Abstract
Non-invasive techniques for quantifying early biochemical and biomechanical changes in articular cartilage may provide a means of more precisely assessing osteoarthritis (OA) progression. The goals of this study were to determine the relationship between T1rho magnetic resonance (MR) imaging relaxation times and changes in cartilage composition, cartilage mechanical properties, and synovial fluid biomarker levels and to demonstrate the application of T1rho imaging to evaluate cartilage composition in human subjects in vivo. Femoral condyles and synovial fluid were harvested from healthy and OA porcine knee joints. Sagittal T1rho relaxation MR images of the condyles were acquired. OA regions of OA joints exhibited an increase in T1rho relaxation times as compared to non-OA regions. Furthermore in these regions, cartilage sGAG content and aggregate modulus decreased, while percent degraded collagen and water content increased. In OA joints, synovial fluid concentrations of sGAG decreased and C2C concentrations increased compared to healthy joints. T1rho relaxation times were negatively correlated with cartilage and synovial fluid sGAG concentrations and aggregate modulus and positively correlated with water content and permeability. Additionally, we demonstrated the application of these in vitro findings to the study of human subjects. Specifically, we demonstrated that walking results in decreased T1rho relaxation times, consistent with water exudation and an increase in proteoglycan concentration with in vivo loading. Together, these findings demonstrate that cartilage MR imaging and synovial fluid biomarkers provide powerful non-invasive tools for characterizing changes in the biochemical and biomechanical environment of the joint.
Keywords: Biomarkers, Imaging, Modulus, T2 mapping, MRI
1. Introduction
Osteoarthritis (OA) is a joint disease characterized by the degeneration of articular cartilage, osteophyte formation, and joint space narrowing (Duvvuri et al., 1997). Articular cartilage is composed of chondrocytes in a dense extracellular matrix that is primarily made up of water, collagen, and proteoglycans, and has a limited capacity for repair following damage. OA progression is associated with increases in synthesis and breakdown of the extra-cellular matrix components, although the precise changes in the biochemical and biomechanical environment of the tissue as OA progresses remain unclear.
Recent data suggests that major contributing factors to early OA are altered mechanical loading and abnormal cartilage physiology (Guilak, 2011; Rivers et al., 2000). However, the specific contributions and interactions of these factors are not fully understood (Guilak, 2011). Both altered loading patterns and abnormal physiology can contribute to degradation of the cartilage matrix, which is reflected in the biochemical and biomechanical properties of the extracellular matrix. Specifically, one of the earliest detectable changes in animal models of OA is a loss of biomechanical properties, including decreased compressive stiffness and increased permeability (Appleyard et al., 1999; Setton et al., 1994). Additionally, in OA cartilage, proteoglycan content decreases and the collagen network becomes disrupted (Keenan et al., 2011). These breakdown products are evident in synovial fluid with varying proteoglycan concentrations and increased collagen type II cleavage (C2C) neoepitope concentrations, depending on the OA severity (Bolam et al., 2006; Prink et al., 2010; Ratcliffe et al., 1988). Additionally, matrix metalloproteinases (MMPs) are elevated in OA cartilage, promoting the enzymatic digestion of extra-cellular matrix components and forming degradation fragments that can be detected in synovial fluid (Catterall et al., 2010; Janusz et al., 2002; Settle et al., 2010).
While molecular biomarkers in the synovial fluid can provide measures of overall joint health (Kraus et al., 2010), it is unclear how these biomarkers relate to local changes in the biochemical or biomechanical properties of cartilage. In this regard, quantitative magnetic resonance (MR) imaging techniques have been used to track early OA in vivo (Tang et al., 2011). T1rho and T2 relaxation times in particular have been shown to be sensitive to disruptions in the organization of proteoglycan and collagen within the cartilage extracellular matrix, respectively (David-Vaudey et al., 2004; Duvvuri et al., 1997). Specifically, previous studies have shown that increased T1rho relaxation times correspond to decreased proteoglycan concentration and increased water content (Keenan et al., 2011; Li et al., 2011; Regatte et al., 2006; Wheaton et al., 2005). T2 relaxation times are sensitive to changes in collagen content, collagen organization, and water content (Choi and Gold, 2011; Chou et al., 2009; Dunn et al., 2004; Jazrawi et al., 2011).
However, there is limited data providing a comprehensive assessment of the compositional, biochemical, and biomechanical properties of OA cartilage related to T1rho relaxation times. Additionally, while synovial fluid biomarkers have been used to reflect the health status of the entire joint, their relationship to T1rho relaxation times is unclear. A comprehensive evaluation relating T1rho relaxation times to the properties of OA cartilage and synovial fluid biomarkers would provide critical information for evaluating the changes in the biochemical and biomechanical environment of the joint as OA progresses in vivo. In addition, these measurements could be used to establish molecular biomarkers that reflect changes in local cartilage biomechanical function and composition.
The goals of this study were to relate T1rho relaxation times to changes in cartilage composition, cartilage mechanical properties, and synovial fluid biomarker levels in vitro. In order to demonstrate the utility of these findings to the study of human subjects, we sought to quantify the effects of in vivo mechanical loading on T1rho relaxation times. We hypothesized that T1rho relaxation times, water content, and permeability would be increased in OA regions compared to normal regions of OA porcine joints, while cartilage sGAG content and aggregate modulus would be decreased in the OA regions. Additionally, we hypothesized that OA joints would contain decreased concentrations of synovial fluid sGAG and increased levels of C2C and MMP activity compared to normal joints. Finally, in human subjects, we hypothesized that in vivo mechanical loading experienced during walking would cause decreased T1rho relaxation times due to compression of the cartilage matrix leading to exudation of water and increased proteoglycan concentrations.
