaccine in routine immunization programs”.13 The recommendation was based on the high rate of anamnestic responses to HBV in most people for many years after priming, even when detectable antibodies had been absent at the time of exposure. Nevertheless, some investigators continue to recommend boosters because of the progressive decline of anti-HBs over time and the associated potential risk of development of HBV infections: approximately 4.1%–6.7% of vaccinees may acquire HBV infections [antibody against hepatitis B core antigen (anti-HBc) positive] after 15 y of HB vaccination,14-16 especially in the children who had a low response to the initial vaccination17 and the diminished immune memory to HB vaccination, which raises concerns regarding the efficacy of HB vaccine booster practices.9 Therefore, HB vaccine boosters might be required for adolescents or adults who lack protective levels of anti-HBs following a primary series of HB vaccination in infancy and live in middle or high HBV-endemic areas where opportunities for HBV exposure exist in the household and community.
Hepatitis B is highly endemic in the western region of China. The State Project of Liver and Mental Health Aid for Children was launched in Shanxi Province, northwestern of China. One of the goals of the State Project was to vaccinate the children and adolescents with anti-HBs < 10 mIU/ml. In combination with this State Project, we conducted a study to evaluate the persistence of protection from HB vaccination among adolescents immunized in infancy as well as the immune response to booster doses of HB vaccine.
Results
Baseline seroprevalence of HBV markers
Of 1479 adolescents tested for HBV markers, 3.38% (50/1479) were HBsAg positive; 4.53% (67/1479) were anti-HBc positive; 63.56% (940/1,479) were positive for anti-HBs alone; and 29.88% (442/1479) were negative for all markers.
Recruitment of participants
Of 1,479 participants assessed for eligibility, 1,300 were excluded because of no or unclear HB vaccination history, or receiving the primary HB vaccination series after the first year of life. As a result, 180 adolescents met the inclusion criteria and were enrolled into the current study.
HBV exposure and infection
Exposure to HBV infection was found in 1.67% (3/180) of participants, with 1 being HBsAg positive alone, and 2 being anti-HBc positive (1 positive for anti-HBc alone; and 1 positive for both anti-HBc and anti-HBs, and negative for HBsAg, HBeAg, anti-HBe, and HBV DNA).
Persistence in protection from hepatitis B vaccine
Of 180 participants, 177 were evaluated for seroprotection of anti-HBs induced by the primary HB vaccination series in infancy. One hundred and 3 participants (58.2%) had an anti-HBs level of < 10 mIU/ml, while 74 (41.8%) participants had protective levels of anti-HBs (≥10 mIU/ml) that indicated continuous protection 15–17 y after a primary series of HB vaccination in infancy with the GMC level of 145.11 ± 5.40 mIU/ml (95% CI: 98.17–214.44 mIU/ml). Among 74 individuals with a seroprotective level of anti-HBs, 48.65% (36/74) were low response (10 ≤ anti-HBs < 100 mIU/ml); 36.49% (27/74) were moderate response (100 ≤ anti-HBs < 1,000 mIU/ml); and14.86% (11/74) were high response (anti-HBs ≥ 1,000 mIU/ml). The overall seroprotection rates did not differ significantly in terms of gender or BMI (P > 0.05, data not shown).
Anamnestic response and factors associated with the response after a booster dose of 20 μg HB vaccine
A total of 103 individuals with anti-HBs < 10 mIU/ml accepted the administration of a booster dose of 20 μg HB vaccine. At one month after the booster, 100 participants were available for the followed-up, and among them, 84% (84/100) showed an anamnestic response (anti-HBs ≥ 10 mIU/ml) with GMC level of 875.19 ± 4.84 mIU/ml (95% CI: 621.58–1231.97 mIU/ml). The rates of low, moderate and high response were 7% (7/100), 39% (39/100), and 38% (38/100), respectively. Sixteen (16%) participants maintained the anti-HBs negative were considered as non-responders.
