Abstract
Purpose
To identify the prevalence and determinants of self-reported eye care use among Chinese Americans.
Design
Population-based, cross-sectional study.
Participants
A total of 4582 Chinese Americans 50 years and older residing in Monterey Park, California.
Methods
Multivariable logistic regression analyses based on Andersen’s Behavioral Model of Health Services Use were conducted to identify predisposing, enabling, and need variables associated with self-reported eye care use.
Main Outcome Measures
Prevalence of self-reported use assessed as eye care visit in the past 12 months, dilated eye examination in the past 12 months, and ever having had a dilated examination, and odds ratios for factors associated with these measures.
Results
Overall, 36% of participants reported an eye care visit and 21% reported a dilated examination in the past 12 months. Forty-eight percent reported ever having had a dilated eye examination. Older age, female gender, preference for English, more education, health and vision insurance, a usual place for health care, currently driving, a greater number of comorbidities, and lower vision-specific quality of life (NEI VFQ-25) scores were associated with higher odds of reporting use of eye care.
Conclusions
Use of eye care among Chinese Americans was found to be as low as what is reported for African Americans and Hispanics, and lower than what is reported for whites. Multiple modifiable factors are associated with use of eye care among the rapidly growing Chinese American population. Culturally sensitive interventions targeting these factors should be a priority. Further research is needed to investigate how findings from this group of Chinese Americans reflect other Asian Americans that are different in language and ethnicity.
Keywords: Chinese American, utilization of care, dilated examination, eye care
INTRODUCTION
Vision has a strong impact on the quality of life among older adults. The 2006–2010 Behavioral Risk Factor Surveillance System (BRFSS) found that individuals with self-reported moderate/severe vision impairment are 80% to 130% more likely to report having fair/poor health, life dissatisfaction, and disability.1 A recent poll of public attitudes about vision health2 found that Americans from all racial/ethnic backgrounds consider losing eye sight equal to or worse than losing memory, hearing, speech, or a limb for their day-to-day lives. Early detection and treatment are critical to prevent or reduce loss of vision and associated quality of life. However, recent survey data indicates that most Americans do not receive eye care at intervals recommended to preserve vision.3 For example, the 2002 National Health Interview Survey (NHIS) found that even among American adults with self-reported diabetes, only 60% visited an eye doctor and 63% had a dilated eye examination in the past 12 months,4 as recommended by the American Academy of Ophthalmology.5 Other studies of high-risk populations also found that rates of adherence to recommended follow-up care were below 50%, even when major barriers to eye care such as cost and accessibility were minimized.6–8 These low rates of eye care utilization can be major obstacles to combating vision problems in the United States (US) such as the high prevalence (~2.4 million Americans9) of undiagnosed and untreated glaucoma.
Despite previous reports on eye care utilization in the US,4, 10–18 to our knowledge, there is no detailed report on eye care utilization among Asian Americans. In general, racial/ethnic minority groups have less access to health care services than non-Hispanic whites,14, 19 but it remains unclear whether Asian Americans receive less eye care as well. The Asian American population is growing rapidly: 10.2 million individuals residing in the US identified themselves as Asian in 2000, and this number increased by 43% to 14.7 million in 2010.20 Asian immigrants are projected to surpass Hispanics and become the largest immigrant group by 2055, and make up 38% of the foreign-born population by 2065.21 In addition, the proportion of the Asian American population 65 years and older is projected to increase from 9% in 2010 to 22% by 2050.22 Given these demographic projections and the known disparities in health and health care across racial/ethnic groups in the US,23 understanding the unique pattern of eye care utilization among Asian Americans is critical to developing appropriate public health interventions to reduce vision loss.
In the present report, using data from the Chinese American Eye Study (CHES), we assessed the use of eye care among Chinese Americans, the largest sub-group of Asian Americans.20 In addition, we identified factors related to eye care use among Chinese Americans based on Andersen’s Behavioral Model, one the most cited model of health services use.26, 27
METHODS
Study Design
Details of the CHES study design, sampling plan, and baseline data have been reported previously.24 In brief, the study population consists of self-identified Chinese Americans 50 years and older residing in Monterey Park, California, at the time of recruitment (2010–2013). A door-to-door census of all dwelling units within the 10 census tracts of the city was completed. All eligible residents were informed of the study objectives and were invited to complete a questionnaire and a clinical examination at the Local Eye Examination Center by a trained ophthalmologist. A detailed in-home interview was conducted to determine demographic factors, level of acculturation, ocular and systemic medical histories, access to medical and ocular care, and other factors. On arrival at the clinic for the eye examination, participants completed an in-clinic questionnaire that consisted primarily of questions about health-related quality of life and physical activity. The comprehensive ocular examination included presenting and best-corrected distance and near visual acuity (VA), refraction, slit lamp examination with the Lens Opacities classification system (LOCS II), intraocular pressure, Swedish Interactive Threshold Algorithm Standard C24 visual field test(s) using automated field analyzer, ocular biometry, dilated fundus examination, and fundus/optic disc photography. Fundus photographs were graded in a masked manner using standardized protocols at the Ocular Epidemiology Grading Center at the University of Wisconsin in Madison. Height, weight, blood pressure, glycosylated hemoglobin levels, and blood glucose levels were also assessed.
Interviews and clinical examinations were conducted in a standardized manner after informed consent had been completed. Institutional review board/ethics committee approval was obtained from the University of Southern California Health Science Review Board. All study procedures adhered to the principles outlined in the Declaration of Helsinki for research involving human subjects.
Vision impairment
Each participant’s vision was measured for each eye with presenting correction (if any) at 4 meters (m) using standard Early Treatment Diabetic Retinopathy Study (ETDRS) protocols with a modified ETDRS distance chart trans-illuminated with a chart illuminator (Precision Vision, La Salle, Illinois, USA).25 Presenting distance VA was first measured for both eyes with existing refractive correction, and then reassessed for each eye, starting with the right, using different charts. If the participant could not read 55 letters at 4 m in either eye, an automated refraction was performed, using the Humphrey Automatic Refractor (Carl Zeiss Meditec, Dublin, California, USA), followed by subjective refraction using standard protocols. After refraction, the eye was retested to measure the best-corrected VA. Vision impairment was defined as presenting binocular VA of 20/40 or worse.
Measures of Eye Care Use
During the in-home interview, participants were asked about the last time that they went for eye care and their last complete eye examination, which included dilating their pupils and a doctor using bright lights to look into the back of their eyes. Three dichotomous self-reported measures of eye care use were analyzed for this study: having had any eye care visits in the past 12 months (yes/no), having had a dilated eye examination in the past 12 months (yes/no), and ever having had a dilated eye examination (yes/no).
