Abstract
Objective
Adiposity rebound (AR) or BMI (body mass index) rebound refers to the increase in BMI following the minimum BMI in early childhood. Early AR (before age 5) is predictive of adult obesity. To determine how 4 domains–demographics, maternal BMI, food security, and behavioral characteristics–may affect timing of AR.
Study design
248 children, ages 2.5 to 3.5, in Latino farmworker families in North Carolina were examined at baseline and every 3 months for 2 years. BMI was plotted serially for each child and the onset of BMI rebound was determined by visual inspection of the graphs. Given the ages of the children, all rebounds were detected prior to age 5 and were deemed “early,” while other children were classified as “non-rebounders.” Classes were then compared in terms of the 4 domains using bivariate analyses and linear mixed models.
Results
131 children demonstrated early rebound, 59 children were non-rebounders, and a further 35 had inconclusive data. Parents of early rebounders were less likely to have documentation permitting legal residence in the United States. Mothers of early rebounders were on average 3 BMI units heavier. Sex, household food security, diet quality, caloric intake, and daily activity did not differ between classes. In multivariable analysis, female sex, limited maternal education, increased maternal BMI, and increased caloric intake were significant predictors of early rebound.
Conclusion
High maternal BMI was the strongest predictor of early BMI rebound, but increased caloric intake was also significant. Limiting excess calories could delay premature AR and lower the risk of future obesity.
Keywords: Early adiposity rebound, determinants of adiposity rebound timing, maternal body mass index, food security, diet, physical activity, Latino farmworker families, childhood obesity
The prevalence of obesity is high among children across the United States, but Latino children experience disproportionate risk. In the 2011–2012 NHANES study, risk of obesity was approximately double for 2–5 year-old Latino children compared with all children in this age category.1 Children of Latino farmworkers may have even higher rates of obesity.2,3
Typically, body mass index (BMI) increases throughout the first year of life and declines to a nadir around age 6. The second rise in BMI following this minimum marks the beginning of the adiposity rebound (AR). In 1984, a French study first noted that early rebounders (before 5.5 years of age) had substantially increased adiposity at age 16 compared with children who rebounded later.5 The association between early AR and subsequent obesity has been demonstrated repeatedly6–10 and increased fat deposition has been confirmed using DEXA scans.11,12 Early AR is also linked to other components of the metabolic syndrome, including insulin resistance14, diabetes15, dyslipidemia14,16, and elevated blood pressure.14,16
Although the onset of AR was initially attributed to an increase in fat mass5, recent research suggests that AR coincides with a cessation of fat mass decline and an increase in lean mass.17,18 An increase in fat mass may actually lag behind 2–3 years, particularly for boys.19 Therefore, a more accurate term for AR would be “BMI rebound.” The term AR should thus be construed to represent an increase in BMI, with an understanding that increase in fat mass may lag behind the increase in lean mass.
This study sough to determine the extent to which modifiable risk factors for obesity and other characteristics influence the timing of AR. We hypothesized that children with risk factors for obesity, such as a high caloric intake and sedentary lifestyle, are more likely to rebound early.
Methods
This analysis included 248 families enrolled in the Niños Sanos study, a longitudinal 2-year study of dietary and physical activity patterns of young children of Latino farmworkers in North Carolina. Eligible participants were self-identified Latinas with a co-resident child aged 2.5 to 3.5 years and at least 1 member of the household engaging in farm work during the previous year. Children with special healthcare needs limiting physical activity were excluded.
A site-based sampling plan with a large contact base was developed to recruit participants.24 “Sites” are organizations or locations with which members of the target community are associated, such as Head Start programs and community health centers. Community data collectors also conducted door-to-door recruitment in Latino neighborhoods and farmworker camps, and contacted previous study participants.
A bilingual data collector screened for inclusion criteria, explained study procedures and incentives, and invited eligible families to take part. All participants provided signed informed consent. The Wake Forest School of Medicine Institutional Review Board approved the study, and a certificate of confidentiality was obtained from the National Institutes of Health.
Study staff interviewed participants at baseline and every 3 months for 2 years. All interviews were conducted in Spanish in participants’ homes or another preferred location between April 19, 2011, and July 30, 2014. Compensation of $10 was provided for each interview. Children’s weight was measured using a Tanita model BSB800 digital scale capable of determining weight to the nearest 0.1 kilogram. Height was determined twice using a portable stadiometer without shoes. If the 2 measurements differed by more than 0.5 centimeters, another measurement was taken and the 2 closest values were averaged.
Physical activity data were collected using Actical accelerometers (Mini Mitter Company, Inc., Bend OR) at baseline, 3, 6, 9, 12 and 24 months. Each device was attached to an elastic belt positioned above the child’s iliac crest.25 The parent and child were oriented to proper placement and usage of the device and written instructions with illustrations were also provided. Children were asked to wear the belt for 7 days and only remove the device for swimming, bathing, and sleeping. A successful wear day was defined as including at least 8 hours of wear data. Eighty-five percent of children provided at least 5 days of data including a weekend day.