Comparison of the demographic characteristics between the anamnestic response group and non-response group revealed no significant differences in age (P = 0.599), sex ratio (P = 0.930), or BMI (P = 0.986, data not shown). Further analyses on seroprotection rates, GMCs and distributions of anti-HBs were performed according to demographic characteristics as shown in Table 1. The overall seroprotection rates did not differ significantly in terms of gender or BMI (P > 0.05). As for GMC, participants with BMI < 18.5 kg/m2 showed significantly higher anti-HBs titers than those with BMI ≥ 25.0 kg/m2 (1387.39 vs. 173.94 mIU/ml, P = 0.048) as well as those with 18.5 kg/m2 ≤ BMI < 25.0 kg/m2 (1387.39 vs. 273.53 mIU/ml, P = 0.055) . At the same time, the rates of low and high response were significantly different among 3 BMI groups: with the rise of BMI, the rates of low response presented a trend of increasing [0% (0/13), 5.63% (4/71), 18.75% (3/16), P = 0.043], while rates of high response declined in turn [69.23% (9/13), 36.62% (26/71), 18.75% (3/16), P = 0.006].
Table 1.
Seroprotection rates, GMCs and distributions of anti-HBs stratified by demographic characteristics after booster vaccination.
| Seroprotection rate, % | GMC, mIU/ml | anti-HBs < 10 mIU/ml, % | 10 ≤ anti-HBs< 100 mIU/ml, % | 100 ≤ anti-HBs< 1000 mIU/ml, % | anti-HBs ≥ 1000 mIU/ml, % | ||
|---|---|---|---|---|---|---|---|
| 20 μg HBV vaccine | |||||||
| Subjects, no. N = 100 | |||||||
| Gender | Male | 83.67 (41/49) | 712.69 | 16.33 (8/49) | 10.20 (5/49) | 36.73 (18/49) | 36.73 (18/49) |
| Female | 84.31 (43/51) | 1064.39 | 15.69 (8/51) | 3.92 (2/51) | 41.18 (21/51 ) | 39.22 (20/51) | |
| P Value | 0.930 | 0.247 | 0.930 | 0.402 | 0.649 | 0.798 | |
| BMI | Underweight (<18.5) (1) | 84.62 (11/13) | 1387.39 | 15.38 (2/13) | 0 (0/13) | 15.38 (2/13) | 69.23 (9/13) |
| Normal (18.5–24.9) (2) | 83.10 (59/71) | 273.53 | 16.90 (12/71) | 5.63 (4/71) | 40.85 (29/71) | 36.62 (26/71) | |
| Overweight and Obese (≥25.0)(3) | 87.50 (14/16) | 173.94 | 12.50 (2/16) | 18.75 (3/16) | 50.00 (8/16) | 18.75 (3/16) | |
| P Value | 0.904 | 0.104 | 0.904 | 0.043 | 0.138 | 0.006 | |
| P12 = 1.000 | P12 = 0.055 | P12 = 1.000 | P12 = 1.000 | P12 = 0.151 | P12 = 0.028 | ||
| P13 = 1.000 | P13 = 0.048 | P13 = 1.000 | P13 = 0.232 | P13 = 0.114 | P13 = 0.006 | ||
| P23 = 0.955 | P23 = 0.557 | P23 = 0.955 | P23 = 0.217 | P23 = 0.503 | P23 = 0.171 | ||
| 60 μg HBV vaccine | |||||||
| Subjects, no. N = 16 | |||||||
| Gender | Male | 100 (8/8) | 153.57 | 0 (0/8) | 50 (4/8) | 37.50 (3/8) | 12.50 (1/8) |
| Female | 100 (8/8) | 478.30 | 0 (0/8) | 37.50 (3/8) | 12.50 (1/8) | 50 (4/8) | |
| P Value | — | 0.312 | — | 1.000 | 0.569 | 0.282 | |
| BMI | Underweight (<18.5) (1) | 100 (2/2) | 51.39 | 0 (0/2) | 50 (1/2) | 50 (1/2) | 0 (0/2) |
| Normal (18.5–24.9) (2) | 100 (12/12) | 527.96 | 0 (0/12) | 33.33 (4/12) | 25 (3/12) | 41.67 (5/12) | |
| Overweight and Obese (≥25.0)(3) | 100 (2/2) | 26.16 | 0 (0/2) | 100 (2/2) | 0 (0/2) | 0 (0/2) | |
| P Value | — | 0.091 | — | 0.329 | 0.264 | 1.000 | |
| P12 = 0.140 | P12 = 1.000 | P12 = 0.505 | P12 = 0.505 | ||||
