Supplemental Digital Content is available in the text
Keywords: executive functions, functional MRI, hypertension, white matter lesions
Abstract
Objectives:
Our study aimed at exploring structural and functional differences in the brain during higher cognitive processing between middle-aged hypertensive patients and controls matched for sex, age and years of education.
Methods:
Two groups of 20 patients took part in MRI examinations. This article reports the results of functional MRI during a Stroop color interference task and structural evaluations based on a modified Fazekas scale.
Results:
No intergroup differences were found in regards to the severity of white matter lesions (Mann–Whitney U test = 150.5, P > 0.1), nor from the task performance in the scanner (t(35) = 0.2, P > 0.1). However, brain activation patterns between patients and controls varied. Hypertensive patients involved significantly more cerebral areas during the processing, regardless of the task difficulty. Differences were found in 26 diverse regions of both primary and associative cortices (with a peak voxel located in the cuneus, Z = 6.94, P < 0.05 family-wise error corrected at voxel level).
Conclusion:
Our findings provide an insight into the brain mechanisms related to essential hypertension and suggest a functional reorganization (neuroplasticity) early in the course of the disease.
INTRODUCTION
Hypertension is known to affect brain structure and cognitive processing [1–3]. It is recognized as a major risk factor for cognitive decline and dementia [4], and the role of proper drug treatment in brain protection has been emphasized [5].
The hypertension–brain relationship is commonly interpreted as a one-way link, in which cardiovascular changes resulting from sustained blood pressure (BP) elevation are the cause and cerebral disorder (and further on – cognitive dysfunction) is the result [6,7]. Many studies support this notion demonstrating accelerated structural brain aging in the hypertensive population, such as white matter lesions (WMLs) [8,9], gray matter thickness reduction [10,11] and increased cerebral atrophy [12].
Furthermore, the cognitive functioning of individuals with hypertension has been widely studied [13]. High BP was reported to affect executive functions [14] as well as working memory tasks performances [15] in patients of different sex [16], race [17] and age [18].
In contrast, there is growing evidence that the hypertension–brain link can also be read as a bidirectional one [19]. It has recently been suggested that the disease may influence cerebral functioning early in its course or even that the brain regulatory dysfunction may be considered the cause of elevated BP [20]. This concept is supported by MRI studies linking exaggerated BP reactivity with altered brain activation patterns in response to psychological stress in normotensive patients [21–23].
However, much less is known about mechanisms underlying this relationship in hypertension. Earlier studies addressing this issue included primarily elderly patients [24,25] in whom concomitant brain structural changes and/or comorbidities are likely to cloud primary functional abnormalities. Other studies evaluated the overall cardiovascular risk contribution to functional differences at the neuropsychological [18] and neuroanatomical [26,27] levels without specific analysis focusing on the effects of hypertension per se.
Functional MRI (fMRI) based on the measurement of blood oxygenation level dependent (BOLD) signal allows us to visualize brain activity. Variations in oxygen consumption and cerebral blood flow (CBF) result in the change of magnetic properties of the tissue (hemoglobin and deoxyhemoglobin are magnetically different) and in the change of MRI signal intensity.
Therefore, we incorporated MRI to examine both cerebral functional and structural differences between the middle-aged patients with uncomplicated essential hypertension and controls matched for sex, age and years of education. We tested the hypothesis that the brain-activation patterns during higher cognitive processing are altered in hypertension. Furthermore, we explored whether this reorganization is reflected in the structural disorder traditionally attributed to BP.
METHODS
Participants
The article reports results from 40 patients. Initially, we screened 66 participants [37 patients with uncomplicated hypertension – the hypertensive group (HTN) and 29 healthy controls – the control group (CON)]. The patients were free of cardiovascular disease and diabetes. Fourteen of the patients were excluded because of previous psychoneurological history (i.e. drug addiction, depression, traumatic brain injury etc.), existing brain disorder (WMLs were not a reason of exclusion) or technical problems during fMRI acquisition (i.e. excessive movement, synchrobox malfunction etc.). All patients with resistant hypertension (seven patients) were excluded as well due to the small sample size. In the end two matched groups (9 men and 11 women each) were formed and balanced for sex, age and educational level (as it is emphasized [16,28]) to control for these variables in the study population. As a result, no significant intergroup differences in these variables were identified (sex: the chi-square test P > 0.1; age: t(38) = 1.6, P > 0.1; years of education: t(38) = 1.1, P > 0.1). The groups were not balanced in regards to BMI, but they were comparable with respect to waist–hip ratio (WHR). The occurrence of hyperlipidemia and smoking status didn’t differentiate the populations either. All participants were right handed as assessed by the Edinburgh Handedness Inventory [29]. Detailed characteristics of the groups are provided in Table 1.
TABLE 1.
Demographic and blood pressure characteristics of the groups
| HTN | CON | P | |
| Sex: female/malea | 11 (55%)/9 (45%) | 11 (55%)/9 (45%) | |
| Age (years)b | 48.9 ± 8.0 (27–64) | 45.4 ± 11.4 (32–61) | 0.270 |
| Years of educationb | 14.8 ± 2.6 | 16.4 ± 3.1 | 0.090 |
| Smokersa | |||
| Current | 3 (15%) | 4 (20%) | |
| Past | 6 (30%) | 5 (25%) | |
| Never | 11 (55%) | 11 (55%) | |
| BMI (kg/m2)b,c | 31.6 ± 6.5 | 26.5 ± 2.8 | 0.007** |
| 28.9 (26.7–36.2) | 26.2 (24.2–28.3) | ||
| Waist-to-hip ratiob | 0.92 ± 0.07 | 0.89 ± 0.06 | 0.138 |
| SBP (mmHg)b | |||
| Office | 131.0 ± 12.7 | 124.5 ± 9.9 | 0.075 |
| Ambulatory daytime | 130.3 ± 9.8 | 123.3 ± 9.3 | 0.026* |
| Ambulatory night-time | 114.1 ± 9.6 | 109.4 ± 8.0 | 0.098 |
| DBP (mmHg)b | |||
| Office | 84.5 ± 7.9 | 77.4 ± 7.8 | 0.007** |
| Ambulatory daytime | 82.8 ± 9.9 | 78.2 ± 4.7 | 0.070 |
| Ambulatory night-time | 69.2 ± 9.7 | 65.7 ± 5.7 | 0.172 |
| Serum lipidsb | |||
| Total cholesterol (mg/dl) | 203.7 ± 44.8 | 214.2 ± 43.0 | 0.452 |
| LDL cholesterol (mg/dl) | 123.9 ± 36.1 | 135.3 ± 42.8 | 0.372 |
| HDL cholesterol (mg/dl) | 53.5 ± 14.0 | 54.5 ± 19.1 | 0.859 |
| Triglycerides (mg/dl) | 131.1 ± 67.7 | 122.8 ± 92.3 | 0.764 |
| Dyslipidemiaa | 14 (70%) | 16 (80%) | 0.465 |
CON, the healthy control group; HTN, the hypertension patients group.
aNumber of patients (percentage).
bMean ± standard deviation (range).
cMedian (quartiles).