2. Materials and methods
2.1. In vitro experimental design
Medial femoral condyles were isolated from 2 to 3-year old skeletally mature female pig knees obtained from a local abattoir. Condyles were visually assessed for cartilage degradation, and divided into groups containing condyles with focal OA regions, which we defined as OA joints (n = 19), and condyles with no visual evidence of OA (healthy joints, n = 9). Healthy joints were included to provide reference data for normal cartilage values. The cartilage was photographed and graded by three blinded graders for OA severity using the Collins scale (Collins and McElligott, 1960). During the dissection, synovial fluid from each joint was collected and stored at −80 °C. The harvested condyles were then adhered to the base of a plastic container, immersed in phosphate buffered saline (PBS), and MR scanned.
Sagittal GRE T1rho- and TSE T2-relaxation MR images were acquired using a 3T scanner (Trio Tim, Siemens) with an 8 channel knee coil using the parameters in Table 1 (Borthakur et al., 2003). After scanning, 5 mm diameter cartilage explants were harvested from the condyles. For condyles from OA joints, three explants (one each for histological, biochemical, and biomechanical testing) were harvested from both the OA region and from the normal region on the same condyle. For condyles from healthy joints, three total cartilage explants were harvested. The relative position of the biopsy punches for histological, biochemical, and biomechanical testing was varied for each joint to reduce site-to-site variability in the outcome measures.
Table 1.
T1rho and T2 MR imaging parameters.
| Parameter |
In vitro imaging
|
In vivo imaging T1rho | |
|---|---|---|---|
| T1rho | T2 | ||
| Repetition time (TR) | 3500 ms | 3500 ms | 3500 ms |
| Echo time (TE) | 5.9 ms | 13.8, 27.6, 41.4, 55.2, 69.0, 82.8, 96.6 ms | 5.9 ms |
| Field of view (FOV) | 140 × 140 mm | 140 × 140 mm | 140 × 140 mm |
| Matrix size | 256 × 256 | 256 × 256 | 256 × 256 |
| Slice thickness | 3 mm | 3 mm | 3 mm |
| Slice gap | 0 mm | 0 mm | 0 mm |
| Spin-lock frequency | 500 Hz | – | 500 Hz |
| Spin lock times (TSL) | 5, 10, 20, 40, 60, 80, 100 ms | – | 5, 10, 40, 80 ms |
2.2. Cartilage assessment
2.2.1. Histology and grading
For histology, the explants were frozen in OCT (Sakura Finetek) and cut into 8 μm thick sections. Tissue sections were formalin fixed and stained with Harris Hematoxylin with glacial acetic acid (Poly Scientific), 0.02% aqueous fast green (Sigma-Aldrich), and Safranin-O (Sigma-Aldrich) to identify cell nuclei, collagens, and proteoglycans, respectively. Stained sections were photographed and cartilage structure and Safranin-O staining were quantified by three blinded graders, using a Modified Mankin grading scale (Lim et al., 2011; Xie et al., 2006).
2.2.2. MR imaging analyses
T1rho and T2 relaxation times were determined for each pixel using a two-parameter least-squares regression for the following equations, respectively (Borthakur et al., 2003):
where S is the signal intensity, TSL is the spin lock time, and TE is the echo time.
For the OA joints, T1rho and T2 analyses were performed as described above on the normal and OA regions of cartilage separately. Normal and OA regions of OA joints were identified visually from the MR images and confirmed from photographs of each condyle. Explants for histology, biochemistry, and mechanical testing were taken from these same visually identified regions. For the healthy joints, T1rho and T2 analyses were performed across the entire condyle.
2.2.3. Biochemical analyses
For biochemical analyses, explants were cut in half, wet weights were determined, explants were lyophilized, and then dry weights were measured to calculate the percent water content of each explant. One half of each explant was digested in 1 mL of papain (Rowland et al., 2013) overnight at 65 °C and then sulfated glycosaminoglycan (sGAG) content in the tissue was measured using the 1, 9-dimethylmethylene blue (DMB) assay (Farndale et al., 1982). Total micrograms of sGAG were corrected for the wet weight of the tissue.
In order to solubilize extractable or cleaved collagen in the tissue, the second half of each explant was digested overnight in 1 mg/mL α-chymotrypsin (Sigma) at 37 °C (Hosseininia et al., 2013). After chymotrypsin digestion, the supernatant was collected and stored at −20 °C and the remaining tissue was papain digested overnight at 65 °C (Rowland et al., 2013). Collagen content in the chymotrypsin and papain fractions was measured using a hydroxyproline assay with trans-4-hydroxyproline standards (Sigma) (Detamore and Athanasiou, 2004; Woessner, 1961). Total collagen content was calculated as the sum of the collagen content in the papain and chymotrypsin digested fractions. The percentage of extractable collagen in each explant was calculated by dividing the collagen content in the chymotrypsin fraction by the total collagen content and multiplying by 100.