| P13 = 0.733 | P13 = 1.000 | P13 = 1.000 | P13 = — | ||||
| P23 = 0.064 | P23 = 0.165 | P23 = 1.000 | P23 = 0.165 | ||||
We tried to investigate factors associated with immune response to a booster dose of 20 μg HB vaccine. A multivariate logistic regression analysis was performed, and the variable of pre-boost anti-HBs level was identified (OR = 4.818, 95% CI: 1.393–16.668, P = 0.013), as shown in Table 2. Furthermore, 0.7 mIU/ml of pre-boost anti-HBs level was determined in the condition of the highest Youden's index through ROC (receiver operating characteristic curve) analysis. The participants with pre-boost anti-HBs levels ≥ 0.7 mIU/ml had a higher probability of achieving immune response (anti-HBs ≥ 10 mIU/ml) to the booster dose of 20 μg HB vaccine than those with anti-HBs levels < 0.7 mIU/ml (OR = 8.071, 95% CI: 2.131–30.571, P = 0.002).
Table 2.
Logistic regression analysis for testing factors associated with immune response to a booster dose of 20 μg HBV vaccine.
| Characteristics | regression coefficient | Standard error | Wald test | P Value | OR | 95%CI for OR |
|---|---|---|---|---|---|---|
| Age | −0.270 | 0.440 | 0.376 | 0.540 | 0.764 | 0.322–1.809 |
| Gender | ||||||
| Male | — | — | — | — | — | — |
| Female | 0.224 | 0.595 | 0.142 | 0.706 | 1.251 | 0.390–4.016 |
| BMI, kg/m2 | ||||||
| Normal (18.5–24.9) | — | — | 0.728 | 0.695 | — | — |
| Underweight (<18.5) | −0.334 | 0.887 | 0.142 | 0.707 | 0.716 | 0.126–4.076 |
| Overweight and Obese (≥25.0) | 0.632 | 0.878 | 0.518 | 0.472 | 1.881 | 0.336–10.520 |
| Pre-boost anti-HBs level, (log transferred) mIU/ml | 1.572 | 0.663 | 6.165 | 0.013 | 4.818 | 1.393–16.668 |
| Constant | 6.101 | 7.248 | 0.709 | 0.400 | 446.471 | — |
Immune response to a booster dose of 60 μg HB vaccine
According to the study protocol, 16 non-responders to a booster of 20 μg HB vaccine were immunized an additional dose of 60 μg HB vaccine, and 100% (16/16) persons demonstrated immune response with anti-HBs levels ≥ 10 mIU/ml assessed at 1.5 month after injection. Mean GMC level was 271.02 ± 8.78 mIU/ml (95% CI: 85.17–862.38 mIU/ml). The rates of low, moderate and high response were 43.75% (7/16), 25% (4/16), and 31.25% (5/16), respectively. As shown in Table 1, all the seroprotection rates, GMCs did not differ significantly in terms of gender or BMI (P > 0.05) after the 60 μg booster dose.
Discussion
The primary HB vaccination series could induce protective antibody in > 95% healthy children. However, there are about 20 million new births every year in China,18 it is estimated that there are still 1 million children showing no response to the primary immunization. Even children who responded with protective antibody also experienced significant decline of anti-HBs concentrations in the following years. All of the individuals who lack protective levels of anti-HBs are at potential risk of HBV infection. Thus, it is necessary to evaluate the duration of protection provided by the primary series of HB vaccination in infancy, especially in highly HBV epidemic areas, and assess the possible need for booster doses.