Independent Variables for Multivariate Analysis
According to Andersen’s Behavioral Model, health service use is a function of a person’s need for such service, predisposition to use the service, and enbling factors that facilicate access to the service.26, 27 We defined 3 categories of independent variables: predisposing variables, enabling variables, and need variables. Predisposing variables were further subcategorized as predisposing demographic variables and predisposing social variables, and need variables were further subcategorized as self-reported need variables and evaluated need variables.
Predisposing demographic variables included age (grouped as in the Preferred Practice Pattern Guidelines5), gender, and marital status (never married, married/living with partner, divorced/separated/widowed).
Predisposing social variables included acculturation, generational status, number of years living in the US, language preference, and educational attainment. Level of acculturation was determined based on the Suinn-Lew Asian Self-Identity Acculturation Scale (SL-ASIA).28, 29 Acculturation was categorized as high (more assimilated to US culture) if the SL-ASIA score was higher than the cohort mean of 1.76; a score of 1.76 or lower was considered low. Generational status was assigned as follows: participants who were foreign-born were categorized as first generation; and participants who were US-born were categorized as second generation or higher.
Enabling variables included household income (< $15 000, $15 000–<$30 000, ≥ $30 000 per year), current driving status (yes/no), insurance (not insured, medical insurance only, both medical and vision insurance), a usual place of care (yes/no), and a usual provider (yes/no).
Evaluated need variables included presenting binocular near and distance vision impairment.
Self-reported need variables included the Short Form-12 Physical Health Composite (SF-12 PHC) score, the Short Form-12 Mental Health Composite (SF-12 MHC) score,30 the National Eye Institute Vision Functioning Questionnaire-25 (NEI VFQ-25)31 composite score, and a comorbidity score. The SF-12 PHC and SF-12 MHC scores were calculated such that a score of 50 (standard deviation of 10) was the average among adults in the US.21 Higher scores represent better health-related quality of life. The NEI-VFQ-25 is composed of 12 vision-specific subscales: general health, general vision, near vision activities, distance vision activities, ocular pain, vision-specific social function, vision-specific role difficulties, vision-specific mental health, vision-specific dependency, driving difficulties, color vision, and peripheral vision. Each subscale contains between 1 and 4 items. The NEI-VFQ-25 was scored using standard algorithms. Each item was first scored on a scale from 0 to 100. Item scores within a subscale were averaged to yield the subscale score (range, 0–100). An overall composite score was calculated using the mean of the vision-targeted subscale scores, but excluding the general health rating question. Higher overall composite scores indicate better visual function. The mean overall composite score was 93 for the normal-vision reference group in the NEI VFQ-25 development article.31 On a 100-point scale, a 5 point or greater change in SF-12 PHC/MCH or VFQ-25 score is considered the minimal difference important to subjects.32–34 The comorbidity score is a composite score of 12 self-reported medical conditions: diabetes mellitus, arthritis, stroke or brain hemorrhage, hypertension, angina, heart attack, heart failure, asthma, skin cancer, other cancers, back problems, and deafness or hearing problems.35–37 Hypertension and diabetes were assessed by a combination of self-report, physical examination, and blood testing. Globe et al36 demonstrated that systemic comorbidities were associated with visual function.
Imputation Procedures for Missing Data
No independent variable was missing more than 1% of its values, with the exception of self-reported annual household income, which was missing 13%. To assess potential bias from the use of complete data only, missing values for self-reported annual household income were imputed through multiple imputations using ordered logistic regression in STATA (StataCorp LP, College Station, Texas, USA). The imputation model of missing values included all dependent and independent variables of logistic models. Twenty-five sets of imputed data were created and combined using Rubin’s rules.38 There were no material differences in analysis results of the complete data and those of the imputed data; therefore, results from analyses with imputed income data were reported.
Statistical Analysis
Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) in order to evaluate potential associations between 5 conceptual model categories (i.e., predisposing demographic, predisposing social, enabling, self-reported need, and evaluated need variables) with each measure of eye care use. Multivariate regression was first completed for all variables in each category of the Behavioral Model. Factors associated with eye care use with a P value equal to .10 in the category-specific analyses were considered for inclusion in the final multivariate analysis, which included variables from all 5 categories. Variables with a P value less than .05 in the final multivariate analysis were retained. For comparison, forward stepwise regression was completed as a secondary analysis to select independent variables from all model categories at the .05 significance level. Independent variables identified as significant for all 3 measures of eye care use were categorized as most important; variables identified as significant independent variables in 1 or 2 models were categorized as moderately important; and variables not identified as significant for any of the 3 measures were categorized as least important. We further evaluated the effect of independent variables separately among working-age (< 65 years old) and retirement-age (≥65 years old) participants.
All analyses were conducted using SAS software 9.4 (SAS, Inc., Cary, North Carolina, USA). All statistical tests were two-sided and results were considered statistically significant when associated P value was less than .05.
RESULTS
Study Cohort
A total of 4582 Chinese Americans completed a full eye examination. Compared with the overall 50+ years old Chinese population living in the US,39 CHES participants were similar in age (47% of CHES participants vs 44% of US Chinese were 50–59 years old), more likely to be female (63% of CHES vs 52% of US Chinese), and less likely to have more than 12 years of education (67% of CHES participants vs 77% of US Chinese). The majority of these participants were first-generation immigrants from mainland China (69.4%) and Taiwan (13.4%). Most (75%) had lived in the US for more than 10 years and (98%) lived in the US year round.
Among the 4582 CHES participants, 9 did not complete the questions about the use of eye care services, and 22 were unable to recall. Therefore, use of eye care data was available for 4551 participants, or 99.3% (Table 1), included in this analysis. Twenty-nine percent were 65 years or older, 63% were female, 76% were married or living with a partner, 98% were first-generation immigrants, 32% had less than 12 years of education, and 88% preferred Asian language. Thirty one percent reported an annual income of less than $15 000, 43% were uninsured, 36% had no usual place of care, 37% had no usual provider, and 30% did not drive. Through comprehensive eye examination, 10% had impaired presenting binocular distance vision, and 12% had impaired presenting binocular near vision. The mean comorbidity score was 1.4, the mean SF-12 PHC score was 49.1, the mean SF-12 MHC score was 55.9, and the mean NEI VFQ score was 89.6 (Table 1).
Table 1.