2.2.4. Biomechanical analyses
For biomechanical testing, creep experiments were performed in a confined-compression configuration, using an ELF 3100 materials testing system (Bose). Explants were confined in a chamber containing PBS and compressive loads were applied with a rigid porous platen. Explants were loaded into the confining chamber to ensure that the samples were flat and parallel to the loading platen. Explants were loaded with a 2 gf tare load, then a step compressive load of 17 gf was applied, and samples were allowed to equilibrate until the change in displacement was less than 0.001 mm over a period of 100 s. The average coefficient of determination (r2) of the resulting creep curves was 0.96. A nonlinear least-squares regression procedure was utilized to determine the compressive modulus (HA) and hydraulic permeability (k) of the tissue (McNulty et al., 2013; Mow et al., 1980).
2.3. Synovial fluid biomarkers
Synovial fluid sGAG concentrations were measured using an alcian blue assay (Kamiya Biomedical). C2C was measured in the synovial fluid using an enzyme-linked immunosorbent assay (Ibex) that detects a neoepitope created by the cleavage of type II collagen by collagenases. Total MMP activity in the synovial fluid was measured using the quenched fluorogenic substrate Dab-Gly-Pro-Leu-Gly-Met-Arg-Gly-Lys-Flu (Sigma-Aldrich), as previously described (Backus et al., 2011; Carter et al., 2015; Liu et al., 2016; McNulty et al., 2009; Wilusz et al., 2008).
2.4. In vivo T1rho imaging
To further explore the relationship of T1rho relaxation times to changes in cartilage properties, we examined the effects of mechanical loading in response to in vivo conditions. In 6 healthy subjects, we measured T1rho relaxation times before and after a walking activity (Lad et al., 2016). Subjects arrived early in the morning (Coleman et al., 2013) to allow for baseline cartilage measurements following a 45 min relaxation period (Lad et al., 2016). Subjects were then imaged with a 3T MRI scanner and an eight-channel knee coil using the parameters shown in Table 1. Subjects then walked on a treadmill for 20 min at 2.5 mph (Lad et al., 2016). Immediately after walking, subjects received a post activity T1rho scan. The bony and articular surfaces of the MR images were traced and stacked to form a wireframe model. Solid modeling software (Geomagic, Studio, Geomagic, Inc.) was used to align the pre- and post-activity models (Abebe et al., 2011) to allow for site-specific comparison of T1rho relaxation times. A grid sampling system was created on the cartilage surface to span the entire tibial and femoral cartilage surfaces (Lad et al., 2016; Okafor et al., 2014). T1rho values were calculated as described in the MR Image Analysis section above.
2.5. Statistical analyses
Statistical analyses were performed using Statistica version 7 (StatSoft). The normal regions and OA regions in the OA joints were compared using the Wilcoxon matched pairs test. Healthy and OA joints were compared using the Mann-Whitney U test. In order to normalize the data to plot linear correlations, we used Mankin grade to categorize the data from the different outcome measures (9 groups ranging from Mankin grades 0–9). Within each group, outcome measures (T1rho relaxation time, sGAG, water content, modulus, permeability, and SF sGAG) were averaged. Pearson’s correlations were then performed to assess the relationship of T1rho with the various outcome measures. Single mean t-tests were used to analyze the effects of treadmill walking on in vivo T1rho relaxation times across the tibiofemoral joint. Differences were considered statistically significant where p < 0.05.
3. Results
3.1. Collins and histological grading
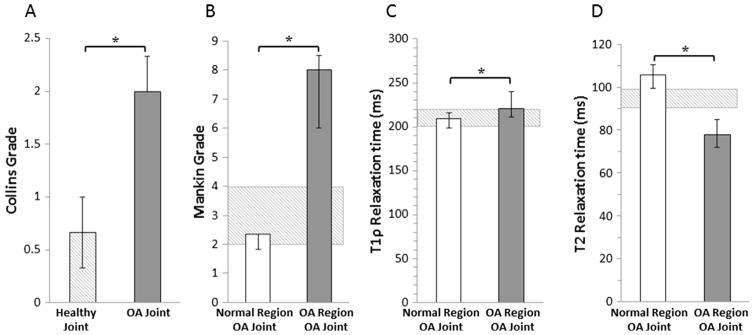
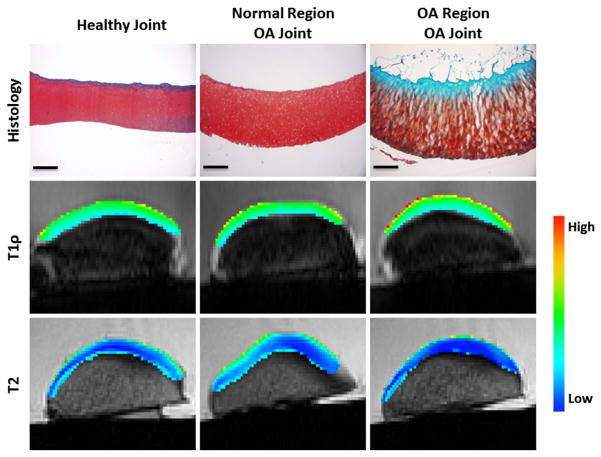
Visual grading of cartilage showed that there was a significant difference in Collins grade between healthy and OA joints (Fig. 1A, p < 0.0001). In healthy joints and the normal regions of OA joints, histological staining of cartilage explants revealed intact cartilage structure and robust Safranin-O staining, indicative of a proteoglycan rich extracellular matrix (Fig. 2). However, the OA regions of OA joints demonstrated substantial tissue degeneration, loss of proteoglycan staining, and disruption of the collagen network (Fig. 2). Modified Mankin grading of the tissue sections revealed significantly higher Mankin scores in the OA regions of the OA joints, compared to the normal regions of the OA joints (Fig. 1B, p = 0.0002).