Universal HB vaccination has been introduced for more than 2 decades in China, and a recombinant HB vaccine was used for immunization since 2000. A seroprevalence of HBV infection and a long-term protection of HB vaccine were investigated in adolescents of 15–17 y old who are living in the western region of China where HB is highly endemic. In the current study, the positive rate of HBsAg in adolescents was 3.38% (50/1,479), which is lower than that of 15–29 y old group out of the general population in the western region of China (5.60%) and higher than that of 5–14 y old group (1.56%). The positive rate of anti-HBs alone in this population was 63.56% (940/1,479), higher than that of 15–29 y old group (54.40%).5
Of 180 participants enrolled in our study, asymptomatic breakthrough infections occurred in 3 participants (1.67%). Many follow-up studies demonstrated that the rate of breakthrough infection was rather low among the vaccine recipients, mainly manifested as anti-HBc positive (1.0%–13.8%), transient HBsAg-positive (0.7%–5.4%) or HBV DNA positive (0.19%–0.9%), but few clinically significant infections diagnosed or few HBsAg carriers reported.8,9,19 Due to the failure to get data of anti-HBs levels at 1- or 2-month after the primary vaccination, it is impossible to tell whether they became infected because of vaccine failure or breakthrough infections in the current study, which is one the limitations of this study.
After 15–17 y following a primary series of HB immunization in infancy, there was > 40% (41.8%) adolescents in our cohort having protective levels of anti-HBs with a GMC of 145.11 mIU/ml, and the overall seroprotection rates did not differ significantly in terms of gender, BMI. Data from a 30-year follow-up study in neonates born to HB carrier mothers in Hong Kong showed the dynamic changes of anti-HBs seroconversion rate: 92.6% (1006/1086, baseline), 91.2% (980/1075, Year 1), 64.2% (612/953, Year 5), 44.8% (355/792, Year 10), 33.3% (203/610, Year 16), 36.3% (130/358, Year 21), 37.4% (92/246, Year 30). The proportion of having protective levels of antibody in our study (41.8%) was higher than 33.3% (Year 16), which could be due to the difference in the study cohorts (general neonates vs. neonates born to HBV carrier mothers).20 A recent meta-analysis confirmed that the duration of protection offered by HB vaccines was negatively influenced by the schedule of vaccine administration (gap time between last and preceding dose of the primary vaccine series < 6 months), vaccine dosage (lower vaccine dosage than presently recommended) and the characteristics of the population (maternal carrier status).21
Vaccine protection, however, may still persist through immune memory. An indirect method to assess immune memory is to look for an “anamnestic response” - a rapid, prominent increase in antibody level and in response to a booster dose of HB vaccine. Many studies having more than 10-year follow-up demonstrated high rates of anamnestic response to booster doses, even when detectable antibodies had been absent at the time of exposure.22-28 We investigated a cohort of adolescents immunized 3-dose HB vaccine in infancy from a highly HBV endemic area, and challenged a group of them (anti-HBs < 10 mIU/ml) with a booster dose of 20 μg HB vaccine 15–17 y later. One month after the booster, 84% (84/100) individuals showed an anamnestic response with GMC of 875.19 mIU/ml, providing strong evidence that the immune memory existed in the majority of adolescents. However, there were also 16% (16/100) individuals did not develop protective antibody levels after the first booster of HB vaccine, which may indicate waning of immune memory. An et al boosted one dose of HB vaccine to the anti-HBs negative children who had been vaccinated 5 or 10 y ago, and found that positive rates of anti-HBs post-boost were 100% (Year 5) vs. 93.62% (Year 10) and GMC were 243.45 (Year 5) vs. 47.24 mIU/ml (Year 10), presenting the GMC level of Year 10 declined 5-fold than that of Year 5.29 Data from another study showed that the anamnestic response rates after receipt of a booster dose of vaccine were 85.3% (64/75, Year 10) vs. 73.6% (39/53, Year 15).17 Thus, population maintained immune memory decreased with an advancing age or increasing interval between booster and initial vaccination. In addition to age, a higher BMI might negatively affect the immune response triggered by HB vaccine: overweight or obese persons (BMI ≥ 25.0 kg/m2) were not easy to produce the protective anti-HBs after vaccination.30 Our study showed that participants with BMI < 18.5 kg/m2 presented significantly higher anti-HBs titers than those with BMI ≥ 25.0 kg/m2 (1387.39 vs. 173.94 mIU/ml), which indicating that individuals with relatively low BMIs tend to have anamnestic response with relatively high levels of GMC to the booster vaccination.