*P < 0.05.
**P < 0.01.
Study protocol was approved by the Ethics Committee of the Medical University of Gdansk (NKEBN/422/2011). All participants were informed about the study merits and signed a written consent.
Medication and blood pressure measures
The diagnosis of hypertension was based on the 2013 European Society of Hypertension/European Society of Cardiology criteria. We have excluded patients with secondary forms of hypertension. The patients were younger than 65 years and free of cardiovascular disease and diabetes. The hypertension was defined as receiving antihypertensive treatment or daytime SBP mean values of higher than 135 mmHg in untreated patients. Twenty-four-hour ambulatory BP monitoring (ABPM) was used in every patient to confirm BP status. ABPM was performed within 3 weeks following an fMRI study with the Spacelabs 90207 recorder (Spacelabs Inc., Snoqualmie, Washington, USA). The recorders were programed to obtain measurements every 20 min from 0600 to 2200 h (day), and every 30 min from 2200 to 0600 h (night). Office BP was assessed at the day of the fMRI study.
In the HTN group, mean time from the diagnosis of hypertension was 10.9 ± 10.5 years. Patients were treated with angiotensin-converting enzyme (ACE) inhibitors (50%), Angiotensin II receptor antagonists (30%), calcium channel blockers (35%), diuretics (30%), β-blockers (25%) and α-blockers (10%). Mean number of antihypertensive drugs was 1.8 (median value = 2). Lipid-lowering drugs (statins) were used in 35% of patients and 20% of controls. The groups did not differ in regards to lipid treatment occurrence (P = 0.29).
Functional MRI task
We chose executive functioning task among the cognitive domains. It is considered to be the most vulnerable to increased BP [30] and sensitive to vascular cognitive impairment [31]. The Stroop color word interference task we selected has been successfully used in previous neuropsychological studies of the hypertensive population [32] and neuroimaging studies of the cerebrovascular reactivity in healthy groups [21–23]. Yet previous fMRI research adapted the task as a block design, in which blocks of demanding cognitive performance alternated with blocks of nondemanding ones. This resulted in transient stressor-evoked cardiovascular reactivity in patients. We were not interested in brain response to stressors, nor in the cardiovascular reactivity but in the differences between the study groups during demanding task processing. Therefore, we used a modified Stroop task in a rapid event design paradigm. In this design, the stimuli of different task conditions are presented continuously in pseudorandom order, thus providing a sustained level of emotional/stressor load throughout the study. Each participant was trained with the task outside the scanner prior the examination. Color recognition was tested as well.
During the task, patients were visually presented with gray boards consisting of the text in the center and four color markers on the bottom of the screen. The texts in all boards were normalized to the same width to compensate for the differences in word lengths. The color markers stood as an answer reminder for participants (to decrease the memory load of the task). The order of the color markers was consistent throughout the study. There were four groups of boards, each representing one of the conditions: control, congruent, incongruent and reading.
The text presented on the board varied between the conditions. It was
-
1.
a colored string of symbols (#, % etc.) in the control condition,
-
2.
a word representing a color name written in the same color in the congruent condition (i.e. ‘BLUE’ written with blue font),
-
3.
a word representing a color name written in a different color in the incongruent condition (i.e. ‘BLUE’ written in red font),
-
4.
a word representing a color name written in contoured black in the reading condition.
The patients were instructed to press the button referring to the font color regardless of the text written [in (1)–(3) conditions] or to press the button referring to the color name written [in (4) condition]. The latter was introduced to ensure nonperceptual processing. Example stimuli are provided in Fig. 1.
FIGURE 1.
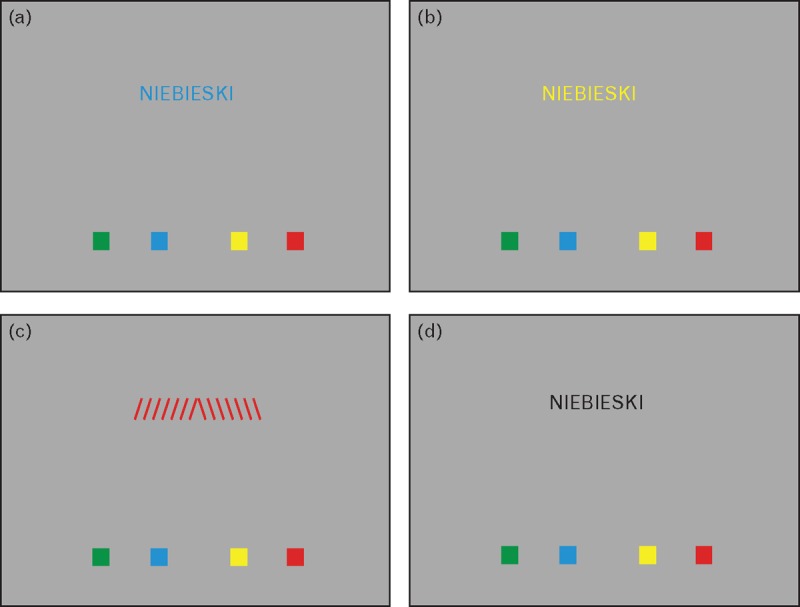
Example boards presenting each of the task's conditions in the functional MRI Stroop paradigm (caption meaning BLUE in Polish language). Panel (a) – the congruent condition, panel (b) – the incongruent condition, panel (c) – the control condition, and panel (d) – the reading condition.
Each board was presented for 2000 ms followed by a 500-ms presentation of a fixation cross. The boards were presented in a fix, pseudo-random order. During the functional run, the total number of boards of each of the conditions was as follows:
-
1.
76 boards of the control condition,
-
2.
80 boards of the congruent condition,
-
3.
77 boards of the incongruent condition,
-
4.
75 boards of the reading condition.
In addition, there was a jitter introduced as a prolonged fixation cross presentation (3000 ms instead of 500 ms). There were 28 such jitters during the whole run. The amount of the boards and the stimuli and jitters order (the onsets) were optimized with the use of a genetic algorithm [33] to maximize the incongruent vs congruent condition contrast. All stimuli were presented by MRI-compatible goggles. Patients’ answers were collected via dedicated response pads (Nordic Neuro Lab) to evaluate the differences in the task performance between the study groups. The log files containing answers of three of the participants (one from the CON group, two from the HTN group) were not registered properly and were excluded from the intergroup task performance comparison. The percentage of correct responses to each of the conditions was calculated and further contrasted between the groups with an independent two-sample t test in the Statistical Package for the Social Sciences (SPSS) software (version 21; IBM, Armonk, New York, USA).