Fig. 1.
Median (A) Collins grades of healthy and OA joints, (B) Modified Mankin grades, (C) T1rho relaxation times, and (D) T2 relaxation times of normal and OA regions of the cartilage from OA joints (*p < 0.05). The error bars show the interquartile range. The hashed box indicates the interquartile range for the healthy joints.
Fig. 2.
Representative histology, T1rho imaging, and T2 imaging for a healthy joint and normal and OA regions of cartilage from an OA joint. Histological staining with Safranin-O (proteoglycan = red), fast green (collagen = blue), and hematoxylin (nuclei = black). Scale bar = 500 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Imaging biomarkers
The T1rho color maps illustrate higher relaxation times, particularly in the surface layer in the OA regions of the OA joints (Fig. 2). Overall, there was a significant 5% increase in median T1rho relaxation times in the OA regions compared to the normal cartilage regions of OA joints (Fig. 1C, p = 0.001). On the other hand, overall median T2 relaxation times were significantly decreased in the OA regions of OA joints by approximately 36% (Fig. 1D, p = 0.002).
3.3. Biochemical composition of cartilage
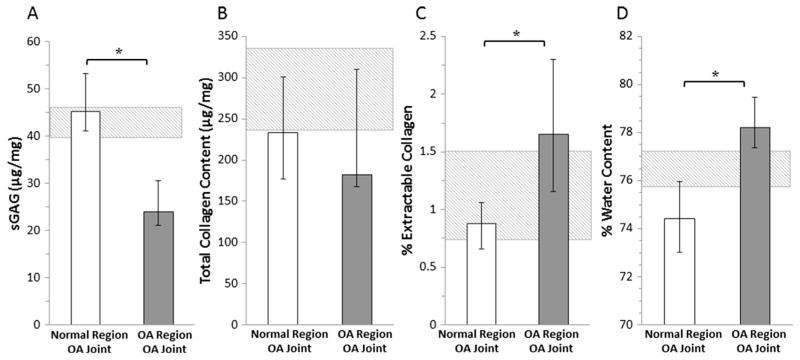
The median cartilage sGAG content was 47% lower in the OA regions of cartilage compared to the normal regions of cartilage in the OA joints (Fig. 3A, p = 0.0002). The total collagen content was not significantly different between these groups (Fig. 3B). However, the percentage of extractable collagen was significantly elevated in the OA regions compared to the normal regions of OA joints (Fig. 3C, p = 0.002). The percent water content was 5% higher in OA regions of cartilage compared to the normal regions of cartilage in OA joints (Fig. 3D, p = 0.0004).
Fig. 3.
Quantification of cartilage biochemical composition showing median (A) sGAG content, (B) Total collagen content, (C) % Extractable collagen, and (D) % Water content of normal and OA regions of cartilage from OA joints (*p < 0.05). The error bars show the interquartile range. The hashed box indicates the interquartile range for the healthy joints.
3.4. Biomechanical properties
The cartilage aggregate modulus was significantly decreased by 56% (Fig. 4A, p = 0.0002) in the OA region compared to the normal region of cartilage in OA joints. Hydraulic permeability was increased by 35% in the OA regions of the OA joints (Fig. 4B, p = 0.06).
Fig. 4.
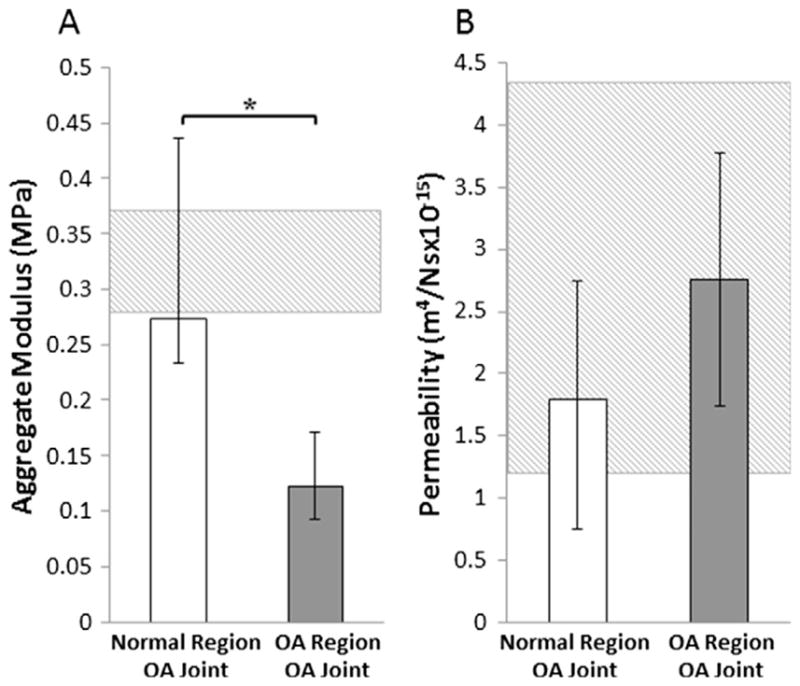
Quantification of cartilage mechanical properties showing median (A) Aggregate modulus and (B) Hydraulic permeability of normal and OA regions of cartilage from OA joints (*p < 0.05). The error bars show interquartile range. The hashed box indicates the interquartile range for the healthy joints.