As for the appropriate doses of booster vaccination, researchers from Taiwan evaluated the efficacy of a 3-dose booster protocol [20 μg (0–1–6 month)] in university students (20–22 y old) vaccinated in infancy with anti-HBs < 10 mIU/ml, and 75.3% (238/316) had anti-HBs ≥ 10 mIU/ml after the first booster dose while the rate rose to 95.3% (122/128) after full series vaccination, demonstrating the 3-dose booster protocol benefits with a high seroconversion of anti-HBs.16 Another investigation in Hong Kong also applied the same booster protocol in university students (17–23 y old) vaccinated in infancy with anti-HBs < 10 mIU/ml, and the seroconversion rate increased from 85.5% (59/69) post one booster dose to 100% (69/69) post 3 doses. And in the seroconversion group, the rates of low, moderate and high response were 29% (20/69), 38% (26/69), 19% (13/69) post one-dose turned to 3% (2/69), 23% (16/69), 74% (51/69) post 3-dose boost, respectively. The data showed that a 3-dose booster, rather than a single dose, is required for the majority to achieve a seroprotection rate of > 90% for anti-HBs negative individuals immunized in infancy. In the current study, we attempted to use the 20 μg-60 μg (0–1 month) regimen as a booster protocol, seroprotection rate of 84% after a 20 μg vaccine and 100% after an additional 60 μg vaccine were achieved, respectively, demonstrating the same immune effect compared with 20 μg (0–1–6 month) vaccine regimen (100% vs. 100%). Because of the higher concentration of HBsAg, the 60 μg vaccine has a better immunogenicity and could simplify the implementation of HB vaccination at the same time. As for the appropriate population of boosting, individuals such as health care workers or persons having HBsAg carriers in family members etc, a booster vaccination might be a reasonable alternative if opportunities for HBV exposure exist.
Some researchers analyzed the factors influencing the effect of booster immunization. A study from USA found that college students (18–23 y old) with pre-boost anti-HBs levels of 1–9 mIU/ml were more likely to respond to a challenge dose than those with 0 mIU/ml (83% vs. 50% for seroprotection rate, P < 0.001; 99.8 vs. 9.8 mIU/ml for GMC, P = 0.042),31 suggesting the presence of any detectable anti-HBs among persons vaccinated in the remote past may indicate the persistence of immune memory. In our study, multivariate logistic regression analysis revealed that pre-boost anti-HBs titers ≥ 0.7 mIU/ml was the only independent predictor of anamnestic response, which is similar to the study above. The cutoff value of anti-HBs level of 0.7 mIU/ml obtained in our study was calculated based on a relative small size of samples (103 participants), which need to be justified in a large number of samples.
There were several shortcomings in our study: (1) We did the cross-sectional study on the persistence of anti-HBs antibody in adolescents of 15–17 y old immunized HB vaccine in infancy. The ideal study design is a cohort study or longitudinal study rather than a cross-sectional study. (2) Due to the limitations of the questionnaire and field work, we failed to get more detailed clinical information from the students who had HBV infection, including whether mothers or any family members were HBsAg carriers and their serological status after the primary vaccination, which led to difficult to identify the cause of HBV infection. (3) Relatively small sample size in our study may not be adequate to support results from the inferential statistical analyses.
In conclusion, although the adolescents aged 15–17 y showed high seronegative rate (58.2%) of anti-HBs (<10 mIU/ml) after primary 3-dose HB vaccination in infancy, the results after booster vaccination suggested that immune memory was well preserved in most (84%) of the adolescents. It demonstrated that vaccine protection continues up to 15–17 years, and 89.3% of participants had evidence of protection (anti-HBs ≥ 10 mIU/ml or response to a booster dose of vaccine). Furthermore, our study also pointed out a high success rate (100%) by administering the booster vaccination regimen [20 μg-60 μg (0–1 month)] in adolescents with anti-HBs < 10 mIU/ml in the HBV epidemic region. We believe this booster protocol could be a useful reference for HB boosting vaccination program for adolescents (>15 y old) in HBV epidemic areas.