Dietary data were collected by bilingual staff members using three 24-hour recalls during a seven day period, including one weekend day and two weekdays. The Nutrition Data System for Research software (version 11) was used.26 The first recall was conducted face-to-face, and subsequent interviews were conducted by telephone or in person when possible. Mothers were given a printed serving size guide, and the interviewer measured the size or volume of their child’s usual bowl, plate, and cup to help facilitate calculation of serving sizes. For children enrolled in preschools or daycares, food intake data were collected directly from the caregivers.
BMI at every observation was plotted for each child. Onset of AR was determined via visual inspection. Visual inspection has advantages over the use of polynomial models.27, 12 If the minimum BMI occurred after age 5 or at the last observation, there was no indication of early rebound. If the minimum BMI occurred before age 5, the remaining data points were examined to determine if sufficient rise in BMI had occurred to constitute a definitive rebound. We stipulated that, after the minimum, BMI must increase at a rate of at least 0.2 units per year, determined by linear regression of the remaining points. This threshold was set to account for the possibility of measurement error and random fluctuation, based on the recommendations of other studies.7,12
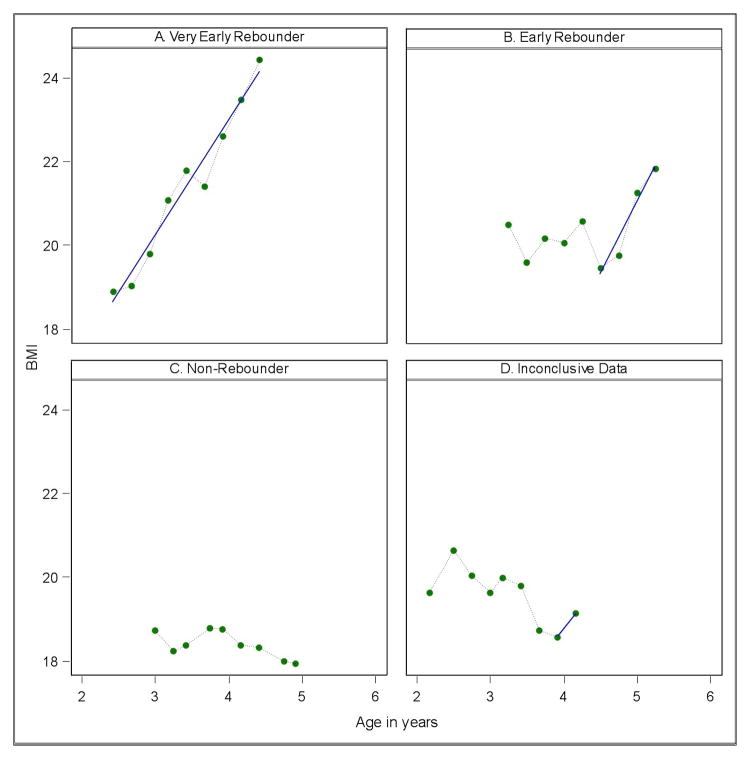
If the AR occurred prior to 3.5 years of age, the child was classified as having a very early rebound. Children whose BMI increased steadily since the first observation were assumed to have already rebounded. If rebound occurred between the ages of 3.5 and 5, the child was said to have an early rebound. Given the timeframe of the study, all rebounds were identified before age 5 and were therefore very early or early. Children without evidence of rebound were considered non-rebounders. A subset of children had growth data that could not be classified, due to insufficient data. We specified that at least 4 observations were necessary to make a classification, and that at least 2 observations had to occur after the minimum in order to establish a trend. Representative growth trajectories for each classification are shown in Figure 1 (available at www.jpeds.com). Although some studies suggest girls’ BMI rebounds earlier than boys’ BMI6,10, due to conflicting evidence28, overall growth curve similarities, and the loss of statistical power when dividing the sample by sex, boys and girls were not considered separately.
Figure 1: online.
Four representative growth trajectories.
Panel A is representative of very early rebounders, with minimum body mass index (BMI) before the age of 3.5 years and steady increase thereafter. Panel B demonstrates early rebound (between the ages of 3.5 and 5 years), with minimum BMI at around 4.5 years followed by an increase. Any increase after the adiposity rebound (AR) must be at least 0.2 BMI units per year. Panel C shows an individual who has not yet rebounded–there is no rise in BMI. Panel D shows data deemed inconclusive, because there is not sufficient data to indicate whether the point of minimum BMI constitutes the beginning of AR. Data was deemed inconclusive if there were either fewer than 4 total observation points or fewer than 2 observations after the BMI minimum. This step was taken to account for the possibility of measurement error and random fluctuation in BMI.
Standard CDC growth charts were used to determine age- and sex-specific BMI percentiles for children.29 Overweight was defined as BMI ≥ 85th percentile, but < 95th percentile, and obese as ≥ 95th percentile. Normal BMI was defined as < 85th percentile.