3.5. Synovial fluid biomarkers
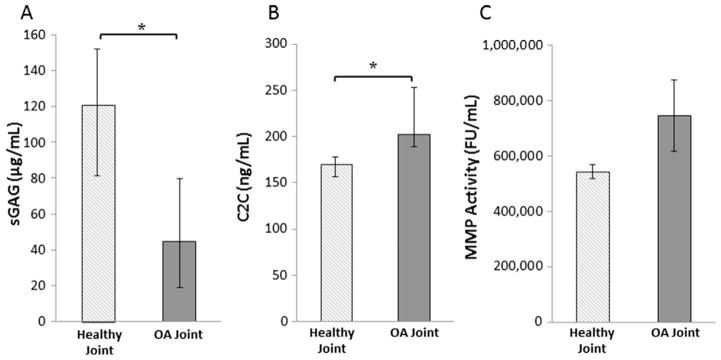
Synovial fluid sGAG concentrations were nearly 63% lower in the OA joints compared to the healthy joints (Fig. 5A, p = 0.01). C2C concentrations, indicative of collagen breakdown by collagenases, in the synovial fluid were significantly elevated in the OA joints compared to the healthy joints (Fig. 5B, p = 0.006). MMP activity trended towards being increased in the synovial fluid from OA joints compared to healthy joints (Fig. 5C, p = 0.12).
Fig. 5.
Quantification of synovial fluid content showing median (A) sGAG content, (B) C2C levels, and (C) MMP activity in fluorescence units/mL from healthy and OA joints (*p < 0.05). The error bars show the interquartile range.
3.6. Correlations
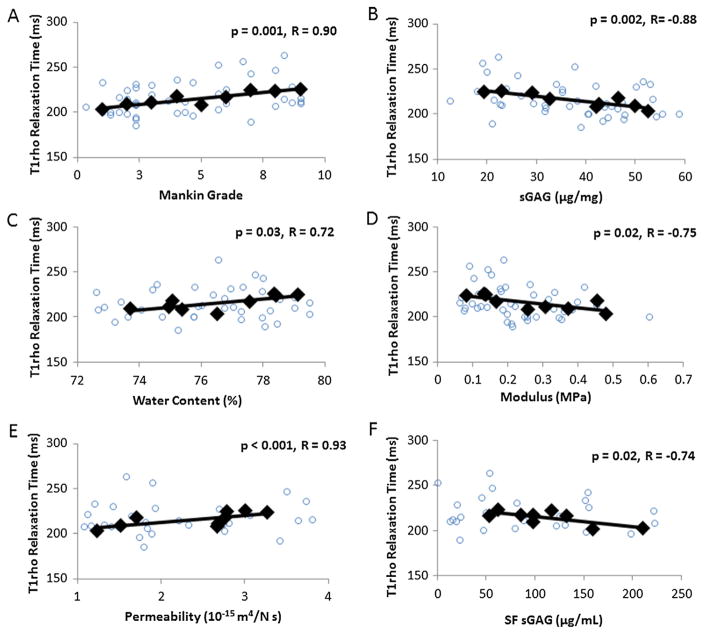
T1rho relaxation times were positively correlated with Mankin grade (Fig. 6A, R = 0.90, p = 0.001). In addition, T1rho relaxation times were negatively correlated with tissue sGAG content (Fig. 6B, R = −0.88, p = 0.002) and positively correlated with water content (Fig. 6C, R = 0.72, p = 0.03). Furthermore, T1rho relaxation times were negatively correlated with aggregate modulus (Fig. 6D, R = −0.75, p = 0.02) and positively correlated with hydraulic permeability (Fig. 6E, R = 0.93, p < 0.001). Finally, synovial fluid sGAG concentrations were negatively correlated with T1rho relaxation times (Fig. 6F, R = −0.74, p = 0.02).
Fig. 6.
Pearson’s correlations demonstrating significant relationships of T1rho relaxation times with (A) Mankin grade, (B) tissue sGAG content, (C) water content, (D) aggregate modulus, (E) hydraulic permeability, and (F) synovial fluid (SF) sGAG. Circles indicate individual data points and diamonds represent the mean data points categorized by Mankin grade.
3.7. In vivo T1rho imaging
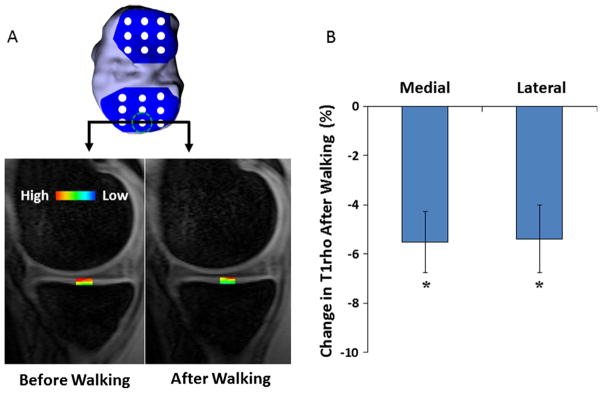
Following 20 min of treadmill walking, T1rho relaxation times decreased in both the medial and lateral tibiofemoral compartments (Fig. 7, p < 0.05), suggesting a decrease in the water content and an increase in the proteoglycan concentration.
Fig. 7.
(A) A grid system was used to span the tibial (depicted here) and femoral cartilage surfaces (Lad et al., 2016) in order to measure T1rho relaxation times across the joint. The color map illustrates changes in T1rho relaxation values before and after walking on a treadmill. (B) Percent change in T1rho relaxation times resulting from the walking activity in both the medial and lateral compartments of the tibiofemoral cartilage. * indicates a statistically significant change in T1rho relaxation times after walking (p < 0.05).