Materials and methods
Study design
A total of 1,479 healthy adolescents aged 15–17 y from 2 senior middle schools in a county of Shanxi province were investigated in October 2015, and checked the HB vaccination history and tested for baseline HBsAg, anti-HBs, and anti-HBc using ELISA. Those participants with evidence of HB immunization series of 3 doses in infancy only (according to the record of immunization card) and no HB booster vaccine given since primary HB immunization were enrolled. A recombinant HB vaccine of 5μg was used for infant immunization. Participants with the screening results of positive HBsAg and/or anti-HBc were further detected HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc using CMIA and HBV DNA by PCR, then HBV infection rate was analyzed. All enrolled participants except persons identified with HBV infection were re-measured anti-HBs quantitatively by CMIA, and anti-HBs ≥ 10 mIU/ml was regarded as protected and evaluated the persistence of protection from HB vaccination. Individuals with anti-HBs < 10 mIU/ml were immunized with a 20 μg booster dose of HB vaccine, and a subsequent 60 μg booster dose was given if anti-HBs < 10 mIU/ml still maintained following the first booster. A positive response to the booster dose was defined as anti-HBs levels ≥ 10 mIU/ml at 30 d or more after the boost. Blood samples for immunogenicity analyses were obtained from participants 1 or 1.5 month following administration of every dose. Figure 1 showed the flow chart of the study.
Figure 1.
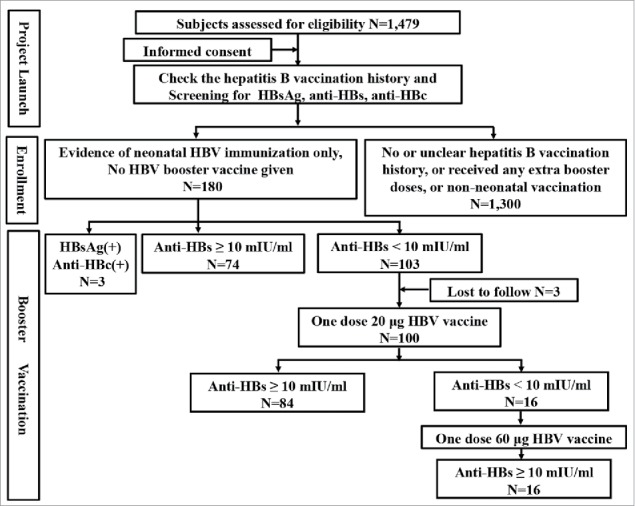
The flow diagram of participants through the study.
The written informed consent was obtained from each participants or his/her guardian prior to enrollment. The project protocol was approved by Peking University Institutional Review Board and in accordance with the ethical standards of the Helsinki Declaration.
Vaccines
The two types of HB vaccine were manufactured by Shenzhen Kangtai Biological Products Co., Ltd., China containing 20 μg (batch number B201408003) or 60 μg (batch number A201408006) of HBsAg per dose (1.0 ml), respectively. The vaccines were stored at 4°C and maintained within the cold chain during transportation. The vaccinees were administered intramuscularly into the deltoid muscle.
HBV serological tests
The baseline HBsAg, anti-HBs, and anti-HBc were qualitatively tested using ELISA kits (Shanghai Kehua Bio-engineering Co., Ltd.). Further, the serum samples of qualified participants were re-measured anti-HBs quantitatively by CMIA using the Architect i2000SR analyzer (Abbott Diagnostics Division).The participants with positive results of ELISA for HBsAg and/or anti-HBc were further tested for HBV DNA using PCR as described previously.32
Throughout the study, we took HBsAg to be the marker of the chronic carrier stage (and/or with anti-HBc/HBV DNA positive), and individuals with positive both anti-HBs and anti-HBc were defined as having protective immunity derived from past natural virus infection, while only those with positive anti-HBc were defined as being infected before as well. Participants with positive anti-HBs alone were defined as being vaccination-induced immunity.