MRI acquisition
The MRI examination was held on a 3T Achieva TX scanner (Philips Healthcare; Best, The Netherlands) with the use of a 32-channel head coil. The structural imaging protocol included T1-weighted turbo field echo [repetition time = 8.1 ms, echo time = 3.7 ms, voxel size 1 × 1 × 1 mm, field of view (FOV) 260 × 252 × 160 mm, flip angle: 8°], T2-weighted turbo spin echo (repetition time = 3683 ms, echo time = 80 ms, voxel size 1.2 × 1.2 × 2 mm), fluid attenuation inversion recovery (FLAIR: repetition time = 9000 ms, echo time = 125 ms, voxel size 1 × 1,1 × 4 mm) and diffusion-weighted imaging (b = 0, 500, and 1000 mm2/s, repetition time = 3951 ms, echo time = 83 ms, voxel size 1.5 × 1.9 × 4 mm) sequences.
The blood oxygen level-dependent signal during the Stroop task was collected with the T2∗ Gradient Echo-Planar Imaging sequence (FFE-EPI: 37 axial slices, repetition time: 2000 ms, echo time: 30 ms, flip angle: 90°, matrix: 64 × 64, slice thickness: 3 mm, 420 volumes, voxel size 3 × 3 × 3 mm, acquisition time 14 min, FOV = 250 mm). Functional imaging was preceded with 4 dummy-scans.
Radiological assessment
The FLAIR sequence was used for the qualitative assessment of WMLs severity in each examined patient. WMLs were graded using modified Fazekas scale [34] without making a distinction between deep and periventricular white matter changes [35]. The chosen method was successfully applied previously in other studies [36,37]. The Fazekas classification is a scale ranging from 0 (no WMLs) to 3 (large confluent areas of WMLs), with punctate foci seen in the 1st grade and a beginning of confluence of WMLs observed in the 2nd grade. Depending on the severity of white matter changes, patients were classified into four groups (0–3) in accordance with the Fazekas scores. The evaluation was performed by two independent readers (a neuroradiologist with a 10-year experience and a general radiologist) that were blinded to the clinical data.
The inter-rater agreement was estimated with the Cohen's kappa coefficient. In addition, the Mann–Whitney U test was calculated to compare and contrast the severity of WMLs in the groups of interest. All statistical analyses were performed with SPSS software (version 21).
Functional MRI data processing
Data preprocessing was performed using Statistical Parametric Mapping software, version 12 (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks Inc.; Sherborn, Massachusetts, USA). Single-patient analyses included slice timing correction, realignment to the first image of the time series, normalization at 2 × 2 × 2 mm to a standard brain atlas (SPM12 Montreal Neurological Institute template space) and smoothing using a 6-mm full width at half maximum Gaussian kernel. Then the data were inspected with the artifact detection toolbox (ART: http://www.nitrc.org/projects/artifact_detect). A timepoint with difference from the previous that exceeded 3 SDs in a general mean signal, of more than 1-mm translation or more than 0.02° rotation was marked as an outlier timepoint. Functional run with more than 10% of the outliers would have been discarded from analyses (no patient in the study met this criterion).
Afterwards, a General Linear Model was fitted to the patients’ data to estimate the parameters for each condition. The four task conditions were modeled as box-cart functions of 2000-ms duration convolved with the standard Hemodynamic Response Function. Six directions of motion parameters from the realignment step as well as outlier timepoints were used as nuisance regressors. The low-frequency noise and the signal drift were high-pass filtered with 1/128-Hz cutoff.
Next a whole-brain full-factorial analysis was performed with four conditions (control, incongruent, congruent and reading) and two groups (patients and controls) as factors. Due to intergroup imbalance in respect to the BMI, as well as several reports regarding the influence of one's weight on the central nervous system [38], the model was further extended to include covariates associated with patients’ BMI and WHR. Finally, the participants’ SBP and DBP values, respectively, based on the ABPM (all day mean and SD of the measurement) were also included as covariates. This procedure ensured that the variance of the fMRI signal associated solely with the differences in patients’ weight or BP was ruled out from the computed intergroup differences.
The main effects (group and condition respectively) and interaction between the factors (group × condition) were calculated, as well as the simple comparisons between the groups of interest (CON vs HTN/HTN vs CON) to determine the direction of the effects. The F-contrasts for specific covariates were calculated as well to identify brain regions in which the variability of fMRI signal during the task was associated with one's weight indices (BMI and WHR) or BP level (SBP or DBP). All results were corrected for multiple comparisons (P < 0.05) using the family-wise error (FWE) correction at the voxel level with minimum cluster size of 10 consecutive voxels.
RESULTS
Demographic and laboratory data
As was described in the ‘Methods’ section, the groups differed in regards to the mean BMI values (the hypertensive patients were more obese than the controls). Yet, no imbalance was found in respect to the mean WHR. This surprising effect may be a result of the dissimilarity in the mentioned coefficients values computation. The BMI includes information regarding one's weight and height, whereas the WHR is also susceptible to one's fat distribution – android vs gynoid type. In our study samples, the BMI did not correlate with the WHR (r = 0.08, P > 0.1) suggesting that although there were differences regarding the overall weight of the participants, those were not reflected in distinct fat distributions of the patients.
Also, the comparison of the overall mean BMI values of the groups is somewhat misleading, as the metric is vulnerable to extreme values. The SD of the weight in the HTN group was pronouncedly higher (6.5 kg/m2 as opposed with the 2.8 kg/m2 in the CON group) indicating higher heterogeneity in the patients’ population. Therefore, it should be noted that the difference between the groups’ median values of the BMI was significantly smaller (around 2.7 points) than the difference between the groups’ mean values of the BMI (around 5.1 points). Nevertheless, due to the issues mentioned, the weight indices were included as confounds in the intergroup fMRI comparisons.
The BP monitoring revealed that the HTN group had significantly higher mean SBP during daytime and DBP during the morning office examination. Other BP measures showed no intergroup differences, thus suggesting relatively good drug induced BP control in the HTN group. As there was no real-time BP monitoring during the MRI examination, the mean values from the ambulatory daytime assessment were included as covariates in the fMRI model.
No intergroup differences were found in regards to participants’ sex, age, education, smoking status, level of serum lipids and nor the dyslipidemia prevalence.
Radiological results
No significant differences were noted between the HTN and the CON groups in the severity of WMLs (Mann–Whitney U test = 150.5, P > 0.1). Punctate WMLs were observed in eight healthy and nine hypertensive patients. Yet 12 controls and 11 patients presented no WMLs at all. Also, no moderate or severe WMLs (second or third grade according to the modified Fazekas scale) were observed in either group.
A substantial inter-rater agreement on the Fazekas scale scores was found (κ = 0.634, P < 0.001).
Functional MRI results
There were no significant differences in the scanner task performance between the groups in the control (t(22) = 1.31, P > 0.1), the incongruent (t(35) = 0.58, P > 0.1) and the reading (t(35) = 1.12, P > 0.1) condition. For the congruent condition, there was a tendency for statistical significance, although it was the HTN group that performed better (t(23) = 1.93, P = 0.06). The means and SDs of the groups are presented in Table 2.
TABLE 2.