Food security was measured at baseline and quarterly for each household using a Spanish-language adaptation of the 18-item US Household Food Security Survey Module (HFSSM).30 Food security was graded 1 – 4 for participants, with 1 = very low, 2 = low, 3 = marginal, and 4 = secure.
The Revised Children’s Diet Quality Index (DQI)31, specifically developed for use among preschoolers, was used to evaluate quality of children’s dietary intake at baseline, 12 months, and 24 months, using data from the three 24-hour food recalls at each time point. This index uses 13 dietary components, such as added sugar and fat intake, to determine diet quality. The range of possible total scores extends from 0 to 90, with higher values indicating higher quality.32
Accelerometers were initialized with 15-second time periods known as epochs, which provided data to determine minutes spent performing activities of varying intensity.25 Epochs with fewer than 12 counts of activity were labeled sedentary, and epochs with more than 714 counts were classified as moderate/vigorous activity. The total number of epochs in each category was divided by the number of observation days to derive average minutes per day for each activity.
Mothers reported their own age, height, and weight, and the child’s age and sex. Mothers reported their current employment and marital status, and education, in terms of years completed. If the mother reported either parent moved from place to place to perform farm work, the family was classified as migrant; otherwise, they were classified as seasonal. Each mother reported whether she and her spouse/partner had documents allowing legal residence in the United States. If either had documents, the household was considered to have legal residence. Energy intake was calculated as average total Calories consumed per day from the 24-hour recalls.
Statistical Analyses
Different rebound classes were analyzed by children’s BMI and weight-for-age percentile (WAPCT) at baseline, year 1, and year 2. Descriptive statistics were then used to examine the relationship between rebound class and variables in the 4 domains of interest. The Waller-Duncan test for pairwise comparison, which minimizes the Bayes risk function, was used to test for significant differences in continuous variables across classes. For categorical data, a Pearson’s Chi-Square test was first used. Pairwise comparison using the Bonferroni adjustment was conducted if the Chi-Square test was significant.
A comprehensive generalized linear mixed model was applied to the longitudinal data using predictors from the 4 domains. For the dependent variable, 2 classes of BMI rebound were used–non-rebounder and combined very early/early. This was necessary because in the ordinal linear mixed effects (logistic) model, the proportionality assumption in a 3-category (very early, early, and non-rebounder) states that the odds ratios across 2 consecutive rebound classes are equal, which is not necessarily valid. The mixed effect model accounted for dependency between repeated measurements from the same individual. All statistical tests were 2-sided with significance levels set at 0.05. All analysis were conducted in SAS v9.4; the program PROC GLIMMIX was used in the longitudinal analysis.
Results
At baseline 248 families were enrolled in the study. Age and at least 1 BMI measurement were available for 245 children, who comprised the initial pool of subjects. Retention was high, with only 15 families lost to follow-up and 12 families withdrawing over the course of the study. On average, 86.3% of families completed each quarterly assessment. Twenty children had fewer than 4 measurements of BMI and were excluded from the study, resulting in a final sample of 225 children. The majority of mothers had less than a high school education, and 42.9% had 6 years or less of schooling (Table I; available at www.jpeds.com). Most were born in Mexico (86.1%) and married (90.2%). Most families worked in seasonal agriculture (72.7%) as opposed to migrant work, and only 15.1% of families had at least 1 parent with documentation allowing legal residence in the United States. Children were split evenly in terms of age and sex and almost all were born in the United States.
Table I.
Selected Demographic Characteristics of Participating Mothers and Children in Latino Farmworker Families (N=245)
| N (%) | |
|---|---|
| Mothers | |
| Age | |
| 18–25 | 72 (29.4%) |
| 26–35 | 136 (55.5%) |
| 36–45 | 37 (15.1%) |
| Education (years) | |
| 0–6 | 105 (42.9%) |
| 7+ | 140 (57.1%) |
| Place of birth | |
| US | 11 (4.5%) |
| Mexico | 211 (86.1%) |
| Other | 23 (9.4%) |
| Marital status | |
| Married | 221 (90.2%) |
| Not married | 24 (9.8%) |
| Children | |
| Age (yr) | |
| 2 | 129 (52.7%) |
| 3 | 116 (47.3%) |
| Sex | |
| Boy | 117 (47.8%) |
| Girl | 128 (52.2%) |
| Place of birth | |
| US | 242 (98.8%) |
| Other | 3 (1.2%) |
| Family | |
| Attends Head Start | |
| No | 186 (77.2%) |
| Yes | 55 (22.8%) |
| Family Status | |
| Migrant | 67 (27.3%) |
| Seasonal | 178 (72.7%) |
| Parental Documentation | |
| Both undocumented | 208 (84.9%) |
| One or both documented | 37 (15.1%) |
Forty-one children (18.2%) were classified as having a very early AR (< 3.5 years of age). Ninety children (40.0%) demonstrated early rebound (between ages of 3.5–5). Fifty-nine children (26.2%) were considered non-rebounders, and 35 children (15.5%) had unclassifiable growth curves for which the presence or absence of AR could not be determined with certainty.