Distribution of Predisposing, Enabling, and Need Variables in the Chinese American Eye Study (n = 4,551)
| N | Percent | |
|---|---|---|
| Predisposing Demographic Variables | ||
| Age (yrs.) | ||
| 50–54 | 1064 | 23% |
| 55–64 | 2164 | 48% |
| 65–74 | 793 | 17% |
| 75+ | 530 | 12% |
| Female Gender | 2880 | 63% |
| Marital Status | ||
| Never Married | 255 | 6% |
| Married/Living with Partner | 3440 | 76% |
| Divorced/Separated/Widowed | 814 | 18% |
| Predisposing Social Variables | ||
| Acculturation | ||
| SL-ASIA score (median, mean ±SD) | 1.76, 1.82 ± 0.46 | – |
| Generational Status | ||
| First | 4476 | 98% |
| Second and Higher | 71 | 2% |
| Number of years living in the US | ||
| >10 years | 3402 | 75% |
| 6–10 years | 597 | 13% |
| ≤5 years | 544 | 12% |
| Language Preferred | ||
| Only Asian | 2256 | 50% |
| Mostly Asian with Some English | 1710 | 38% |
| Asian and English Equally | 454 | 10% |
| Mostly or Only English | 128 | 3% |
| Level of Education (yrs.) | ||
| 1–5 (some elementary school) | 205 | 5% |
| 6–11 (some middle/high school) | 1241 | 27% |
| 12 (high school graduate) | 1304 | 29% |
| > 12 (some college or college graduate) | 1772 | 39% |
| Enabling Variables | ||
| Annual Household Income | ||
| < $15000 | 1430 | 31% |
| $15 000–<$30 000 | 1472 | 32% |
| ≥ $30000 | 1062 | 23% |
| Refused to Answer/Don’t Know | 587 | 13% |
| Insurance Status | ||
| Not Insured | 1960 | 43% |
| Medical Only | 2033 | 45% |
| Medical + Vision | 550 | 12% |
| Having a Usual Place of Care | 2929 | 64% |
| Having a Usual Provider | 2856 | 63% |
| Currently Driving | 3153 | 70% |
| Evaluated Need Variables | ||
| Impaired Binocular Distance Vision | 474 | 10% |
| Impaired Binocular Near Vision | 542 | 12% |
| Self-reported Need Variables | ||
| Comorbidity Score (median, mean ± SD) | 1, 1.4 ± 1.4 | – |
| 0 | 1455 | 32% |
| 1–2 | 2267 | 50% |
| 3+ | 826 | 18% |
| SF-12 PHCa (Median, Mean ±SD) | 51.7, 49.1 ± 9.2 | – |
| SF-12 MHCa(Median, Mean ±SD) | 60.1, 55.9 ± 10.1 | – |
| NEI VFQ-25 Composite Scoreb (median, mean ± SD) | 94.3, 89.6 ± 12.3 | – |
Abbreviation: SD (standard deviation); SL-ASIA= Suinn-Lew Asian Self-Identity Acculturation Scale; US (United States).
Short Form-12 Physical Health Composite (SF-12 PHC) and Short Form-12 Mental Health Composite (SF-12 MHC) are scored such that a score of 50 (standard deviation of 10) is the average score among adults in the US.
National Eye Institute Visual Functioning Questionnaire-25 (NEI VFQ-25) is scored using the standard algorithm to calculate the subscale scores that have a possible range from 0 to 100. Then 11 of the 12 subscale scores (excluding the general health rating question) are averaged to yield a composite score.
Use of Eye Care
Overall, 36% of CHES participants reported an eye care visit in the past year, 48% reported ever having had a dilated eye examination, and 21% reported a dilated examination in the past year (Table 2). The prevalence of eye care use became higher among individuals of older age, with the steepest increase occurring after age 65. For example, when stratified by age groups, 8% of those participants 50 to 54 years old, 16% of those 55 to 64 years old, 36% of those 65 to 74 years old, and 44% of those 75 years and older received a comprehensive dilated examination in the past year. The crude overall prevalence of eye care use was higher among participants with vision impairment than among those without (Table 2; Ps < .001); however, this difference did not remain after age standardization. Age-specific prevalence of eye care use was similar between participants with vision impairment and those without (Ps > .05).
Table 2.
Prevalence of Eye Care Use in the Chinese American Eye Study by Age, Gender, and Vision Impairment Status
| All Participants (N = 4551) |
Participants without Vision Impairment (N = 4077)
|
Participants with Vision Impairmenta (N = 474)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 1 eye care visits in past 12 months | Ever had a dilated eye exam | ≥ 1 dilated eye exams in the past 12 months | ≥ 1 eye care visits in the past 12 months | Ever had a dilated eye exam | ≥ 1 dilated eye exams in the past 12 months | ≥ 1 eye care visits in the past 12 months | Ever had a dilated eye exam | ≥1 dilated eye exams in the past 12 months | |
| Overall | 36.1% | 47.9% | 21.1% | 35.3% | 46.6% | 20.1% | 43.1% | 59.1% | 30.2% |
| Age (yrs.) | |||||||||
| 50–54 | 21.7% | 29.7% | 8.3% | 21.8% | 29.6% | 8.1% | 21.1% | 32.1% | 12.3% |
| 55–64 | 30.8% | 42.8% | 16.4% | 31.2% | 43.1% | 16.7% | 24.2% | 38.2% | 12.1% |
| 65–74 | 53.9% | 65.2% | 36.1% | 54.2% | 65.4% | 35.9% | 51.9% | 63.5% | 37.7% |
| 75+ | 60.0% | 79.6% | 43.9% | 60.6% | 79.3% | 43.4% | 59.0% | 80.3% | 44.9% |
| Gender | |||||||||
| Male | 32.7% | 46.0% | 20.6% | 32.7% | 45.1% | 20.2% | 32.9% | 54.9% | 24.5% |
| Female | 38.0% | 49.0% | 21.4% | 36.8% | 47.5% | 20.0% | 48.1% | 61.1% | 32.9% |
Vision impairment was defined as presenting binocular distance visual acuity of 20/40 or worse.
We also evaluated eye care use by history of recent routine physical examination (Table 3). In CHES, 57% of participants reported having had a routine physical examination in the past 12 months. Among participants without a recent physical examination, 22% reported an eye care visit in the past year, 35% reported ever having had a dilated eye examination, and 10% reported a dilated examination in the past year. Among participants with a recent physical examination, the corresponding figures were higher (Ps < .001): 47%, 58%, and 30%, respectively. These patterns were consistent for all age groups and both genders (Table 3), and remained even after adjustment for age, gender, level of education, possession of medical/vision insurance, and number of comorbidities (Ps < .001).
Table 3.