Mean percentages of correct responses during the functional MRI Stroop task
| HTN | CON | P | |
| Controla | 98.39 ± 1.53 | 96.88 ± 4.65 | 0.194 |
| Congruenta | 98.54 ± 1.98 | 96.05 ± 5.24 | 0.067 |
| Incongruenta | 83.98 ± 30.30 | 88.38 ± 13.35 | 0.568 |
| Readinga | 96.07 ± 5.50 | 93.61 ± 7.56 | 0.268 |
| Totala | 94.26 ± 7.87 | 93.75 ± 7.36 | 0.837 |
CON, the healthy control group; HTN, the hypertension patients group.
aMean ± SD.
To account for the obesity indices influence (the BMI and the WHR), prior investigating the intergroup differences, an F-contrast focused on the variability of the BOLD signal induced by those covariates was calculated. It resulted in a scattered pattern of small clusters across the whole brain [see the figure, Supplemental Digital Content (SDC) 1, which illustrates the significant clusters and the table, SDC 2, which lists direct coordinates and Z-statistics for the peak voxels of the significant clusters). This image (thresholded at P < 0.05 FWE at voxel level with no extended cluster minimum) was next used as an exclusive mask for all the following analyses presented. In other words – every voxel, which BOLD response variability stayed in line with one's BMI or WHR, was excluded from further analyses. We performed this procedure to ensure that non of the results discussed below are related to the patients’ weight.
The significance threshold for fMRI results presented below was set at P value less than 0.05 FWE voxel-wise corrected for multiple comparisons. This approach, being the most restrictive available, ensures a minimum risk of false positive errors. Also it concurrently reduces the cohesion of activation patterns, making the results seem scattered rather than continuous. Therefore, we also inspected the data uncorrected for multiple comparisons, to evaluate if the significant peaks of activation observed from any continuous clusters when threshold is lowered. The data proved so with intergroup differences forming continuous clusters along the brain midline (see the figure, SDC 3, which illustrates the uncorrected results of the full factorial fMRI analysis).
Despite no differences in task performance, the activation patterns between the groups varied. The whole-brain full-factorial fMRI analysis revealed significant main effects of the factors (group and condition separately) with no significant interaction between them (group × condition). This pattern of results (significant main effects with no significant interaction between them) determines that the factors influence the brain-activation patterns independently – no matter what the task's level of difficulty was (color recognition, reading, naming congruent color and naming incongruent color), the hypertensive patients and the healthy control patients demonstrated different brain activation patterns.
The main effect of the group showed diverse brain regions involving frontal, parietal and occipital lobes and the limbic system. To determine the direction of the effect, two simple intergroup t test comparisons were performed. The CON vs HTN contrast showed only one small cluster in the frontal subgyral area of the right hemisphere (Z = 5.12, PFWE < 0.05). It was the opposite comparison (HTN vs CON) that mostly contributed to the main effect observed. The HTN group presented additional activation in 25 clusters that covered the areas of both primary and associative cortices. What is noticeable – those clusters centered around medial surface of the brain with the biggest areas concentrated in the posterior cingulate as well as cuneus and precuneus. It should be noted that as the factorial model also included the SBP and the DBP as covariates; therefore, the intergroup differences discovered cannot be attributed to the patients’ distinct BP levels.
The main effect of the condition reflected the differences in cognitive load between the task's conditions (color recognition, reading, naming congruent color and naming incongruent color). Significant brain clusters were found in all cortical areas as well as the thalamus of both hemispheres.
Direct coordinates of the peak significant voxels are reported in Table 3. The anatomical regions were defined with the Automated Anatomical Labeling software [39]. The activation maps are shown in Fig. 2.
TABLE 3.
Significant clusters (P < 0.05 family-wise error corrected at voxel level) of the whole brain factorial functional MRI analysis presented in alphabetical order
| Anatomical region | x | y | z | Z | No. of voxels |
| Group main effect (controls vs patients) | |||||
| RH frontal lobe subgyral area | 34 | −6 | 32 | 5.12 | 12 |
| Group main effect (patients vs controls) | |||||
| LH calcarine fissure and surrounding cortex | −22 | −76 | 8 | 6.62 | 143 |
| LH calcarine fissure and surrounding cortex | 2 | −78 | 12 | 5.43 | 32 |
| LH cuneus | −2 | −86 | 26 | 5.97 | 27 |
| LH cuneus | −16 | −82 | 36 | 5.38 | 20 |
| LH cuneus | −8 | −74 | 26 | 5.35 | 26 |
| LH hippocampus | −14 | −44 | 8 | 6.06 | 120 |
| LH hippocampus | −18 | −26 | −10 | 5.29 | 21 |
| LH lingual gyrus | −6 | −60 | −2 | 5.78 | 62 |
| LH lingual gyrus | −12 | −40 | −2 | 5.62 | 21 |
| LH median cingulate and paracingulate gyri | −12 | 6 | 38 | 6.23 | 45 |
| LH median cingulate and paracingulate gyri | −2 | 22 | 36 | 5.86 | 53 |
| LH postcentral gyrus | −30 | −34 | 44 | 5.71 | 21 |
| LH precental gyrus | −38 | −4 | 58 | 5.90 | 44 |
| LH precental gyrus | −26 | −4 | 40 | 5.27 | 11 |
| LH precuneus | −2 | −48 | 54 | 6.79 | 153 |
| LH precuneus | −14 | −64 | 58 | 6.10 | 16 |
| LH superior frontal gyrus, medial | −6 | 52 | 34 | 5.19 | 11 |
| RH anterior cingulate and paracingulate gyri | 14 | 34 | 22 | 5.94 | 20 |
| RH calcarine fissure and surrounding cortex | 24 | −64 | 16 | 6.43 | 193 |
| RH cuneus | 12 | −92 | 16 | 6.94 | 60 |
| RH lingual gyrus | 6 | −62 | 4 | 6.43 | 86 |
| RH median cingulate and paracingulate gyri | 10 | −22 | 34 | 5.79 | 20 |
| RH superior parietal gyrus | 18 | −76 | 50 | 5.77 | 22 |
| RH supplementary motor area | 6 | −2 | 62 | 5.59 | 14 |
| RH supplementary motor area | 4 | −8 | 60 | 5.44 | 11 |
| Condition main effect | |||||
| LH anterior cingulate and paracingulate gyri | −2 | 34 | 16 | 5.46 | 29 |
| LH inferior frontal gyrus, triangular part | −40 | 22 | 26 | 7.49 | 895 |
| LH inferior parietal, but supramarginal and angular gyri | −12 | −88 | −2 | Inf | 12 019 |
| LH superior frontal gyrus, medial | −8 | 54 | 10 | 5.37 | 14 |
| LH superior frontal gyrus, medial orbital | 0 | 54 | −10 | 6.47 | 190 |
| LH thalamus | −12 | −16 | 4 | 6.14 | 33 |
| RH inferior frontal gyrus, opercular part | 40 | 6 | 26 | 6.18 | 65 |
| RH insula | 34 | 26 | 0 | 5.60 | 12 |
| RH median cingulate and paracingulate gyri | 0 | −38 | 46 | 5.95 | 57 |
| RH middle frontal gyrus | 38 | 28 | 34 | 5.47 | 10 |
| RH middle occipital gyrus | 22 | −92 | 10 | 7.76 | 201 |
| RH precental gyrus | 54 | 6 | 36 | 5.18 | 10 |
Only clusters exceeding 10 voxels are reported. Anatomical labels based on the Automated Anatomical Labeling software. Results of the second level factorial analysis, P < 0.05 FWE corrected at voxel level, x, y, z are MNI coordinates of the most significant center of the activation within the activated cluster. FEW, family-wise error; LH, left hemisphere; MNI, Montreal Neurological Institute; RH, right hemisphere; Z, z value.