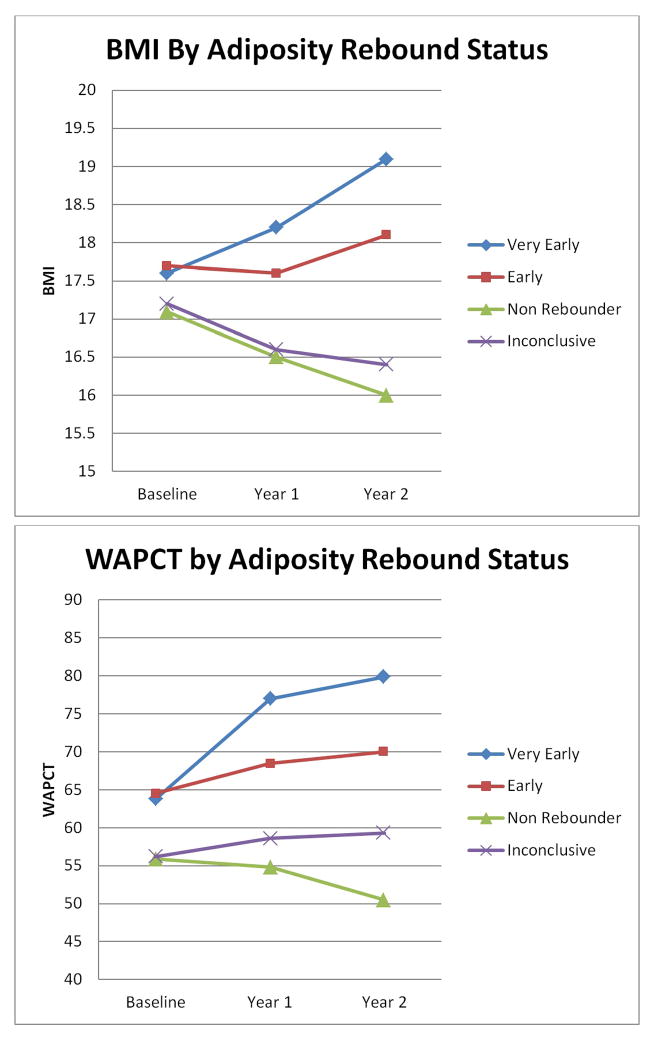
Children across categories were similar in terms of BMI at baseline, ranging from 17.1 in non-rebounders to 17.7 in early rebounders (Figure 2). At baseline, WAPCT was slightly higher in very early and early rebounders compared with non-rebounders and the inconclusive group. At the end of year 1 and year 2, BMI and WAPCT declined for non-rebounders. In contrast, BMI and WAPCT increased at year 2 for very early and early rebounders, with the biggest increase seen in the very early rebounders–BMI of 18.1 and WAPCT of 79.8. Over the course of the study, proportion of children with obesity increased significantly among early rebounders (Table II). Despite being fairly similar at baseline, at the end of year 2, 67.5% of very early rebounders and 56.6% of early rebounders were classified as overweight or obese versus only 27.5% of non-rebounders.
Figure 2.
Longitudinal Change in Body Mass Index (BMI) and Weight-for-Age Percentile (WAPCT). BMI and WAPCT are shown for each class of children at baseline, the end of year 1, and the end of year 2.
Table II.
Body Mass Index Status by Adiposity Rebound Class
| Very Early Rebounder (N=41) | Early Rebounder (N=90) | Non-Rebounder (N=59) | Inconclusive (N=35) | |
|---|---|---|---|---|
|
|
||||
| Baseline | ||||
| Normal | 20 (50.0%) | 48 (55.2%) | 32 (56.1%) | 26 (76.5%) |
| Overweight | 9 (22.5%) | 13 (14.9%) | 16 (28.1%) | 2 (5.9%) |
| Obese | 11 (27.5%) | 26 (29.9%) | 9 (15.8%) | 6 (17.7%) |
| Year One | ||||
| Normal | 12 (31.6%) | 43 (50.0%) | 39 (69.6%) | 23 (67.7%) |
| Overweight | 7 (18.4%) | 20 (23.3%) | 10 (17.9%) | 7 (20.6%) |
| Obese | 19 (50.0%) | 23 (26.7%) | 7 (12.5%) | 4 (11.8%) |
| Year Two | ||||
| Normal | 13 (32.5%) | 39 (43.3%) | 42 (72.4%) | 22 (66.7%) |
| Overweight | 5 (12.5%) | 22 (24.4%) | 10 (17.2%) | 5 (15.2%) |
| Obese | 22 (55.0%) | 29 (32.2%) | 6 (10.3%) | 6 (18.2%) |
Very early and early rebounders were compared with non-rebounders across 4 domains that may affect timing of AR (Table III). Demographic variables included sex, age, mother’s marital status and education, documentation status, and status as a seasonal or migrant farmworker. Maternal BMI was considered to be a proxy variable reflecting the child’s genetic predisposition for obesity. Food security was intended to represent household environment, and behavior was represented by DQI, daily caloric intake, and minutes per day spent sedentary or engaged in moderate-to-vigorous activity. Few significant differences were noted, with the exception of age, documentation status, and maternal BMI. Very early rebounders were on average 3 months younger than non-rebounders and early rebounders were on average 4 months older than non-rebounders. Non-rebounders were more likely to be part of documented families (25.4%) compared with early rebounders (8.8%). Mean maternal BMI was 31.4 for both very early and early rebounders, versus 28.4 for non-rebounders.