Prevalence of Eye Care Use in the Chinese American Eye Study by History of Recent Physical Examination
| Participants who did NOT have a routine physical examination in the past 12 months (N = 1959a) |
Participants who had a routine physical examination in the past 12 months (N = 2585a) |
|||||
|---|---|---|---|---|---|---|
| ≥ 1 eye care visits in the past 12 months | Ever had a dilated eye exam | ≥ 1 dilated eye exams in the past 12 months | ≥ 1 eye care visits in the past 12 months | Ever had a dilated eye exam | ≥ 1 dilated eye exams in the past 12 months | |
| Overall | 21.6% | 35.0% | 9.6% | 47.0% | 57.7% | 29.9% |
| Age (yrs.) | ||||||
| 50–54 | 14.3% | 24.2% | 4.4% | 30.8% | 36.4% | 13.1% |
| 55–64 | 20.0% | 33.8% | 9.0% | 40.6% | 51.1% | 23.2% |
| 65–74 | 34.6% | 49.1% | 17.4% | 61.0% | 71.1% | 43.1% |
| 75+ | 48.7% | 74.2% | 27.4% | 63.5% | 81.2% | 48.8% |
| Gender | ||||||
| Male | 18.7% | 32.5% | 9.2% | 44.3% | 57.3% | 30.1% |
| Female | 23.4% | 36.6% | 9.9% | 48.5% | 58.0% | 29.7% |
Data on routine physical examination were missing for 7 participants.
Among CHES participants, 8.3% (N=381) participants reported having difficulty getting eye care when needed during the past 12 months. The corresponding figures among participants who did not have any eye care in the past 12 months and those who did was 9.8% and 5.7% respectively (P for difference <.001).
Comparison with Eye Care Use by Mexican Americans in the Los Angeles Latino Eye Study (LALES)
We also compared the prevalence of eye care use in CHES with that in LALES, since both utilized the same study protocols (Figure 1). Compared with Mexican Americans in LALES, Chinese Americans in CHES reported a lower prevalence of ever having had a dilated eye examination (age-standardized to the 2010 US Census population: 51.7% vs. 67.0%; P adjusted for age and gender< .001). Specifically for each age group, Chinese Americans in CHES reported a lower prevalence of ever having had a dilated eye examination at younger ages (29.7% vs. 52.4% for 50–54 years old; P<0.001) but reached similar prevalence at 75 years and older (79.6% vs. 81.7%; P=0.49).). In terms of recent dilated eye examination, even though the overall prevalence was similar between CHES and LALES (age-standardized: 24.0% vs. 24.7%; P=0.50), there were differences in age-specific prevalence: Chinese Americans in CHES reported a lower prevalence of recent dilated eye examinations before age 65 (P adjusted for age and gender < .001) and a higher prevalence among those 65 years and older (P adjusted for age and gender = .001). A similar pattern of inter-study differences was observed for recent eye care.
Figure 1.
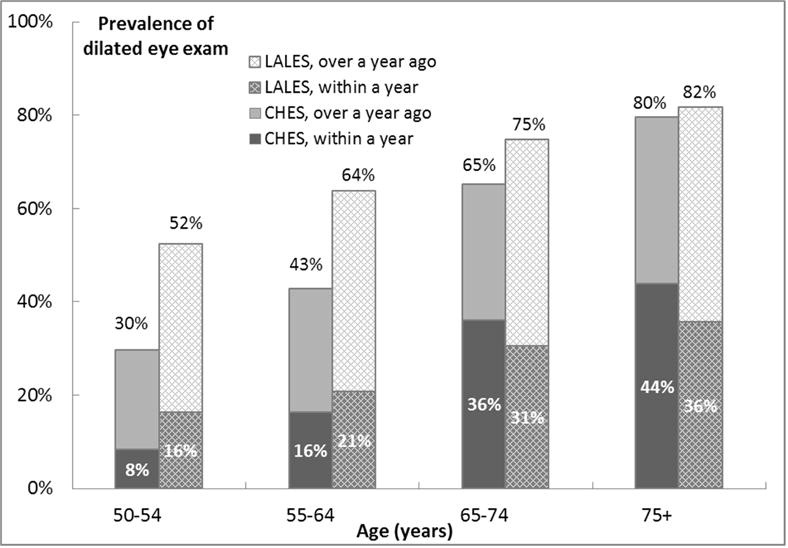
Comparison of Self-reported History of Having Had a Dilated Eye Examination between the Chinese American Eye Study (CHES) and the Los Angeles Latino Eye Study (LALES).
Factors Associated with Eye Care Use
A conceptual model approach was used to identify independent associations between variables from 3 categories (i.e., predisposing, enabling, and need variables) of Andersen’s Behavioral Model and each measure of eye care use. Table 4 presents the statistically significant associations identified in the multivariate analyses. Older age, female gender, English preference, higher level of education, health and/or vision insurance coverage, a usual place for medical care, current driver status, a greater number of comorbidities, and a lower vision-specific quality of life score were all associated with greater use of eye care based on all 3 measures (Ps < .05). To evaluate if any of the 12 medical conditions included in the comorbidity score calculation (e.g., diabetes mellitus and hypertension) were essential to the association with eye care use, we also performed leave-one-out analysis and found that the association between comorbidity score and use of eye care did not depend on any one particular condition. Further analysis of the NEI VFQ-25 subscales revealed that vision-specific mental health subscale score was the most important NEI VFQ-25 indicator of eye care use in CHES (Supplemental Table 1). Lower scores of vision-specific mental health (i.e., more mental distress due to vision) were associated with greater use of eye care.
Table 4.