FIGURE 2.
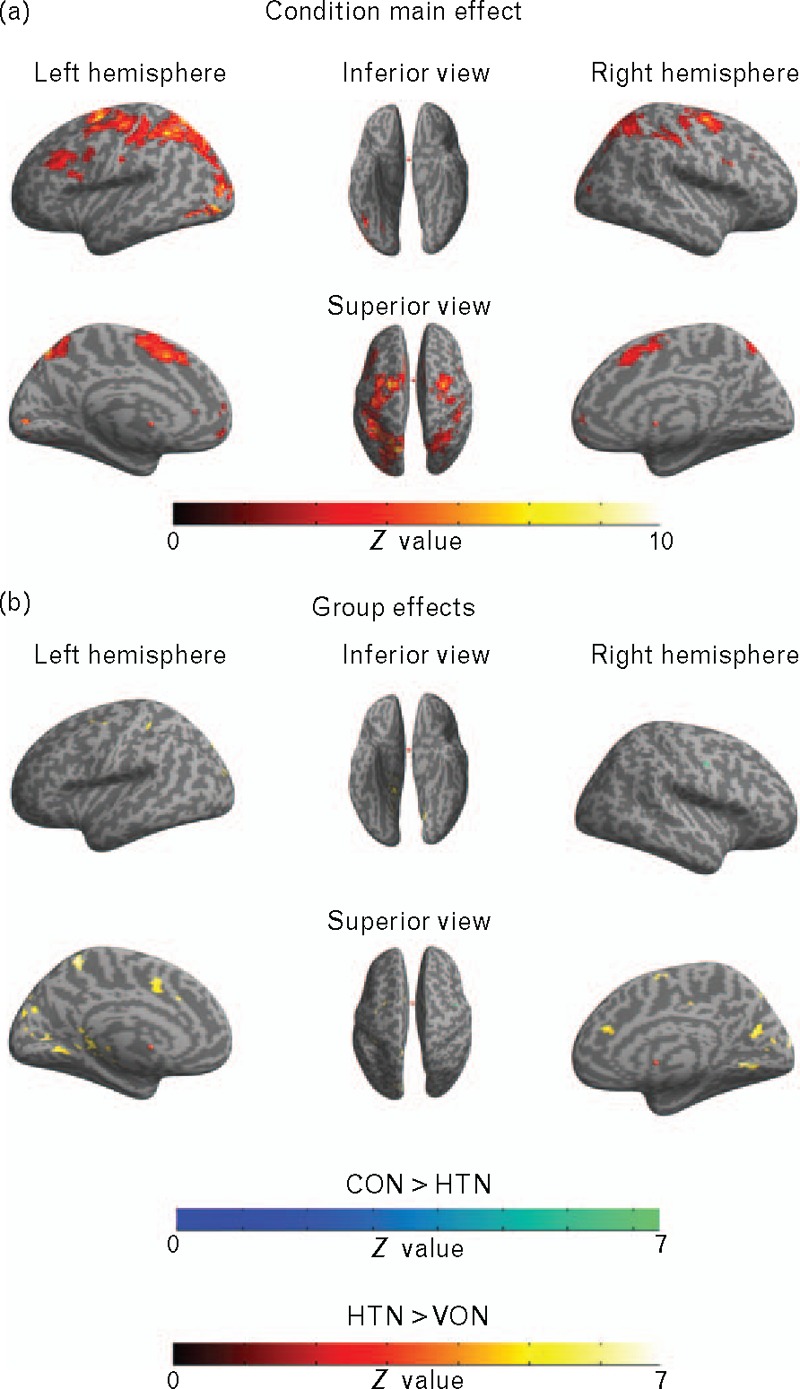
A surface template brain presentation of the significant clusters of the functional MRI full factorial analysis with task conditions (control, reading, congruent, incongruent) and groups (the control group and the hypertensive group) as factors (P < 0.05, family-wise error corrected at voxel level, masked with covariate BMI and waist–hip ratio dependent map – see the Results section and Supplemental Digital Content 1 and Supplemental Digital Content 2 for details). Panel (a) represents the main effect of the condition factor (the brain areas corresponding to the differences in the cognitive load of conditions), panel (b) represents the compound main effect of the group factor divided into simple intergroup comparisons (the brain areas that the control patients involved more during the processing in blue/green – the control group vs the hypertensive group, the brain areas that the hypertensive patients involved more during the processing in red/yellow – the hypertensive group vs the control group).
DISCUSSION
The major finding of the present fMRI study is that uncomplicated hypertension in middle-aged patients is associated with significantly greater areas of cerebral activation during demanding task processing. Significantly, this difference occurs in a reasonably effectively drug-treated population, irrespective of the task difficulty, and also in the absence of a task performance drop or existence of brain disorder in the HTN. In other words, in the study population neither decrease in cognitive outcome, nor overt cerebral damage accompanied a major functional reorganization observed at a neuronal level.
Brain structure
Both age and duration of the hypertension contribute significantly to the changes in the cerebral circulation and structure over time [40,41]. However, we have not found significant differences between HTN and CON groups according to the severity of WMLs. Several explanations to this phenomenon may be offered. Our patients were middle-aged with a relatively short duration of a reasonably successfully treated hypertension, which might account for the lack of differences in traditional measures of brain structural damage. This interpretation is further supported by the fact that in previous studies, the structural changes were mostly reported in populations over the age of 60 years [9,24,25] and if younger populations were examined, an adequate BP control was not achieved [42,43].
Functional study
The modification of the Stroop task used in our study included four conditions of different cognitive load – varying from easy color perception/reading to the most difficult – naming incongruent font color.
Although the percentage of correct responses to each of the conditions did not differ between the controls and patients, in fMRI study the hypertensive patients showed additional activations in many brain areas regardless of the conditions’ cognitive demands. Those areas included lingual gyrus, parahippocampal gyrus, cuneus and precuneus, frontal and cingulate cortex as well as the primary and secondary motor areas. This remains consistent with previous studies as some of these regions already revealed alternations in populations at risk of hypertension (i.e. the posterior cingulate cortex [44], the cingulum [45] and frontal cortex [15]), populations with heightened cardiovascular reactivity (i.e. cingulate cortex [23,46,47], medial prefrontal cortex [22,21]) as well as in the hypertensive patients (i.e. hippocampus [48]).