Table III.
Characteristics of Adiposity Rebound Classes by Mean or Percent
| Very Early (N=41) | Early (N=90) | Non-Rebounder (N=59) | |
|---|---|---|---|
|
|
|||
| Demographics | |||
| Males | 46.3% | 43.3% | 52.5% |
| Age in Months+ | 31.2* | 38.2* | 34.2 |
| Mother Married | 80.5% | 94.4% | 88.1% |
| Less than 7 Years of Education | 46.3% | 50.0% | 33.9% |
| Any Household Documentation+ | 17.1% | 8.8%* | 25.4% |
| Migrant Farmworker Status | 22.0% | 23.3% | 27.1% |
| Maternal BMI | |||
| Maternal BMI+ | 31.4* | 31.4* | 28.4 |
| Household Context | |||
| Food Security | 2.27 | 2.40 | 2.22 |
| Behavior | |||
| DQI Score | 61.7 | 59.6 | 59.4 |
| Calories per Day | 1185 | 1226 | 1143 |
| Minutes Sedentary Per Day | 410 | 420 | 439 |
| Minutes Moderate-Vigorous Activity Per Day+ | 5 | 7 | 6 |
indicates significant difference with non-rebounder class as reference, and
indicates overall significant difference;
BMI = Body Mass Index, DQI = Diet Quality Index
A generalized linear mixed model was used to determine if variables in any of the domains predicted early AR (Table IV). Higher maternal BMI predicted early rebound (p < 0.01), as did higher caloric intake (p < 0.05). For every 1 unit increase in maternal BMI, a child was 15% more likely to have an early rebound. For every 100 additional calories consumed, a child was 22% more likely to have an early rebound. Female children were also more likely to undergo early rebound (p < .05). Limited maternal education (< 7 years) was associated with higher likelihood of early rebound (p < .05). The interaction between maternal BMI and sex was also significant (p < 0.05). Minutes of moderate-vigorous physical activity and DQI were not included due to consideration of multicollinearity with other terms.
Table IV.
Odds ratio estimates of risk factors for early adiposity rebound
| Effect | Odds Ratio | Confidence Interval | P value |
|---|---|---|---|
| Age | 1.04 | (0.98, 1.11) | 0.227 |
| Sex (male) | 18.1 | (1.90, 172.8) | 0.012 |
| Marital Status (married) | 1.14 | (0.37, 3.48) | 0.821 |
| Education (< 7 years) | 0.40 | (0.18, 0.87) | 0.020 |
| Documentation Status (no documentation) | 0.64 | (0.24, 1.71) | 0.374 |
| Migrant Status (migrant) | 1.18 | (0.50, 2.80) | 0.708 |
| Maternal BMI | 1.15 | (1.07, 1.24) | <0.001 |
| Food Security (insecure) | 1.02 | (0.49, 2.10) | 0.963 |
| Energy Intake | 1.22 | (1.03, 1.43) | 0.021 |
| Minutes Sedentary Per Day | 1.00 | (0.99, 1.01) | 0.475 |
| Maternal BMI and Gender Interaction | 0.91 | (0.85, 0.99) | 0.020 |
Odds ratio > 1 indicates higher likelihood of being in early rebound class; reference class is shown in parentheses. BMI = Body Mass Index, DQI = Diet Quality Index; Energy Intake = daily caloric intake/100
Discussion
Children classified as early and very early rebounders demonstrated markedly elevated prevalence of obesity even within the short time frame of the study. Approximately 2/3 of children with classifiable growth curves experienced BMI rebound before age 5, an important difference relative to many other studies. The original French study linking early AR to obesity showed that only 30% of children experienced AR before 5.5 years of age.5 In a New Zealand study, average age of rebound was 6.6 for boys and 6.0 for girls.10 A more recent cohort study done in the United Kingdom indicated 72.8% of participants rebounded after age 5.12 On the other hand, the relatively early AR seen in children of Latino farmworkers appears fairly consistent with data from NHANES, which showed that Mexican-American boys and girls rebounded at age 5 on average.26 Studies from Chile14 and Japan16 also suggest AR occurs earlier relative to the samples from Europe5,12 and New Zealand.10 Given the diversity of results, timing of AR may vary substantially between populations. The preponderance of rebounds before age 5 in children of Latino farmworkers may increase their risk of adult obesity.