Multivariable Associations of Predisposing, Enabling, and Need Variables Stratified by Use of Eye Care in the Chinese American Eye Study
| ≥ 1 eye care visits in the past 12 months
|
Ever had a dilated eye exam
|
≥ 1 dilated eye exams in the past 12 months
|
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P | OR (95% CI)a | P | OR (95% CI)a | P | ||
| Predisposing Demographic Variables | |||||||
| Age (yrs.) | |||||||
| 50–54 | 1.00 | – | 1.00 | – | 1.00 | – | |
| 55–64 | 1.42 (1.18–1.71) | <.001 | 1.59 (1.33–1.95) | <.001 | 1.73 (1.33–2.24) | <.001 | |
| 65–74 | 2.84 (2.24–3.59) | <.001 | 2.85 (2.25–3.60) | <.001 | 3.30 (2.45–4.43) | <.001 | |
| 75+ | 3.33 (2.48–4.47) | <.001 | 5.24 (3.83–7.16) | <.001 | 3.67 (2.59–5.21) | <.001 | |
| Gender | |||||||
| Male | 1.00 | – | 1.00 | – | 1.00 | – | |
| Female | 1.64 (1.41–1.90) | <.001 | 1.44 (1.25–1.66) | <.001 | 1.28 (1.07–1.52) | .006 | |
| Marital Status | |||||||
| Never Married | 1.00 | – | – | – | 1.00 | – | |
| Married/Living with Partner | 1.76 (1.27–2.43) | <.001 | – | – | 1.60 (1.06–2.42) | .027 | |
| Divorced/separated/widowed | 1.79 (1.26–2.55) | .001 | – | – | 1.66 (1.07–2.58) | .024 | |
| Predisposing Social Variables | |||||||
| Acculturation | |||||||
| High | – | – | 1.00 | – | – | – | |
| Low | – | – | 0.85 (0.71–1.01) | .062 | – | – | |
| Generational Status | – | – | – | – | – | – | |
| Number of years living in the US | |||||||
| >10 years | 1.00 | 1.00 | |||||
| 6–10 years | 0.67 (0.54–0.84) | <.001 | 0.57 (0.41–0.78) | <.001 | |||
| ≤5 years | 0.74 (0.58–0.95) | .017 | 0.67 (0.48–0.95) | .024 | |||
| Language preferred | – | – | – | – | – | – | |
| Only Asian | 1.00 | – | 1.00 | – | 1.00 | – | |
| Mostly Asian, Some English | 1.02 (0.87–1.21) | .77 | 1.15 (0.96–1.37) | .13 | 1.00 (0.82–1.22) | .99 | |
| Asian and English Equally | 1.22 (0.95–1.58) | .12 | 1.21 (0.92–1.59) | .18 | 1.37 (1.03–1.82) | .031 | |
| Mostly or Only English | 2.11 (1.38–3.21) | <.001 | 3.19 (1.89–5.38) | <.001 | 1.93 (1.26–2.97) | .003 | |
| Level of Education (years) | |||||||
| 1–5 | 1.00 | – | 1.00 | – | 1.00 | – | |
| 7–11 | 1.33 (0.94–1.89) | .11 | 1.36 (0.96–1.93) | .81 | 1.51 (1.00–2.29) | .050 | |
| 12 | 1.32 (0.93–1.88) | .12 | 1.58 (1.11–2.25) | .012 | 1.52 (1.00–2.31) | .050 | |
| > 12 | 2.00 (1.41–2.84) | <.001 | 2.45 (1.72–3.50) | <.001 | 1.75 (1.16–2.65) | .008 | |
| Enabling Variables | |||||||
| Annual Household Income | – | – | – | – | – | – | |
| Insurance Status | |||||||
| Not Insured | 1.00 | – | 1.00 | – | 1.00 | – | |
| Medical Only | 1.32 (1.10–1.59) | .003 | 1.43 (1.20–1.70) | <.001 | 1.34 (1.06–1.69) | .014 | |
| Medical + Vision | 1.99 (1.55–2.56) | <.001 | 2.25 (1.73–2.92) | <.001 | 2.25 (1.69–3.00) | <.001 | |
| Usual Place of Care | |||||||
| No | 1.00 | – | 1.00 | – | 1.00 | – | |
| Yes | 1.89 (1.58–2.27) | <.001 | 1.63 (1.37–1.93) | <.001 | 1.97 (1.54–2.52) | <.001 | |
| Usual Provider | – | – | – | – | – | – | |
| Currently Driving | |||||||
| No | 1.00 | – | 1.00 | – | 1.00 | – | |
| Yes | 1.50 (1.25–1.80) | <.001 | 1.33 (1.11–1.61) | 0.003 | 1.42 (1.13–1.77) | .002 | |
| Evaluated Need Variables | |||||||
| Binocular Distance Vision | – | – | – | – | – | – | |
| Binocular Near Vision | – | – | – | – | – | – | |
| Self-reported Need Variables | |||||||
| Comorbidity Score | |||||||
| 0 | 1.00 | – | 1.00 | – | 1.00 | – | |
| 1–2 | 1.26 (1.07–1.48) | .005 | 1.20 (1.03–1.40) | .023 | 1.51 (1.23–1.86) | <.001 | |
| 3+ | 1.82 (1.45–2.27) | <.001 | 1.96 (1.57–2.45) | <.001 | 2.52 (1.95–3.25) | <.001 | |
| SF-12 PHC | 1.039 (0.996–1.084) | .077 | – | – | – | – | |
| SF-12 MHC | – | – | – | – | – | – | |
| NEI VFQ-25 Composite Score b | 0.917 (0.890–0.944) | <.001 | 0.920 (0.893–0.949) | <.001 | 0.912 (0.885–0.940) | <.001 | |
Abbreviation: CI=confidence interval; NEI VFQ-25=the National Eye Institute Visual Functioning Questionnaire-25; OR=odds ratio; SF-12 PHC=Short Form-12 Physical Health Composite; SF-12 MHC =Short Form-12 Mental Health.
Estimated from multivariate logistic regression.
ORs associated with a 5 point difference.
Marital status and number of years living in the US were associated with 2 of the 3 measures of eye care use (any eye care visit in the past 12 months and dilated eye examination in the past 12 months, ever having a dilated eye examination and dilated eye examination in the past 12 months respectively), and acculturation level and the SF-12 PHC score were associated with only one measure of eye care use (ever having a dilated eye examination and any eye care in the past 12 months respectively) (Table 4). Higher household income (>$30k per year) was associated with higher prevalence of all three measures of eye care use when adjusted for age only, and these associations were limited to younger participants (<65 years old). However, in multivariate analyses adjusted for other covariates, household income was no longer associated with eye care use in the overall analyses of all participants or in subset analyses stratified by age, except that higher household income (>$30k per year) was associated with lower prevalence of dilated eye examination in the past 12 months among 65 years and older participants.
Similar associations between the above-identified factors and eye care use were observed among participants with vision impairment. Similar associations were also observed among participants of working-age (<65 years old) and retirement age (≥65 years old) except insurance (Supplemental Table 2). Having medical insurance as well as vision coverage seemed to have a greater impact on improving eye care use among retirement-age participants than working-age participants (p for interaction=0.003, 0.039, and 0.20 for any eye care in the past 12 months, ever having a dilated eye examination, and having a dilated eye examination in the past 12 months).
DISCUSSION
We presented the first report of the prevalence and associated factors for eye care use among a population-based sample of 50+ years old Chinese Americans residing in the Los Angeles County, California, USA.
Our data suggest that this group of Chinese Americans is not using eye care at recommended levels. The American Academy of Ophthalmology3 recommends that, even in the absence of signs or risk factors for eye disease, adults should receive at least 1 comprehensive eye examination by age 40, and have regular eye examinations every 2 to 4 years between the ages of 40 and 54 years, every 1 to 3 years between the ages of 55 and 64 years, and every 1 to 2 years after the age of 64 years. In this population-based study of Chinese Americans 50 years and older living in a Southern California neighborhood, which has 58 ophthalmologists within a 5-mile radius,40 only 48% reported ever having had a comprehensive (dilated) eye examination, only 36% reported having an eye care visit of any kind in the past year, and 21% reported having a dilated eye examination in the past year. When stratified by age groups, only 8% of participants 50 to 54 years old, 16% participants 55 to 64 years old, and 39% of participants 65 years and older received a comprehensive dilated eye examination in the past year. These levels of eye care use by older Chinese Americans are not in keeping with the recommendations of the American Academy of Ophthalmology and are particularly notable given the high prevalence of undiagnosed eye disease among this population. In CHES, 47% of eye conditions/diseases were undetected prior to completing the CHES clinical examination, and 18% of participants with undetected eye diseases were visually impaired based on presenting visual acuity (ARVO Abstract 5560-D0065, 2016). There is also a substantial gap in eye care use among participants with visual impairment, with only 30% having had a dilated eye examination in the past year.