4. Discussion
Our results demonstrate that non-invasive MR imaging correlates to changes in the compositional, biochemical, and biomechanical properties of cartilage in OA joints. In OA regions of cartilage, T1rho relaxation times increased, corresponding to decreased sGAG content in the tissue. Additionally, these changes in the T1rho relaxation times were associated with alterations in the mechanical properties of the tissue. Elevated T1rho relaxation times were also correlated to decreased levels of sGAG in the synovial fluid, demonstrating an important relationship between imaging and molecular biomarkers of cartilage health. Finally, we demonstrated an in vivo application of T1rho in human subjects, indicating that walking results in decreased T1rho relaxation times, consistent with water exudation and a corresponding increase in proteoglycan concentration with in vivo loading.
OA regions and normal regions of cartilage within the same porcine joint showed significant differences in both biomechanical and biochemical properties. Furthermore, the similarities between the cartilage from healthy joints and the normal regions of cartilage from OA joints suggest that cartilage degeneration in this porcine animal model is relatively focal and is not occurring uniformly throughout the condyle. In a previous study, the properties of healthy appearing cartilage from the patellar groove of porcine knee joints were compared to the arthritic cartilage of the medial femoral condyle within the same joint (Hennerbichler et al., 2008). The authors found relatively small changes in the modulus and histologic appearance of the normal appearing regions of cartilage across a wide range of OA severities. These results are consistent with our findings of localized changes in cartilage composition and properties within the OA regions, and similar values between cartilage in the normal regions of OA joints and healthy joints.
The alterations in the biochemical and biomechanical properties reported in this study align with changes in cartilage that have been previously associated with OA (Appleyard et al., 1999; Guilak et al., 1994; Rivers et al., 2000; Setton et al., 1994). We found that the OA cartilage showed a decrease in sGAG content, an increase in degraded collagen, a decrease in the aggregate modulus, and an increase in water content. In the synovial fluid of OA joints, decreased sGAG concentrations and increased C2C concentrations corresponded with joint degradation. A study using enzymatic depletion to induce the degradation of articular cartilage found results that are consistent with our study, showing a decrease in sGAG content as cartilage degraded and no change in the total collagen content (Grenier et al., 2014). For the biomechanical properties, a decrease in the aggregate modulus and an increase in the permeability of enzymatically degraded cartilage was also found. Similarly, Wheaton et al. used cytokine-induced cartilage degradation and found a decrease in sGAG content and compressive modulus, an increase in hydraulic permeability, and only small changes in collagen content (Wheaton et al., 2005).
Our results show that T1rho relaxation times were increased in the OA regions compared to normal regions of OA joints and were negatively correlated with sGAG content in the tissue. These results are consistent with a previous study reporting that proteoglycan-degraded specimens had a 33% increase in T1rho relaxation time over collagen-degraded or normal articular cartilage (Duvvuri et al., 1997). Regatte et al. (2004) demonstrated higher T1rho relaxation times for OA subjects when compared to their healthy counterparts, and the T1rho values varied depending on the degree of cartilage degeneration. In another study, T1rho relaxation times were also correlated with OA severity (Li et al., 2007). In the present study, we confirmed that T1rho relaxation times positively correlated with OA severity, but also found that T1rho relaxation times negatively correlated with mechanical properties.
Additionally, we demonstrated that T2 mapping has significantly lower relaxation times in the OA regions of cartilage compared to the normal regions of cartilage in the OA joints. Histology of the OA cartilage showed that the superficial layer was missing in most samples; therefore, the lower T2 relaxation times may be due to the loss of the highly organized superficial zone (Nieminen et al., 2000). T2 values in the superficial zone are significantly higher than the deep zone values (Hannila et al., 2009; Nieminen et al., 2001), so the absence of the superficial layer in the OA cartilage would likely cause lower T2 relaxation times in these samples. Others have also found that T2 values were higher in tibial cartilage with moderate OA compared to severe OA (David-Vaudey et al., 2004). Interestingly, we did not detect any changes in the total collagen content of the cartilage in the OA regions. However, the percentage of degraded collagen in the tissue, as measured using chymotrypsin digestion, and in the synovial fluid, as measured using the C2C assay, was increased. Previous studies have also shown increased collagen degeneration in OA tissue using chymotrypsin digestion (Bank et al., 1997; Hosseininia et al., 2013). Our findings highlight the complexity of factors and interactions that contribute to the T2 relaxation time. Since T2 relaxation times are sensitive to changes in water content, collagen content, and the organization of collagen fibrils in the extracellular matrix (Choi and Gold, 2011), our findings suggest that in this model, the disruption of the collagen network due to loss of the superficial zone in the OA regions is contributing more strongly to the T2 signal than the contribution of water content changes alone.
Although many studies have investigated the changes in synovial fluid during OA progression, very little data is available on synovial fluid biomarker levels related to MR imaging biomakers. Previous data has shown that sGAG concentrations are decreased in synovial fluid from OA patients compared to their healthy counterparts (Hollander et al., 1991). In our study, there was a negative correlation between synovial fluid levels of sGAG and T1rho relaxation times. The decreased sGAG concentrations in the synovial fluid of the OA joints in this study reflected the severe degradation that was also seen histologically, by T1rho imaging, and in sGAG tissue content. Overall, in this study the synovial fluid biomarkers reflect the focal changes in tissue composition and mechanical properties that were correlated with changes in the imaging biomarkers. Together, these imaging and molecular biomarkers provide powerful tools to non-invasively measure tissue changes associated with OA. Future studies will help to further elucidate the relationship of MR imaging biomarkers and synovial fluid biomarkers during OA progression. Such data is critical to establishing molecular biomarkers that reflect changes in the local tissue environment and could be used to identify those at high risk for the development and progression of OA.