The strongest determinant of AR onset appears to be maternal BMI, with early rebounders having heavier mothers. Multiple studies have reported a similar association. For example, children with early BMI rebound had significantly heavier (2 BMI units) mothers and fathers compared with late rebounders in a New Zealand study.10 In a United Kingdom study, 23.3% of very early rebounders had at least 1 obese parent, compared with 10.4% of children who experienced AR after age 5.7 Supporting this hypothesis, a genome-wide scan identified 3 chromosomal regions that modulate the age of AR.33 However, maternal BMI is not a pure representation of genetic potential–a mother is likely to share an environment and habits with her child that influence weight. Further research is needed to clarify the pathways through which maternal BMI affects children’s growth, and whether the relationship is due predominately to environmental or genetic factors
A unique finding was that presence of documentation allowing legal residence in the United States was more common in families of children without an early rebound. Although more research is needed to better understand the implications of this result, undocumented status may be a marker of other risk factors linked to obesity, such as limited access to health care, lack of safe and appropriate places for physical activity, and inadequate diet.34 Undocumented workers earn lower wages, live in lower quality housing, and enjoy fewer benefit programs compared with documented workers.35 Although documentation status did not predict early AR in the logistic regression, maternal education was significant. Limited education could also be characteristic of families with lower income and poorer living conditions. Less educated mothers may be less aware of healthy weight targets for their children and how a healthy weight can be achieved.36
One study has shown that children with the highest levels of physical activity experienced AR on average 1 year later than less active children.41 In contrast, levels of physical activity did not appear to differ significantly between children in different classes in our study. This may be due to the fact the sample as a whole was highly sedentary.42 Caloric intake did appear to affect AR timing, however, with higher intake predicting earlier AR.
Latino farmworker families contend with multiple socioeconomic disadvantages that further increase risk of obesity.51 Parents earn low wages, often live in substandard housing, may need to relocate frequently, experience anti-immigrant and anti-Latino bias, and have limited access to health care and government benefit programs. As a result, children may rely on inexpensive, but nutritionally poor, food.36,52,53 Children may live in unsafe neighborhoods and lack appropriate spaces to walk and play.34,54 Many Latino mothers rely on television as a “babysitter” and tool to teach children English.55 Given the deficiency of health services and limited formal education, parents may receive little guidance regarding appropriate weight for their children.
This study has several limitations. The duration of the study was only 2 years, and longer follow-up would have allowed for the identification of AR in more children. Due to the short study period, 35 growth curves were not classifiable, and not all children labeled non-rebounders had data extending at least to the age of 5. If the children with unclassifiable data differed in significant ways from the rest of the sample, this could bias the results. Though not a limitation, the study’s focus on an underserved and understudied population (Latino farmworkers of North Carolina) lessens the generalizability of the results.
Clinicians can use BMI rebound to identify a child at risk of obesity at an early age, even before significant excess weight gain has occurred. Our results indicate Latino farmworker children are likely to experience what has traditionally been considered an early BMI rebound. Timing of BMI rebound is influenced by maternal BMI, but energy intake also appears important. Delaying AR by maintaining a healthy lifestyle may have significant long-lasting benefits for children’s weight.
Acknowledgments
Supported by the National Institute for Child Health and Human Development (R01HD059855), the National Heart, Lung, and Blood Institute (1R01HL101066), and the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS) UL1TR001420. Dietary data analysis was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK56350) to the University of North Carolina Chapel Hill. The authors declare no conflicts of interest.
Abbreviations
- AR
Adiposity Rebound
- BMI
Body Mass Index
- WAPCT
Weight-for-Age-Percentile
- DQI
Diet Quality Index
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosado JI, Bennett Johnson S, McGinnity KA, Cuevas JP. Obesity among Latino children within a migrant farmworker community. Am J Prev Med. 2013;44:S274–81. doi: 10.1016/j.amepre.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Kilanowski JF, Ryan-Wenger N. Health status of an invisible population: carnival and migrant worker children. West J Nurs Res. 2007;29:100–20. doi: 10.1177/0193945906295484. [DOI] [PubMed] [Google Scholar]