Compared with the national average of all race/ethnicities combined,4, 12 Chinese Americans in CHES reported less eye care use. The 2002 NHIS of 18 years and older4 found that among American adults at high risk for serious vision loss (including those with self-reported diabetes, those having self-reported vision or eye problems, or those 65 years and older), approximately half visited an eye doctor in the past 12 months and half had a dilated eye examination; and among those not at high risk, approximately one-third visited an eye doctor and one-third had a dilated eye examination. In CHES, the corresponding figures were lower: 42% and 26% among similarly-defined high risk individuals, and 19% and 7% among low risk individuals (data not shown). The 2006–2009 BRFSS survey found that among visually impaired Americans 40 years and older, the age-adjusted state-level prevalence of annual eye care visits ranged from 48% to 69%, depending on the state.12 The corresponding age-adjusted prevalence among visually impaired CHES participants was much lower: 38%. Given that all CHES participants were 50 years or older and both the NHIS and the BRFSS included younger participants, the actual difference in eye care use between Chinese Americans and the national average may be even larger. However, it is important to note that the method for diagnosing vision impairment was different between studies: comprehensive eye examination in CHES but self-report in the BRFSS.12
Our data suggested that the level of eye care use by Chinese Americans is as low as that reported for older African Americans and Hispanics, and lower than that reported for older whites.10, 17 The Salisbury Eye Evaluation Project, a population-based survey of 65 to 84 year olds in Salisbury, Maryland, reported10 that 50% of African Americans and 69% of whites in their study visited an eye care professional in the past year. The corresponding figure among CHES participants 65 years and older was 56%. When compared with Mexican Americans in LALES,17 Chinese Americans in CHES reported a lower prevalence of ever having had a dilated eye examination at younger ages, before reaching similar prevalence at age 75. This suggests that Chinese Americans may delay their dilated eye examinations until they are older. However, this surprisingly low eye care use among Chinese Americans is in keeping with previous reports of infrequent medical visits by Asian Americans in general.41 For example, Asian Americans are less likely than other racial/ethnic groups to get screened for cancer.
Based on the Andersen’s Behavioral Model, we found a number of variables independently associated with use of eye care by Chinese Americans in CHES, and the impact of these variables also persisted among visually impaired participants, who may benefit the most from using eye care. It has been suggested that barriers to the use of eye care may be similar to barriers to the use of general health care.14 Consistent with this, in CHES, factors that have been associated with the use of general health care such as older age, female gender, more education, a usual place of care, and more comorbidities were also associated with more eye care use. Similar associations have also been reported by previous eye care studies in other populations.10, 13 For example, among Medicare beneficiaries diagnosed with diabetes or chronic eye diseases, a higher score in the Charlson Comorbidity Index was associated with more frequent eye examinations.13 In addition, in CHES, we found that individuals who had a routine physical examination in the past year were also more likely to have a recent dilated eye examination. However, even among those who did have routine physical examinations in the past year, the level of eye care use remained low: only 47% had any eye care visit in the past year, and 30% had a dilated eye examination in the past year. This indicates that there are additional factors associated with eye care use or that some factors may influence eye care use differently than general health care use. For example, in CHES, we found that current driving was associated with higher prevalence of eye care use, but not with the prevalence of routine physical examinations in the past 12 months (data not shown).
Lack of insurance was a key factor limiting accessing eye care by Chinese Americans: insured Chinese Americans were more likely to receive eye care than those who were not, and providing additional insurance coverage for vision care further increased eye care use. Unfortunately, among 65 years and older CHES participants, 11% did not have medical insurance and 78% did not have vision coverage; and among CHES participants younger than 65 years, the corresponding figures were 56% and 92% respectively. This difference in insurance coverage before and after age 65 is most likely due to qualification for Medicare at the age of 65 years, because 76% of 65 years and older CHES participants with medical insurance reported having Medicare. Higher likelihood of having insurance coverage as well as having a usual place of care, comorbidities, and more years living in the US contributed in part to more use of eye care reported by 65 years and older CHES participants, albeit older age itself was also associated with more eye care use independent of these factors. These findings, together with the observed greater impact of insurance coverage on eye care use among 65 years and older participants, underline the importance of gaining insurance coverage through Medicare for eye care use by older Chinese Americans.
Language, rather than cultural factors, may present more of a barrier to getting eye care for Chinese Americans. Even though language preference is often used as a proxy measure for acculturation,42 we found that when analyzed together, language preference itself was a more important predictor, indicating that language preference may be more of a proxy for English proficiency and barriers to access care.43 As 85% of Chinese Americans 50 years or older in CHES speak mostly Asian languages with limited English proficiency, removing this barrier will have a substantial impact on improving eye care use among Chinese Americans. Consistently, previous studies have identified language as the most formidable barrier in accessing health care for Asian American immigrants, especially elderly individuals who are more likely to lack English proficiency.19 Among foreign-born Asian Americans, only 49% say that they can carry on a conversation in English “very well”, according to data from the Pew Research Center.44 However, in CHES, language preference was not significantly associated with getting a routine physical examination in the past 12 months, indicating that limited English proficiency may be more of a barrier to eye care than primary care. In addition to language preference, another predisposing immigration-related factor - the length of time that a Chinese American had been living in the US was also associated with eye care use. Living in the US for less than 10 years was associated with lower prevalence of ever having a dilated eye examination and having a dilated eye examination in the past year, indicating that newcomers may be more likely to be underserved.