In order to relate our in vitro findings from the porcine joints to analysis of human subjects, we examined the effects of mechanical loading on T1rho relaxation times in vivo. In this study, we found that walking in human subjects results in decreased T1rho relaxation times. Our previous work has shown that walking causes compressive strains of the cartilage (Lad et al., 2016), consistent with water exudation from the matrix (Lai et al., 1981). Thus, the decreased T1rho relaxation times observed in the present study are likely reflecting increased apparent proteoglycan concentrations due to loss of water with in vivo loading, which are consistent with the in vitro correlative relationships that we demonstrated in this study with porcine cartilage. In addition, our findings are consistent with previous work demonstrating decreased T1rho and T2 relaxation times following running (Mosher et al., 2005; Subburaj et al., 2012). These studies highlight the utility of T1rho in quantifying physiologically relevant changes in proteoglycan concentration experienced in vivo and in tracking changes associated with OA. Additionally, these results also emphasize the importance of controlling for loading history prior to imaging when using T1rho relaxation times in clinical studies monitoring cartilage health. Finally, when coupled with in vivo measures of cartilage strains (Carter et al., 2015; Coleman et al., 2013; Sutter et al., 2015; Widmyer et al., 2013), these imaging and synovial fluid biomarkers could be used to track the relationship between altered mechanical loading and changes in the composition of the tissue. Such information could provide invaluable data characterizing the interplay between mechanical loading and biological processes that occur with OA.
The MR imaging and molecular biomarkers of both cartilage and synovial fluid presented in this study have the potential to provide new insights into the mechanisms leading to OA development and progression. Specifically, these measurements provide powerful non-invasive tools for comprehensively characterizing changes in the biochemical and biomechanical environment of the joint. Additionally, by comparing molecular biomarkers to imaging biomarkers of cartilage composition and mechanical properties, we can establish new non-invasive clinically relevant molecular biomarker measurements of cartilage health. Ultimately, a better understanding of the changes associated with OA development and progression may lead to new interventions aimed at slowing this devastating disease.
Acknowledgments
The authors thank Drs. Steven Grambow and Adam Goode for statistical expertise. Thanks to Dr. Ari Borthakur at the University of Pennsylvania for providing technical assistance for the pulse sequence used in acquiring the data. This work was supported in part by the National Institutes of Health grants AR63325, AR65527, AR48182, AR48852, AG15768, AR50245, AG46927, AR066477, and AG028716, the Collaborative Research Center of the AO Foundation, Davos, Switzerland, the Arthritis Foundation, and a VA Rehabilitation Research Service Award.
Footnotes
Conflict of interest statement
The authors have no relevant conflicts of interest regarding this manuscript but disclose that Farshid Guilak is an employee of Cytex Therapeutics, Inc.
References
- Abebe ES, Kim JP, Utturkar GM, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effect of femoral tunnel placement on ACL graft orientation and length during in vivo knee flexion. J Biomech. 2011;44:1914–1920. doi: 10.1016/j.jbiomech.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard RC, Ghosh P, Swain MV. Biomechanical, histological and immunohistological studies of patellar cartilage in an ovine model of osteoarthritis induced by lateral meniscectomy. Osteoarthritis Cartilage. 1999;7:281–294. doi: 10.1053/joca.1998.0202. [DOI] [PubMed] [Google Scholar]
- Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, Guilak F, Olson SA. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011;29:501–510. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FP, te Koppele JM. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997;16:233–243. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- Bolam CJ, Hurtig MB, Cruz A, McEwen BJ. Characterization of experimentally induced post-traumatic osteoarthritis in the medial femorotibial joint of horses. Am J Vet Res. 2006;67:433–447. doi: 10.2460/ajvr.67.3.433. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Wheaton A, Charagundla SR, Shapiro EM, Regatte RR, Akella SV, Kneeland JB, Reddy R. Three-dimensional T1rho-weighted MRI at 1.5 Tesla. J Magn Reson Imag. 2003;17:730–736. doi: 10.1002/jmri.10296. [DOI] [PubMed] [Google Scholar]
- Carter TE, Taylor KA, Spritzer CE, Utturkar GM, Taylor DC, Moorman CT, 3rd, Garrett WE, Guilak F, McNulty AL, DeFrate LE. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech. 2015;48:1461–1468. doi: 10.1016/j.jbiomech.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Research & Therapy. 2010:12. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imag Clin N Am. 2011;19:249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MC, Tsai PH, Huang GS, Lee HS, Lee CH, Lin MH, Lin CY, Chung HW. Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage. 2009;17:441–447. doi: 10.1016/j.joca.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Guilak F, DeFrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DH, McElligott TF. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imag. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49:577–583. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: Comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47:645–652. doi: 10.1016/j.jbiomech.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994;12:474–484. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- Hannila I, Raina SS, Tervonen O, Ojala R, Nieminen MT. Topographical variation of T2 relaxation time in the young adult knee cartilage at 1.5 T. Osteoarthritis Cartilage. 2009;17:1570–1575. doi: 10.1016/j.joca.