Statistical analysis
Continuous variables were presented as Mean ± SD depending on the underlying normal distribution, and Student's t-test or one-way ANOVA (analysis of variance) was used to analyze continuous data. Pearson Chi-square tests or Fisher's exact tests were used to compare categorical data. Multivariate logistic regression analyses were performed for testing variables associated with immune response to the booster dose. A P value of < 0.05 (2-tailed) was considered statistically significant. All statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Abbreviations
- Anti-HBc
antibody against hepatitis B core antigen
- Anti-HBs
antibody against hepatitis B surface antigen
- ANOVA
analysis of variance
- BMI
body mass index
- CI
confidence interval
- CMIA
Chemiluminescent Microparticle Immunoassay
- ELISA
Enzyme-Linked Immunosorbent Assay
- GMC
geometric mean concentration
- HBcAg
hepatitis B core antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HB vaccine
hepatitis B vaccine
- OR
odds ratio
- ROC
receiver operating characteristic curve
- S/CO
sample signal-to-cutoff ratio
- SD
standard deviation
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr. Shumei Yun, from Missouri Department of Health and Senior Services, Jefferson City, MO, USA for proofreading and editing the manuscript. Authors are also grateful to Zhonglian Center for Liver Health and China Pharmaceutical Culture Society for supporting the project.
Funding
This study was supported by the State Project of Liver and Mental Health Aid for Children, Major Science and Technology Special Project of China twelfth Five-year Plan (2012ZX10002001) and Guangxi Key Laboratory for the Prevention and Control of Viral Hepatitis.
Author contributions
Dr. H. Zhuang designed the study and critically proofread the manuscript. Dr. X. E. Liu and T. Li organized the field work. Dr. Z. Z. Wang, Y. H. Gao, W. Lu, F. Ding and L. Yan performed the experiments. Dr. Z. Z. Wang analyzed the data and wrote the manuscript. Dr. X. E. Liu revised the manuscript for important contents. All author approved the final version of the manuscript, including the authorship list.
References
- [1].WHO Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection Geneva: WHO, 2015. [PubMed] [Google Scholar]
- [2].Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 2009; 122:98-102; PMID:19187625 [PubMed] [Google Scholar]
- [3].Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al.. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/ 10.1086/599332 [DOI] [PubMed] [Google Scholar]
- [4].Chinese Academy of Medical Sciences, CAMS Chinese medical science and technology development reports Beijing: Science Publishing House, 2016. [Google Scholar]
- [5].National HBV serosurvey for Chinese population aged 1–29 years China medical tribune. August 06, 2015; Sect D-01. [Google Scholar]
- [6].Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008; 26:6266-73; PMID:18848855; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.056 [DOI] [PubMed] [Google Scholar]
- [7].Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev Vaccines 2004; 3:119-29; PMID:15056038; http://dx.doi.org/ 10.1586/14760584.3.2.119 [DOI] [PubMed] [Google Scholar]
- [8].Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68-75; PMID:21653306; http://dx.doi.org/ 10.1093/cid/cir270 [DOI] [PubMed] [Google Scholar]
- [9].Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, et al.. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis 2008; 197:1419-26; PMID:18444799; http://dx.doi.org/ 10.1086/587695 [DOI] [PubMed] [Google Scholar]
- [10].Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis 2003; 187:134-8; PMID:12508157; http://dx.doi.org/ 10.1086/345871 [DOI] [PubMed] [Google Scholar]
- [11].Lee PI, Lee CY, Huang LM, Chang MH. Long-term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B e antigen. J Pediatr 1995; 126:716-21; PMID:7751994; http://dx.doi.org/ 10.1016/S0022-3476(95)70398-5 [DOI] [PubMed] [Google Scholar]
- [12].European Consensus Group on Hepatitis B Immunity Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 2000; 355:561-5; PMID:10683019; http://dx.doi.org/ 10.1016/S0140-6736(99)07239-6 [DOI] [PubMed] [Google Scholar]
- [13].WHO Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec 2009; 84:405-19; PMID:19817017 [PubMed] [Google Scholar]