It is interesting to note that, in CHES, neither of the evaluated need variables (binocular distance vision impairment and binocular near vision impairment) was associated with eye care use, but self-reported need variables—such as NEI VFQ-25 composite score, designed to capture the influence of vision on multiple dimensions of health-related quality of life31—were strongly associated. In addition, among the NEI VFQ-25 subscales, vision-specific mental health score was the most important NEI VFQ-25 indicator of eye care use in CHES, regardless of whether the participant was visually impaired or not. These observations indicate that, among Chinese Americans in CHES, perceived rather than actual need was the more important determinant of eye care use.14 This is consistent with the observations from the Salisbury Eye Evaluation project, in which the perception of an eye problem (self-report of being told about vision problems, mostly early cataract), regardless of VA level, was the most important factor promoting visits to eye care professionals.10
This study has several limitations. First, although we believe the Chinese Americans included in this study are representative of Chinese Americans in the US despite small differences, caution is warranted when extrapolating our estimates to Chinese populations of different cultural heritages or Chinese residing in other regions of the US. Because factors such as cultural beliefs and socioeconomic / environmental contextual factors (e.g. availability of eye care providers in an area and access to public transportation) can also influence health care utilization. For example, the 2002 NHIS4 has found that the western region typically has a lower prevalence of eye care use than the northeast and Midwest. Second, the study’s results may not be generalizable to other Asian American subgroups, which can be very different from Chinese Americans with their unique health care needs and population characteristics in terms of immigration status, English proficiency, cultural assimilation, health beliefs, and health literacy.19, 44 Third, use of eye care was assessed through self-report without confirmation by medical records and it has been shown that patients tend to report less use of care than what was recorded in provider records.45 However, it was also found that the discrepancy between self-report and record reports did not vary by demographic or health variables. Therefore, recall bias is unlikely to have a big impact on the identification of factors associated with eye care use. Similarly, even though our data on systemic comorbidities was also collected through self-report without confirmation by medical records or testing, it has been shown that self-reported comorbidity data can be used as a reliable risk adjustment measure in comparison to medical record data.46 Fourth, we did not collect details on the type of eye care services received (e.g. whether our participants visited an ophthalmologist, optometrist, optician, or alternative medicine clinic) and therefore we are not able to distinguish potential differences between different types of eye care services. In addition, our cross-study comparison of the prevalence of eye care use may be confounded by temporal trends as the studies compared were not conducted concurrently. Lastly, this is cross-sectional study. Longitudinal follow up of the CHES cohort is needed to re-examine factors associated with eye care use and establish the direction of causation.
In conclusion, our data suggest that older Chinese Americans are not using eye care at the levels recommended by the American Academy of Ophthalmology.5 This underuse of eye care found in Chinese Americans is similar to that in African Americans and Latinos, and more than that in whites. These findings call public attention to the vision care disparity experienced by this unique population. Furthermore, several modifiable factors, such as insurance coverage, were associated with eye care use for Chinese Americans. Intervention programs targeting these factors should be a high priority, because vision impairment has significant public health impacts and Chinese Americans are one of the fastest growing populations in the US. Public health programs targeting Chinese Americans or Asian Americans should take into account patient characteristics, such as English proficiency and the psychological aspects of eye care need. In addition, our data support that coordinating eye examinations with routine physical examinations may be one effective approach to increase eye care use.
Supplementary Material
Acknowledgments
a. Funding/Support: This work was supported by grant EY-017337 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and an unrestricted Departmental grant from Research to Prevent Blindness, New York, NY 10022.
c. Other acknowledgements: See Appendix for members/affiliations of the Chinese American Eye Study Group
APPENDIX
The Chinese American Eye Study Group
USC Roski Eye Institute, University of Southern California, Rohit Varma, MD, MPH (Principal Investigator); Roberta McKean-Cowdin, PhD (Co-Investigator); Stanley P. Azen, PhD (Co-Investigator); Mina Torres, MS (Project Director); Chunyi Hsu, MPH (Project Manager); David Dinh, BA (Research Assistant); Ruzhang Jiang, MD (Examiner); Jie Sun, MD, PhD, MPH (Examiner); Dandan Wang, MD (Examiner); YuPing Wang, COT (Examiner); Justine Wong, BA (Clinical interviewer); Shuang Wu, MS (Statistician): Rucha Desai, MS (Programmer);
Battelle Survey Research Center
Lisa V. John, PhD (Recruitment Director); Michelle Cheng, MS (Field Supervisor).
Ocular Epidemiology Reading Center, School of Medicine and Public Health, University of Wisconsin
Ronald Klein, MD, MPH; Lisa M. Grady, BS; Stacy M. Meuer, BS; Michael W. Neider, BA, FOPS
CHES Data Monitoring and Oversight Committee
The Chinese American Eye Study investigators would like to thank the following members of the National Eye Institute’s Data Monitoring and Oversight Committee for their helpful advice and support: Alfred Sommer, MD, MHS (Chair); Anne Coleman, MD, PhD; Dennis Han, MD; Craig Hanis, PhD; Louise Wideroff, PhD; and Terri Young, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available atAJO.com
b. Financial Disclosures: No financial disclosures.
References
- 1.Crews JE, Chou CF, Zhang X, Zack MM, Saaddine JB. Health-related quality of life among people aged ≥65 years with self-reported visual impairment: Findings from the 2006–2010 behavioral risk factor surveillance system. Ophthalmic Epidemiol. 2014;21:287–96. doi: 10.3109/09286586.2014.926556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott AW, Bressler NM, Ffolkes S, Wittenborn JS, Jorkasky J. Public attitudes about eye and vision health. JAMA Ophthalmology. 2016;134(10):1111–1118. doi: 10.1001/jamaophthalmol.2016.2627. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology BoD. Frequency of ocular examinations. 2015 Available at: http://www.aao.org/clinical-statement/frequency-of-ocular-examinations–november-2009. Accessed: Nov 1, 2015.