- 4.Power C, Lake JK, Cole TJ. Measurement and long-term health risks of child and adolescent fatness. Int J Obes Relat Metab Disord. 1997;21:507–26. doi: 10.1038/sj.ijo.0800454. [DOI] [PubMed] [Google Scholar]
- 5.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129–35. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.3.e5. [DOI] [PubMed] [Google Scholar]
- 7.Dorosty AR, Emmett PM, Cowin Sd, Reilly JJ. Factors associated with early adiposity rebound. ALSPAC Study Team. Pediatrics. 2000;105:1115–8. doi: 10.1542/peds.105.5.1115. [DOI] [PubMed] [Google Scholar]
- 8.Prokopec M, Bellisle F. Adiposity in Czech children followed from 1 month of age to adulthood: analysis of individual BMI patterns. Ann Hum Biol. 1993;20:517–25. doi: 10.1080/03014469300002922. [DOI] [PubMed] [Google Scholar]
- 9.Siervogel RM, Roche AF, Guo SM, Mukherjee, Chumlea WC. Patterns of change in weight/stature2 from 2 to 18 years: findings from long-term serial data for children in the Fels longitudinal growth study. Int J Obes. 1991;15:479–85. [PubMed] [Google Scholar]
- 10.Williams SM, Goulding A. Patterns of growth associated with the timing of adiposity rebound. Obesity. 2009;17:335–41. doi: 10.1038/oby.2008.547. [DOI] [PubMed] [Google Scholar]
- 11.Ohlsson C, Lorentzon M, Norjavaara E, Kindblom JM. Age at adiposity rebound is associated with fat mass in young adult males-the GOOD study. PLoS One. 2012;7:e49404. doi: 10.1371/journal.pone.0049404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AR, Sherriff A, Ness AR, Reilly JJ. Timing of adiposity rebound and adiposity in adolescence. Pediatrics. 2014;134:e1354–61. doi: 10.1542/peds.2014-1908. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RW, Goulding A, Lewis-Barned NJ, Williams SM. Rate of fat gain is faster in girls undergoing early adiposity rebound. Obes Res. 2004;12:1228–30. doi: 10.1038/oby.2004.155. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez L, Corvalan C, Pereira A, Kain J, Garmendia ML, Uauy R. Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int J Obes. 2014;38:1299–304. doi: 10.1038/ijo.2014.97. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46:190–4. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 16.Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Pediatrics. 2014;133:e114–9. doi: 10.1542/peds.2013-0966. [DOI] [PubMed] [Google Scholar]
- 17.Plachta-Danielzik S, Bosy-Westphal A, Kehden B, Gehrke MI, Kromeyer-Hauschild K, Grillenberger M, et al. Adiposity rebound is misclassified by BMI rebound. Eur J Clin Nutr. 2013;67:984–9. doi: 10.1038/ejcn.2013.131. [DOI] [PubMed] [Google Scholar]
- 18.Campbell MW, Williams J, Carlin JB, Wake M. Is the adiposity rebound a rebound in adiposity? Int J Pediatr Obes. 2011;6:e207–15. doi: 10.3109/17477166.2010.526613. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RW, Williams SM, Carter PJ, Goulding A, Gerrard DF, Taylor BJ. Changes in fat mass and fat-free mass during the adiposity rebound: FLAME study. Int J Pediatr Obes. 2011;6:e243–51. doi: 10.3109/17477166.2010.549488. [DOI] [PubMed] [Google Scholar]
- 20.Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Antropol. 2002;(Suppl 35):159–84. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 21.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–9. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004;4:6. doi: 10.1186/1471-2431-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz WH. “Adiposity rebound”: reality or epiphenomenon? Lancet. 2000;356:2027–8. doi: 10.1016/S0140-6736(00)03396-1. [DOI] [PubMed] [Google Scholar]
- 24.Ip EH, Saldana S, Arcury TA, Grzywacz JG, Trejo G, Quandt SA. Profiles of food security for US farmworker households and factors related to dynamic of change. Am J Public Health. 2015;105:e42–7. doi: 10.2105/AJPH.2015.302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531–43. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RK. Dietary intake—how do we measure what people are really eating? Obes Res. 2002;10:63S–8S. doi: 10.1038/oby.2002.192. [DOI] [PubMed] [Google Scholar]
- 27.Kroke A, Hahn S, Buyken AE, Liese AD. A comparative evaluation of two different approaches to estimating age at adiposity rebound. Int J Obes. 2006;30:261–6. doi: 10.1038/sj.ijo.0803143. [DOI] [PubMed] [Google Scholar]
- 28.Boonpleng W, Park CG, Gallo AM. Timing of adiposity rebound: a step toward preventing obesity. Pediatr Nurs. 2012;38:37–42. [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 30.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to measuring household food security. Alexandria, VA: US Department of Agriculture, Food and Nutrition Service; 2000. Measuring food security in the United States. [Google Scholar]
- 31.Kranz S, Hartman T, Siega-Riz AM, Herring AH. A diet quality index for American preschoolers based on current dietary intake recommendations and an indicator of energy balance. J Am Diet Assoc. 2006;106:1594–1604. doi: 10.1016/j.jada.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Quandt SA, Trejo G, Suerken CK, Pulgar CA, Ip EH, Arcury TA. Diet quality among preschool-age children of Latino migrant and seasonal farmworkers in the United States. J Immigr Minor Health. 2016;18:505–12. doi: 10.1007/s10903-015-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyre D, Lecoeur C, Delplanque J, Fracke S, Vatin V, Durand E, et al. A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes. 2004;53:803–11. doi: 10.2337/diabetes.53.3.803. [DOI] [PubMed] [Google Scholar]