- [14].Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 2009; 27:1858-62; PMID:19186203; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.027 [DOI] [PubMed] [Google Scholar]
- [15].Chang HC, Yen CJ, Lee YC, Chiu TY, Jan CF. Seroprevalence of hepatitis B viral markers among freshmen-20 years after mass hepatitis B vaccination program in Taiwan. J Formos Med Assoc 2007; 106:513-9; PMID:17660140; http://dx.doi.org/ 10.1016/S0929-6646(07)60001-1 [DOI] [PubMed] [Google Scholar]
- [16].Su FH, Chu FY, Bai CH, Lin YS, Hsueh YM, Sung FC, Yeh CC. Efficacy of hepatitis B vaccine boosters among neonatally vaccinated university freshmen in Taiwan. J Hepatol 2013; 58:684-9; PMID:23207141; http://dx.doi.org/ 10.1016/j.jhep.2012.11.036 [DOI] [PubMed] [Google Scholar]
- [17].Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, Stevenson K, Yano VM, Armstrong GL, Samandari T, et al.. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine 2012; 30:1644-9; PMID:22245310; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.106 [DOI] [PubMed] [Google Scholar]
- [18].National Bureau of Statistics of China China statistical yearbook. Available at: http://www.stats.gov.cn/tjsj/ndsj/2015/indexch.htm. Last accessed 8June2016. [Google Scholar]
- [19].Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28:730-6; PMID:19892043; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.074 [DOI] [PubMed] [Google Scholar]
- [20].Lin AW, Wong KH. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J Hepatol 2013; 59:1363-4; PMID:23994385; http://dx.doi.org/ 10.1016/j.jhep.2013.08.021 [DOI] [PubMed] [Google Scholar]
- [21].Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J 2013; 32:307-13; PMID:23249904; http://dx.doi.org/ 10.1097/INF.0b013e31827bd1b0 [DOI] [PubMed] [Google Scholar]
- [22].Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother 2013; 9:1679-84; PMID:23732904; http://dx.doi.org/ 10.4161/hv.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van der Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, Kahn A, Jeffries D, Akum AA, Hall AJ, et al.. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. Plos One 2007; 2:e753; PMID:17710152; http://dx.doi.org/ 10.1371/journal.pone.0000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jafarzadeh A, Montazerifar SJ. Persistence of anti-HBs antibody and immunological memory in children vaccinated with hepatitis B vaccine at birth. J Ayub Med Coll Abbottabad 2006; 18:4-9; PMID:17591001 [PubMed] [Google Scholar]
- [25].Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, Bell BP. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics 2007; 120:e373-81; PMID:17636112; http://dx.doi.org/ 10.1542/peds.2007-0131 [DOI] [PubMed] [Google Scholar]
- [26].Gabbuti A, Romano L, Blanc P, Meacci F, Amendola A, Mele A, Mazzotta F, Zanetti AR. Long-term immunogenicity of hepatitis B vaccination in a cohort of Italian healthy adolescents. Vaccine 2007; 25:3129-32; PMID:17291637; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.045 [DOI] [PubMed] [Google Scholar]
- [27].Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010; 51:1547-54; PMID:20209603; http://dx.doi.org/ 10.1002/hep.23543 [DOI] [PubMed] [Google Scholar]
- [28].Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al.. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis 2016; 214:16-22; PMID:26802139; http://dx.doi.org/ 10.1093/infdis/jiv748 [DOI] [PubMed] [Google Scholar]
- [29].An JH, Xing XS, Yan YR, He XH, Guang M, Li GY, Li TS, Chang SY, Fan FY, Zhai RF. Observation on the immune persistence of recombinant yeast-derived hepatitis B vaccine (YDV) after 10 years vaccination. Chin J Vaccine Immunization 2005; 06:470-2. [Google Scholar]
- [30].Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol 2006; 78:169-77; PMID:16372285; http://dx.doi.org/ 10.1002/jmv.20524 [DOI] [PubMed] [Google Scholar]
- [31].Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, Twu SJ, Su IJ, Huang LM, Lee CY. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology 2004; 40:1415-20; PMID:15565627; http://dx.doi.org/ 10.1002/hep.20490 [DOI] [PubMed] [Google Scholar]
- [32].Zhao CY, Ding H, Yan CH, Yan L, Peng YQ, Jin PY, Li T, Zhuang H. A novel reverse transcriptase mutation in genotype C hepatitis B virus associated with rtM204I/V drug resistant mutation. Chin J Viral Dis 2012; 04:260-6. [Google Scholar]