- 4.Zhang X, Saaddine JB, Lee PP, et al. Eye care in the united states: Do we deliver to high-risk people who can benefit most from it? Arch Ophthalmol. 2007;125:411–418. doi: 10.1001/archopht.125.3.411. [DOI] [PubMed] [Google Scholar]
- 5.Feder RS, Olsen TW, Prum BE, Jr, et al. Comprehensive adult medical eye evaluation Preferred Practice Pattern® guidelines. Ophthalmology. 2016;123:P209–P236. doi: 10.1016/j.ophtha.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Keenum Z, McGwin G, Jr, Witherspoon CD, Haller JA, Clark ME, Owsley C. Patients’ adherence to recommended follow-up eye care after diabetic retinopathy screening in a publicly funded county clinic and factors associated with follow-up eye care use. JAMA Ophthalmol. 2016;134:1221–1228. doi: 10.1001/jamaophthalmol.2016.3081. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Park CK, Tracey PA, Pollack IP. Community screening for eye disease by laypersons: the Hoffberger program. Am J Ophthalmol. 2002;133:386–92. doi: 10.1016/s0002-9394(01)01380-0. [DOI] [PubMed] [Google Scholar]
- 8.Zheng CX, Hu WD, Tran J, et al. Barriers to receiving follow-up eye care and detection of non-glaucomatous ocular pathology in the Philadelphia Glaucoma Detection and Treatment Project. J Community Health. 2016;41:359–67. doi: 10.1007/s10900-015-0104-3. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158:1121–1129 e1. doi: 10.1016/j.ajo.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Orr P, Barrón Y, Schein OD, Rubin GS, West SK. Eye care utilization by older americans: The SEE project. Ophthalmology. 1999;106:904–909. doi: 10.1016/s0161-6420(99)00508-4. [DOI] [PubMed] [Google Scholar]
- 11.Baker RS, Bazargan M, Bazargan-Hejazi S, Calderon JL. Access to vision care in an urban low-income multiethnic population. Ophthalmic Epidemiol. 2005;12:1–12. doi: 10.1080/09286580590921330. [DOI] [PubMed] [Google Scholar]
- 12.Chou CF, Barker LE, Crews JE, et al. Disparities in eye care utilization among the United States adults with visual impairment: Findings from the behavioral risk factor surveillance system 2006–2009. Am J Ophthalmol. 2012;154:S45–52 e1. doi: 10.1016/j.ajo.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Sloan FA, Yashkin AP, Chen Y. Gaps in receipt of regular eye examinations among medicare beneficiaries diagnosed with diabetes or chronic eye diseases. Ophthalmology. 2014;121:2452–60. doi: 10.1016/j.ophtha.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Eye Institute. Identification of variables that influence access to eye care- Final report. 2005 [Google Scholar]
- 15.Ellish NJ, Royak-Schaler R, Passmore SR, Higginbotham EJ. Knowledge, attitudes, and beliefs about dilated eye examinations among African-Americans. Invest Ophthalmol Vis Sci. 2007;48:1989–1994. doi: 10.1167/iovs.06-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DJ, Lam BL, Arora S, et al. Reported eye care utilization and health insurance status among US adults. Arch Ophthalmol. 2009;127:303–10. doi: 10.1001/archophthalmol.2008.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales LS, Varma R, Paz SH, et al. Self-reported use of eye care among Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2010;117:207–15 e1. doi: 10.1016/j.ophtha.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RN, Indian RW, Zhang X, Geiss LS, Duenas MR, Saaddine JB. Visual impairment and eye care among older adults - five States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1321–5. [PubMed] [Google Scholar]
- 19.Kim W, Keefe RH. Barriers to healthcare among Asian Americans. Soc Work Public Health. 2010;25:286–95. doi: 10.1080/19371910903240704. [DOI] [PubMed] [Google Scholar]
- 20.Hoeffel EM, Rastogi S, Kim MO, Shahid H. 2010 Census Briefs. U.S. Census Bureau; 2012. The Asian Population: 2010. [Google Scholar]
- 21.Pew Research Center. Modern immigration wave brings 59 million to U.S., driving population growth and change through 2065: views of immigration’s impact on U.S. society mixed. Available at: http://www.pewhispanic.org/2015/09/28/modern-immigration-wave-brings-59-million-to-u-s-driving-population-growth-and-change-through-2065/. Accessed Decmber 17, 2016.
- 22.Vincent GK, Velkoff VA. Population Estimates and Projections. U.S. Census Bureau; 2010. The next four decades - the older population in the United States: 2010 to 2050. [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC Health disparities & inequalities report - United States, 2013. MMWR. 2013;62:1–187. [PubMed] [Google Scholar]
- 24.Varma R, Hsu C, Wang D, Torres M, Azen SP. The Chinese American Eye Study: Design and methods. Ophthalmic Epidemiol. 2013;20:335–47. doi: 10.3109/09286586.2013.823505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 26.Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 27.Zhang X, Andersen R, Saaddine JB, Beckles GL, Duenas MR, Lee PP. Measuring access to eye care: A public health perspective. Ophthalmic Epidemiol. 2008;15:418–25. doi: 10.1080/09286580802399102. [DOI] [PubMed] [Google Scholar]
- 28.Suinn RM, Ahuna C, Khoo G. The Suinn-Lew Asian Self-Identity Acculturation Scale: Concurrent and factorial validation. Educational and Psychological Measurement. 1992;52:1041–1046. [Google Scholar]
- 29.Suinn RM, Khoo G, Ahuna C. The Suinn-Lew Asian Self-Identity Acculturation Scale: Cross-cultural information. Journal of Multicultural Counseling and Development. 1995;23:139–148. [Google Scholar]
- 30.Ware JEJ, Kosinski M, Keller SD. A 12-Item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-list-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 32.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–55. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 33.McKean-Cowdin R, Varma R, Hays RD, Wu J, Choudhury F, Azen SP. Longitudinal changes in visual acuity and health related quality of life. The Los Angeles Latino Eye Study. Ophthalmology. 2010;117:1900–1907 e1. doi: 10.1016/j.ophtha.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Submacular Surgery Trials Research G. Responsiveness of the National Eye Institute visual function questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization—SST Report No. 1. Arch Ophthalmol. 2003;121:531–539. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108:1893–1900. doi: 10.1016/s0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 36.Globe DR, Varma R, Torres M, et al. Self-reported comorbidities and visual function in a population-based study: The Los Angeles Latino Eye Study. Arch Ophthalmol. 2005;123:815–821. doi: 10.1001/archopht.123.6.815. [DOI] [PubMed] [Google Scholar]
- 37.Linn BS, Linn MW, Gurel LEE. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 38.Royston P. Multiple imputation of missing values. Stata Journal. 2004;4:227–241. [Google Scholar]
- 39.U.S. Census Bureau. Profile of general population and housing characteristics: 2010 - Census summary file 2. Available at: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF2_SF2DP1&prodType=table. Accessed: April 25, 2013.
- 40.American Academy of Ophthalmology. Find an ophthalmologist. Available at: https://secure.aao.org/aao/find-ophthalmologist. Accessed: Dec 12, 2016.
- 41.Centers for Disease Control and Prevention. Asian American populations. Available at: http://www.cdc.gov/minorityhealth/populations/REMP/asian.html. Accessed: Nov 9, 2015.
- 42.Lee S, Nguyen HA, Tsui J. Interview language: A proxy measure for acculturation among Asian Americans in a population-based survey. J Immigr Minor Health. 2011;13:244–52. doi: 10.1007/s10903-009-9278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee GC, Walsemann KM, Takeuchi DT. English proficiency and panguage preference: testing the equivalence of two measures. Am J Public Health. 2010;100:563–569. doi: 10.2105/AJPH.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pew Research Center. The rise of Asian Americans. 2013 Apr 4; Updated edition: Available at: http://www.pewsocialtrends.org/files/2013/04/Asian-Americans-new-full-report-04-2013.pdf. Accessed: Dec 20, 2016.
- 45.Ritter PL, Stewart AL, Kaymaz H, Sobel DS, Block DA, Lorig KR. Self-reports of health care utilization compared to provider records. J Clin Epidemiol. 2001;54:136–41. doi: 10.1016/s0895-4356(00)00261-4. [DOI] [PubMed] [Google Scholar]
- 46.Olomu AB, Corser WD, Stommel M, Xie Y, Holmes-Rovner M. Do self-report and medical record comorbidity data predict longitudinal functional capacity and quality of life health outcomes similarly? BMC Health Serv Res. 2012;12:398. doi: 10.1186/1472-6963-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