The hypertension-related hyperactivations in our study covered mostly areas of the medial parietooccipital cortex. The occipital regions are widely known for their involvement in the visual perception and processing [49]. Significantly, there have been shown a decrease in CBF in the occipital lobes in patients with hypertension, especially the elderly ones [50]. Hence, the hyperactivity of the mentioned areas, proved by our results, may seem puzzling. One explanation is the age range of our study population. Former studies showed that the decrease in the CBF progresses linearly with age [51]. Perhaps the change in the tissue metabolism in our middle-aged patients did not start yet, and the hyperactivity of the region is a functional marker of future dysfunction. It is also possible that there is an undergoing pathological process in the region, which impairs both the CBF and region functionality, thus resulting in the hyperactivity pattern we found.
Also, notably large clusters localized in the precuneus differentiated the study groups. The precuneus is a region showing diverse functional and structural connectivity covering widespread linkage to many brain areas including all major associative cortical regions, as well as the subcortical and the limbic structures [52]. But distinctively – no connectivity towards primary sensory regions is reported, suggesting the precuneus’ role not in the core perception, but rather in the integration of higher level cognitive processes. Cavanna and Trimble [53] divided main functions of the precuneus into four categories: the visuospatial imaginary, the episodic memory retrieval, the self-processing and the consciousness. Given the Stroop task's specificity, the processes that most likely triggered the hyperactivation in our study were the visual attention orientation and shifting between stimuli features (categorized within the visuospatial imaginary by the authors). Significantly, the precuneus also shows altered CBF pattern in patients with hypertension accompanying type 2 diabetes melitus [54], as well as in patients with cognitive impairment and dementia [55,56]. Therefore, it is likely that the hyperactivation of the region is an early indicator of an underlying neurodegenerative process, as suggested earlier.
At the same time contrary to earlier reports [46,57–59], we did not observe significant differences regarding the insula, nor the limbic structures (apart from the parahippocampal gyrus). This does not rule out the existence of dysfunction of those regions in hypertensive patients. Previous reports emphasize the importance of the mentioned areas mainly in the stressor-evoked reactivity. In our case, the design of the study should have resulted in a continuous stress and a more sustained activation of the regions. That could lead to heightened overall baseline brain activity that might cloud the direct activation differences.
Neural plasticity
Despite the considerations mentioned, our findings clearly confirm functional alternations in cognitive processing in hypertensive patients. Although there were no direct behavioral differences between the groups, the brain-activation patterns varied. The patients had to incorporate more cerebral regions to perform at the equal level as the controls. A similar effect was already noted in the studies of patients with multiple sclerosis [60,61] and is being interpreted as an early marker of neural plasticity. In response to a task's cognitive demand, the brain balances the tissue damage with an involvement of additional areas in processing. Significantly, compensatory changes in regional CBF were already noted in a PET study of hypertensive patients [62]. However, the authors inferred about compensation indirectly (increased correlation between cerebral regions), whereas our results revealed an explicit neural reorganization.
In our study, the functional changes occurred in the absence of direct macrostructural damage. These findings support the concept that the brain is affected by factors inducing hypertension concurrently or even prior to the BP rise (’brain as essential’ hypothesis [19]). This interpretation might provide a better explanation of the fact that the beneficial effect of hypotensive treatment is not clear [12,63].
Limitations
Vascular brain lesions might include microinfarcts, with a typical size of about 0.2–1.0 mm that are often invisible to a conventional structural MRI protocol [64]. Therefore, we cannot exclude the possibility that our functional findings might be related to the underlying small microvascular brain lesions not yet detectable by the radiologic examination. Furthermore, the Fazekas rating system used for the radiological evaluation in our study does not always show a high correlation with volumetric evaluation of WMLs [37] or the neuropsychological outcome [65]. The scale was developed for clinical usage. It is fast and simple, and it shows high reliability in comparison with other visual rating scores [66]. However, due to the broadness of the categories used in the scale, it may lack the sensitivity in the subnormal group we examined in the study.
FMRI technique is blood-oxygenation dependent and thus vulnerable to any vascular abnormalities. Among others, the hypertension being mentioned is one of the factors possibly confounding the fMRI outcome [67]. Still animal models reveal that exaggerated BP changes the Blood Oxygen Level Dependent response only transiently – during the periods of modified hemodynamics [68,69]. We attempted to overcome this problem using the event design. In our study, possibly stressing conditions altered rapidly with nonstressing ones and should result in overall, more continuous vascular response.
In addition, some of the study participants were on lipid-lowering treatment. Statins are widely administered in patients with increased cardiovascular risk, but their effect on brain function is controversial. Both beneficial effects (lowering cholesterol levels, neurovascular protection, reduction of oxidative stress, promotion of neurons survival and plasticity) as well their neurotoxic potential (negative impact on neuronal survival and plasticity, cognitive deficits, adverse psychiatric effects and impairment of neurotransmission) were found [70]. In our study, there was no significant difference in total, LDL and HDL cholesterol as well as triglycerides levels between groups. Furthermore, percentage of participants on statin treatment did not differ between the two groups. Therefore, we expect that the lipid-lowering drugs use did not significantly affect our results.
Furthermore, our patients were treated with at least one antihypertensive drug, and 80% of them received ACE inhibitor or AT1 blocker. Angiotensin-II (both systemic and centrally generated) was shown to act in the brain what might induce oxidative stress, impact endoplasmic reticulum homeostasis, modulate inflammatory processes and transcription factors, and these actions can promote rise in BP [71]. Therefore, drugs acting on the renin–angiotensin system might alter brain function in many ways and could have impact our results. However, we would expect a mostly protective role of this group of drugs [72]. Other classes of drugs could also influence our results, but their effect is less clear.
Also, the recruitment for the group of hypertensive patients was based on rigorous selection of patients with no other cardiovascular disease, diabetes, psychoneurological history (i.e. drug addiction, depression, traumatic brain injury etc.) or existing brain disorder. A good quality of fMRI images was also essential. However, we did not select patients according to the duration of hypertension. Furthermore, assessment of previous BP control is difficult in patients with long-term hypertension. Thus, both hypertension duration and level of BP control might have influenced our results. However, these factors (a few patients with a short history of hypertension – ‘almost normotensive patients’) should make the differences between HTN and CON less significant. It may also suggest that the functional brain reorganization is significant even at the beginning of the disease, which in fact supports our main hypothesis.
In conclusion, our study revealed that middle-aged patients with hypertension show a significant neural functional reorganization, when faced with a demanding cognitive task. We argue that this result is a marker of a neural plasticity in the group. Our findings unveil a number of further questions. Are the functional changes also detectable in the group of high-normotensive individuals or are they restricted to overt clinical hypertension? One of the previous studies already reported altered cognitive performance in the high-normotensive patients [73] suggesting indirectly the existence of an early functional reorganization in the group. Similarly, are patients with family history of hypertension also at risk [44] ? This ought to be confirmed by future fMRI-based studies. Finally, do altered brain mechanisms of higher cognitive processing independently predispose to progression of hypertension or to acceleration in brain aging? Clearly, more studies are needed as better understanding of hypertension-related brain functional reorganization has important implications for the prevention of both cardiovascular disease and cognitive impairment.