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Hennerbichler A, Rosenberger R, Arora R, Hennerbichler D. Biochemical, biomechanical and histological properties of osteoarthritic porcine knee cartilage: implications for osteochondral transplantation. Arch Orthop Trauma Surg. 2008;128:61–70. doi: 10.1007/s00402-007-0360-5. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Atkins RM, Eastwood DM, Dieppe PA, Elson CJ. Degradation of human cartilage by synovial fluid but not cytokines in vitro. Ann Rheum Dis. 1991;50:57–58. doi: 10.1136/ard.50.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseininia S, Lindberg LR, Dahlberg LE. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet Disord. 2013;14:18. doi: 10.1186/1471-2474-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10:785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- Jazrawi LM, Alaia MJ, Chang G, Fitzgerald EF, Recht MP. Advances in magnetic resonance imaging of articular cartilage. J Am Acad Orthop Surg. 2011;19:420–429. doi: 10.5435/00124635-201107000-00005. [DOI] [PubMed] [Google Scholar]
- Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, Delp SL, Beaupre GS, Gold GE. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus VB, Kepler TB, Stabler T, Renner J, Jordan J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS ONE. 2010;5:e9739. doi: 10.1371/journal.pone.0009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad NK, Liu B, Ganapathy PK, Utturkar GM, Sutter EG, Moorman CT, 3rd, Garrett WE, Spritzer CE, DeFrate LE. Effect of normal gait on in vivo tibiofemoral cartilage strains. J Biomech. 2016;49:2870–2876. doi: 10.1016/j.jbiomech.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WM, Mow VC, Roth V. Effects of nonlinear strain-dependent permeability and rate of compression on the stress behavior of articular cartilage. J Biomech Eng. 1981;103:61–66. doi: 10.1115/1.3138261. [DOI] [PubMed] [Google Scholar]
- Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imag. 2011;29:324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim NS, Hamed Z, Yeow CH, Chan C, Huang Z. Early detection of biomolecular changes in disrupted porcine cartilage using polarized Raman spectroscopy. J Biomed Opt. 2011;16:017003. doi: 10.1117/1.3528006. [DOI] [PubMed] [Google Scholar]
- Liu B, Goode AP, Carter TE, Utturkar GM, Huebner JL, Taylor DC, Moorman CT, III, Garrett WE, Kraus VB, Guilak F, DeFrate LE, McNulty AL. Matrix metalloproteinase activity and prostaglandin E2 are elevated in the synovial fluid of meniscus tear patients. Connect Tissue Res. 2016 doi: 10.1080/03008207.2016.1256391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31:1039–1045. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467:1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, Smith MB. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative mri and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, Jurvelin JS. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med. 2000;43:676–681. doi: 10.1002/(sici)1522-2594(200005)43:5<676::aid-mrm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech. 2014;47:96–101. doi: 10.1016/j.jbiomech.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prink A, Hayashi K, Kim SY, Kim J, Kapatkin A. Evaluation of a collagenase generated osteoarthritis biomarker in the synovial fluid from elbow joints of dogs with medial coronoid disease and unaffected dogs. Vet Surg. 2010;39:65–70. doi: 10.1111/j.1532-950X.2009.00604.x. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Doherty M, Maini RN, Hardingham TE. Increased concentrations of proteoglycan components in the synovial-fluids of patients with acute but not chronic joint disease. Ann Rheum Dis. 1988;47:826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: Comparison of T1rho with T2. J Magn Reson Imag. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, Reddy R. 3d–T1rho-relaxation mapping of articular cartilage: In vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Rivers PA, Rosenwasser MP, Mow VC, Pawluk RJ, Strauch RJ, Sugalski MT, Ateshian GA. Osteoarthritic changes in the biochemical composition of thumb carpometacarpal joint cartilage and correlation with biomechanical properties. J Hand Surg Am. 2000;25:889–898. doi: 10.1053/jhsu.2000.16358. [DOI] [PubMed] [Google Scholar]
- Rowland CR, Lennon DP, Caplan AI, Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 2013;34:5802–5812. doi: 10.1016/j.biomaterials.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settle S, Vickery L, Nemirovskiy O, Vidmar T, Bendele A, Messing D, Ruminski P, Schnute M, Sunyer T. Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis: confirmation by multivariate analysis that modulation of type II collagen and aggrecan degradation peptides parallels pathologic changes. Arthritis Rheum. 2010;62:3006–3015. doi: 10.1002/art.27596. [DOI] [PubMed] [Google Scholar]
- Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12:451–463. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, Majumdar S. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40:2134–2141. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE, Jr, DeFrate LE. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am J Sports Med. 2015;43:370–376. doi: 10.1177/0363546514559821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SY, Souza RB, Ries M, Hansma PK, Alliston T, Li X. Local tissue properties of human osteoarthritic cartilage correlate with magnetic resonance T(1) rho relaxation times. J Orthop Res. 2011;29:1312–1319. doi: 10.1002/jor.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54:1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT, 3rd, DeFrate LE, Guilak F. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65:2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res. 2008;26:504–512. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Xie T, Guo S, Zhang J, Chen Z, Peavy GM. Determination of characteristics of degenerative joint disease using optical coherence tomography and polarization sensitive optical coherence tomography. Lasers Surg Med. 2006;38:852–865. doi: 10.1002/lsm.20394. [DOI] [PubMed] [Google Scholar]