- 34.Grzywacz JG, Arcury TA, Trejo G, Quandt SA. Latino mothers in farmworker families’ beliefs about preschool children’s physical activity and play. J Immigr Minor Health. 2016;18:234–42. doi: 10.1007/s10903-014-9990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshikawa H, Kalil A. The effects of parental undocumented status on the developmental contexts of young children in immigrant families. Child Development Perspectives. 2011;5:291–7. [Google Scholar]
- 36.Quandt SA, Grzywacz JG, Trejo G, Arcury TA. Nutritional strategies of Latino farmworker families with preschool children: identifying leverage points for obesity prevention. Soc Sci Med. 2014;123:72–81. doi: 10.1016/j.socscimed.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nord M, Andrews MS, Carlson S. Household food security in the United States, 2008. Washington, DC: U.S. Dept. of Agriculture, Economic Research Service; 2009. [Google Scholar]
- 38.Franklin B, Jones A, Love D, Puckett S, Macklin J, White-Means S. Exploring mediators of food insecurity and obesity: a review of recent literature. J Community Health. 2012;37:253–64. doi: 10.1007/s10900-011-9420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryu JH, Bartfeld JS. Household food insecurity during childhood and subsequent health status: The Early Childhood Longitudinal Study—kindergarten cohort. Am J Public Health. 2012;102:e50–5. doi: 10.2105/AJPH.2012.300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janz KF, Levy SM, Burns TL, Torner JC, Willing MC, Warren JJ. Fatness, physical activity, and television viewing in children during the adiposity rebound period: the Iowa Bone Development Study. Prev Med. 2002;35:563–71. doi: 10.1006/pmed.2002.1113. [DOI] [PubMed] [Google Scholar]
- 41.Moore LL, Gao D, Bradlee ML, Cupples LA, Sundarajan-Ramamurti A, Proctor MH, et al. Does early physical activity predict body fat change throughout childhood? Prev Med. 2003;37:10–7. doi: 10.1016/s0091-7435(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 42.Ip EH, Saldana S, Trejo G, Marshall SA, Suerkan CK, Lang W, et al. Physical activity states of pre-school aged children in farmworker families: predictive factors and relationship with BMI percentile. J Phys Act Health. 2016;13:726–32. doi: 10.1123/jpah.2015-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolland-Cachera MF, Deheeger M, Akrout M, Bellisle F. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 7 years of age. Int J Obes Relat Metab Disord. 1995;19:573–8. [PubMed] [Google Scholar]
- 44.Johnson W, Soloway LE, Erickson D, Choh AC, Lee M, Chumlea WC, et al. A changing pattern of childhood BMI growth during the 20th century: 70 y of data from the Fels Longitudinal Study. Am J Clin Nutr. 2012;95:1136–43. doi: 10.3945/ajcn.111.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowal M, Kryst L, Woronkowicz A, Sobiecki J, Brudeck J, Zarow R. Long-term changes in BMI and adiposity rebound among girls from Krakow (Poland) over the last 30 years (from 1983 to 2010) Am J Hum Biol. 2013;25:300–6. doi: 10.1002/ajhb.22359. [DOI] [PubMed] [Google Scholar]
- 46.Vignerova J, Humenikova L, Brabec M, Riedlova J, Blaha P. Long-term changes in body weight, BMI, and adiposity rebound among children and adolescents in the Czech Republic. Econ Hum Biol. 2007;5:409–25. doi: 10.1016/j.ehb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in US between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–8. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 48.Piernas C, Popkin BM. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in US children and adolescents: patterns and trends by age group and sociodemographic characteristics, 1977–2006. Am J Clin Nutr. 2011;94:1324–32. doi: 10.3945/ajcn.110.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piernas C, Popkin BM. Snacking increased among US adults between 1977 and 2006. J Nutr. 2010;140:325–32. doi: 10.3945/jn.109.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson PM, Butcher KE. Childhood obesity: trends and potential causes. Future Child. 2006;16:19–45. doi: 10.1353/foc.2006.0001. [DOI] [PubMed] [Google Scholar]
- 51.Hovey JD, Magaña C. Acculturative stress, anxiety, and depression among Mexican immigrant farmworkers in the Midwest United States. J Immigr Health. 2000;2:119–31. doi: 10.1023/A:1009556802759. [DOI] [PubMed] [Google Scholar]
- 52.Drewnowski A, Darmon N. Food choices and diet costs: an economic analysis. J Nutr. 2005;135:900–4. doi: 10.1093/jn/135.4.900. [DOI] [PubMed] [Google Scholar]
- 53.Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower-income adults. J Acad Nutr Diet. 2014;114:1943–53. doi: 10.1016/j.jand.2014.06.353. [DOI] [PubMed] [Google Scholar]
- 54.Brewer M, Kimbro RT. Neighborhood context and immigrant children’s physical activity. Soc Sci Med. 2014;116:1–9. doi: 10.1016/j.socscimed.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 55.Lindsay AC, Sussner KM, Greaney ML, Peterson KE. Influence of social context on eating, physical activity, and sedentary behaviors of Latina mothers and their preschool-age children. Health Educ Behav. 2009;36:81–96. doi: 10.1177/1090198107308375. [DOI] [PMC free article] [PubMed] [Google Scholar]