ACKNOWLEDGEMENTS
We thank Wiesława Kucharska, Marcelina Skrzypek-Czerko and Alicja Sutyniec for their excellent research assistance, Joanna Pokusa for administrative support and Tomasz Łączkowski for kind graphical support.
Also, we would like to thank the anonymous reviewers for useful comments and valuable suggestions on earlier versions of the manuscript.
Previous presentations of part of the work:
-
(1)
Naumczyk P, Sabisz A, Witkowska M, Jodzio K, Gąsecki D, Szurowska E, Narkiewicz K (18–22.06.2015). Hypertension and the brain – preliminary results from an fMRI study. OHBM, Honolulu, Hawaii, USA.
-
(2)
Naumczyk P, Graff B, Gąsecki D, Sabisz A, Witkowska M, Jodzio K, et al. (12–15.06.2015). Altered brain mechanisms of higher cognitive processing in essential hypertension 25th European Meeting on Hypertension and Cardiovascular Protection, Milan.
-
(3)
Naumczyk SA, Szurowska P, Witkowska E, Gasecki M, Jodzio D, Narkiewicz K (4–8.03.2015). Hypertension and the brain: preliminary results from an fMRI and DTI study. European Congress of Radiology, Vienna.
The work was supported by the National Science Centre MAESTRO UMO-2011/02/A/NZ5/00329 grant. K.N. is supported by European Regional Development Fund – Project FNUSA-ICRC (no. CZ.1.05/1.1.00/02.0123) and by the European Union – project ICRC-ERA-HumanBridge (no. 316345).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
Hypertension represents a well recognized risk factor for cognitive impairment and dementia but the underlying pathophysiological mechanisms are still not completely understood. The present work sheds new light on this topic by providing the interesting evidence of a possible functional response of the brain to high blood pressure. Indeed, hypertensive subjects manifested with a significant neuronal reorganization during higher cognitive processing in comparison to normotensive subjects in the absence of structural brain damage. This neural plasticity seems to be a response to hypertensive stimulus and likely represents an adaptive response. The strength of the study is the rigorous methodological approach while the main weakness is represented by the impossibility to definitely demonstrate a causal relationship between hypertension and the observed functional brain changes.
Reviewer 2
STRENGTHS. Incorporation of functional magnetic resonance imaging that allows the evaluation of both cerebral functional and structural characteristics of the brain, and inclusion of uncomplicated middle-aged hypertensive patients without previous cardiovascular disease or diabetes.
This is one of the first studies reporting that in response to a task's cognitive demand, the patients had to incorporate more cerebral regions to perform at the equal level as the controls. Thus the authors hypothesize a functional reorganization of the brain early in the course of the disease.
WEAKNESS. Methodological issues about sample size, reproducibility of results, differences between groups about some clinical characteristics that may influence the study.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; BOLD, blood oxygenation level dependent; CBF, cerebral blood flow; CON, the control group; corr., corrected; DWI, diffusion-weighted imaging; FLAIR, fluid attenuation inversion recovery; fMRI, functional MRI; FOV, field of view; FWE, family-wise error; HTN, the hypertensive group; LH, left hemisphere; MNI, Montreal Neurological Institute; RH, right hemisphere; SDC, Supplemental Digital Content; SPSS, Statistical Package for the Social Sciences; TE, echo time; TR, repetition time; WHR, waist–hip ratio; WML, white matter lesion
REFERENCES
- 1.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, et al. Longitudinal study of blood pressure and white matter hyperintensities. The EVA MRI cohort. Neurology 2001; 56:921–926. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions. 6-year results of the Austrian stroke prevention study. Lancet 2003; 361:2046–2048. [DOI] [PubMed] [Google Scholar]
- 3.Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension 2000; 36:1079–1082. [DOI] [PubMed] [Google Scholar]
- 4.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013; 62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia. A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 2008; 7:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension 2014; 63:894–903. [DOI] [PubMed] [Google Scholar]
- 8.Kozera GM, Dubaniewicz M, Zdrojewski T, Madej-Dmochowska A, Mielczarek M, Wojczal J, et al. Cerebral vasomotor reactivity and extent of white matter lesions in middle-aged men with arterial hypertension: a pilot study. Am J Hypertens 2010; 23:1198–1203. [DOI] [PubMed] [Google Scholar]
- 9.Basile AM, Pantoni L, Pracucci G, Asplund K, Chabriat H, Erkinjuntti T, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. Cerebrovas Dis 2006; 21:315–322. [DOI] [PubMed] [Google Scholar]
- 10.Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens 2014; 8:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchistiakova E, Anderson ND, Greenwood CE, MacIntosh BJ. Combined effects of type 2 diabetes and hypertension associated with corthical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. Neuroimage Clin 2014; 5:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, et al. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens 2012; 26:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension 2012; 60:260–268. [DOI] [PubMed] [Google Scholar]
- 14.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: voulnerability of the prefrontal regions and executive functions. Behav Neurosci 2003; 117:1169–1180. [DOI] [PubMed] [Google Scholar]
- 15.Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer CC, Townsend DW, et al. Cerebral blood flow in hypertensive patients: an initial report of reduced and compensatory blood flow responses during performance at two cognitive tasks. Hypertension 1998; 31:1216–1222. [DOI] [PubMed] [Google Scholar]
- 16.Cherubin N, Mortby ME, Janke AL, Sachdev PS, Abhayaratna WP, Anstey KJ. Blood pressure, brain structure and cognition: opposite associations in men and women. Am J Hypertens 2015; 28:225–231. [DOI] [PubMed] [Google Scholar]
- 17.Chuang YF, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, et al. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging 2014; 35:1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosten H, van Eersel MEA, Gansevoort RT, Bilo HJG, Slaets JPJ, Izaks GJ. Cardiovascular risk profile and cognitive function in young, middle-aged and elderly subjects. Stroke 2013; 44:1543–1549. [DOI] [PubMed] [Google Scholar]
- 19.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? NeuroImage 2009; 47:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings JR, Heim AF. From brain to behaviour: hypertension's modulation of cognition and affect. Int J Hypertens 2012; 2012:701385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianaros PJ, Jennings JR, Shen LK, Derbyshire WG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension 2007; 49:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianaros PJ, Shen LK, Remo AM, Christie IC, Critchley HD, Weng J. Heightened resting neural activity predicts exaggerated srtessor-evoked blood pressure reactivity. Hypertension 2009; 53:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan JP, Sheu LK, Gianaros PJ. Resting state functional connectivity within the cingulate cortex jointly predicts agreeableness and stressor-evoked cardiovascular reactivity. NeuroImage 2011; 55:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JCM, Hofman A, van Gijn J, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002; 125:765–772. [DOI] [PubMed] [Google Scholar]
- 25.Murray AD, Staff RT, McNeil CJ, Salarirad S, Starr JM, Deary IJ, et al. Brain lesions, hypertension and cognitive ageing in the 1921 and 1936 Aberdeen birth cohorts. Age 2012; 34:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beason-Held W, Thambisetty M, Deib G, Sojkova J, Lendman BA, Zonderman AB, et al. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke 2012; 43:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings JR, Heim AF, Kuan DCH, Gianaros PJ, Muldoon MF, Manuck SB. Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke 2013; 44:2480–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias MM, Robbins MA, Schultz NR, Streeten DH, Elias PK. Clinical significance of cognitive performance by hypertensive patients. Hypertension 1987; 9:192–197. [DOI] [PubMed] [Google Scholar]
- 29.Oldfield R. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 30.Gorelick PB, Nyenhuis D. Blood pressure and treatment of persons with hypertension as it relates to cognitive outcomes including executive function. J Am Soc Hypertens 2012; 6:309–315. [DOI] [PubMed] [Google Scholar]
- 31.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006; 37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 32.Vicario A, Martinez CD, Baretto D, Casale AD, Nicolosi L. Hypertension and cognitive decline: impact on executive function. J Clin Hypertens 2005; 7:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using genetic algorithm. NeuroImage 2003; 18:293–309. [DOI] [PubMed] [Google Scholar]
- 34.Fazekas F, Chawluk JB, Alavi A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987; 149:351–356. [DOI] [PubMed] [Google Scholar]
- 35.Fazekas F, Kleinert R, Olfenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43:1683–1689. [DOI] [PubMed] [Google Scholar]
- 36.Inzitari M, Simoni G, Pracucci A, Poggesi A, Basile AM, Chabriat H, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med 2007; 167:81–88. [DOI] [PubMed] [Google Scholar]
- 37.Van Straaten ECW, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke 2006; 37:836–840. [DOI] [PubMed] [Google Scholar]
- 38.Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, d’Hellencourt CL. Secret talk between adipose tissue and central nervous system via secreted factors – an emerging frontier in the neurodegenerative research. J Neuroinflammation 2016; 13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Étard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15:273–289. [DOI] [PubMed] [Google Scholar]
- 40.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensice brain. Stoke 2007; 38:1766–1773. [DOI] [PubMed] [Google Scholar]
- 41.Heijer TD, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, et al. Association between blood pressure levels over time and brain athropy in the elderly. Neurobiol Aging 2003; 24:307–313. [DOI] [PubMed] [Google Scholar]
- 42.Sierra C, de la Sierra A, Salamero M, Sobrino J, Gomez-Angelats E, Coca A. Silent cerebral white matter lesions and cognitive function in middle-aged essential hypertensive patients. Am J Hypertens 2004; 17:529–534. [DOI] [PubMed] [Google Scholar]
- 43.Sierra C, de la Sierra A, Pare JC, Gomez-angeltas E, Coca A. Correlation between silent cerebral white matter lesions and left ventricular mass and geometry in essential hypertension. Am J Hypertens 2002; 15:507–512. [DOI] [PubMed] [Google Scholar]
- 44.Haley AP, Gurstad J, Cohen RA, Jerskey BA, Mulligan RC, Sweet LH. Neural correlates of visuospatial working memory in health young adults at risk of hypertension. Brain Imaging Behav 2008; 2:192–199. [Google Scholar]
- 45.Braskie MN, Small GW, Bookheimer SY. Vascular risks and fMRI activation during a memory task in older adults. Neurobiol Aging 2010; 31:1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginty AT, Gianaros PJ, Derbyshire AWG, Phillips AC, Carroll D. Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology 2013; 50:219–239. [DOI] [PubMed] [Google Scholar]
- 47.Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosom Med 2005; 67:31–39. [DOI] [PubMed] [Google Scholar]
- 48.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Wiliamson A, et al. Regional brain changes in ageing healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- 49.Zeki S, Watson JD, Lueck CJ, Friston KJ, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci 1991; 11:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens 2014; 8:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 2007; 38:1766–1773. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Li CR. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage 2012; 59:3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129:564–583. [DOI] [PubMed] [Google Scholar]
- 54.Xia W, Rao H, Spaeth AM, Huang R, Tian S, Cai R, et al. Blood pressure is associated with cerebral blood flow alternations in patients with T2DM as revealed by perfusion functional MRI. Medicine 2015; 94:e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and Alzheimer disease patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bailly M, Destrieux C, Hommet C, Mondon K, Cottier JP, Beaufils E, et al. Precuneus and cingulate cortex atrophy and hypometabolism in patients with Alzheimer's disease and mild cognitive impairment: MRI and (18)F-FDG PET quantitative analysis using Freesurfer. Biomed Res Int 2015; 2015:583931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagai M, Hoskide S, Kario K. The insula cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens 2010; 4:174–182. [DOI] [PubMed] [Google Scholar]
- 58.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume and functional connectivity of the amygdala. J Neurosci 2008; 28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease. NeuroImage 2009; 47:922–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forn C, Baros-Loscerdeles A, Wscurendo J, Belloch V, Campos S, Percet MA, et al. Cortical reorganization during PASAT task in MS patients with preserved working memory functions. NeuroImage 2006; 31:686–691. [DOI] [PubMed] [Google Scholar]
- 61.Audoin B, Ibarrola D, Ranjeva JP, Confort-Gouny S, Malikova I, Ali-Cherif AM, et al. Compensatory cortical activation observed by fMRI during cognitive test at the earliest stage of MS. Hum Brain Mapp 2003; 20:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensice patients is altered by blood pressure treatment. Hypertension 2008; 52:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens 2009; 23:86–96. [DOI] [PubMed] [Google Scholar]
- 64.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts, the invisible lesion. Lancet Neurol 2012; 11:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrett KD, Cohen RA, Paul RH, Moser DJ, Malloy PF, Shah P, et al. Computer mediated measurement and subjective ratings of white matter hyperintensities in vascular dementia: relationships to neuropsychological performance. Clin Neuropsychol 2004; 18:50–62. [DOI] [PubMed] [Google Scholar]
- 66.Kapeller P, Barber R, Vermeulen RJ, Ader H, Scheltens P, Freidl W, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, inter-rater agreement and correlations with quantitative measurements. Stroke 2003; 34:441–445. [DOI] [PubMed] [Google Scholar]
- 67.D’Eposito M, Deonell LY, Cazzaley A. Alternations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci 2003; 4:863–872. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Foniok T, Wansteeler JI, Qiao M, Tomanek B, Vivanco RA, et al. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. NeuroImage 2006; 31:1–11. [DOI] [PubMed] [Google Scholar]
- 69.Tuor UI, Weng R, Zhao Z, Foniok T, Rushforth D, Warnsteeler JI, et al. Transient hypertension concurrent with forepaw stimulation enhances functional MRI responsiveness in infarct and periinfarct regions. J Cereb Blood Flow Metab 2007; 27:1819–1829. [DOI] [PubMed] [Google Scholar]
- 70.Mendoza-Oliva A, Zepeda A, Arias C. The complex actions of statins in brain and their relevance for Alzheimer's disease treatment: an analytical review. Curr Alzheimer Res 2014; 11:817–833. [PubMed] [Google Scholar]
- 71.Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension 2015; 66:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension 2014; 63:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension 2008; 51:663–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


