Abstract
Background:
Management of high blood pressure (BP) in people over 80 years is controversial, but there is limited information available concerning the uptake of hypertension treatment at this age.
Objective:
To evaluate use of antihypertensive drugs and changes in SBP and DBP from 2001 to 2014 in men and women aged 80 years and over.
Methods:
Cohort study using primary care electronic health records of 265 225 participants from the UK Clinical Practice Research Datalink. Records of BP and antihypertensive medications were analysed. Linear trends were estimated by frailty category in multiple regression models.
Results:
Data were analysed for 116 401 men and 148 824 women. The proportion with BP recorded increased from 51% in 2001 to 78% in 2014. The proportion of patients prescribed antihypertensive medications increased from 64 to 76%. Mean SBP declined from 150 (SD 20) mmHg in 2001 to 135 (16) mmHg in 2014. In ‘fit’ participants, the decline in SBP was 12.4 (95% confidence interval 11.9–13.0) mmHg/decade in those treated for hypertension and 8.5 (7.8–9.1) mmHg in those not treated. The decline in SBP was smaller as frailty increased. The proportion of all participants with BP less than 140/90 mmHg increased from 14 to 44% in the study period.
Conclusion:
In octogenarians, BP treatment has intensified between 2001 and 2014. BP values have declined in both treated and untreated participants, with a substantial increase in the proportion achieving conventional BP targets.
Keywords: 80 and over, aged, antihypertensive drugs, blood pressure, frailty, hypertension, primary care
INTRODUCTION
High blood pressure (BP) represents a major contributor to the global burden of disease, being a leading risk factor for cardiovascular diseases (CVDs) [1,2]. BP tends to increase with age [3], and the highest incidence rates for cardiovascular events and greatest cardiovascular mortality are observed at older ages [4]. Individuals aged 80 years and over represent the most rapidly increasing section of the population [5]. In very old people, hypertension remains a key risk factor for CVD, but other health concerns including the accumulation of deficits leading to frailty [6], functional and cognitive decline, [7] dementia [8] and falls and fractures assume increasing importance at this age [9].
BP management in very old people is controversial [9]. The results of the Hypertension in the Very Elderly Trial (HYVET) suggested that good control of BP with antihypertensive therapy (AHT) in people over 80 years of age was associated with fewer strokes, less heart failure and lower all-cause and cardiovascular mortality [10]. There was no evidence for effect modification according to level of frailty [11]. A recent trial of hypertension management in community dwelling people aged 75 years and older, the SBP Intervention Trial (SPRINT) showed that individuals who were treated to attain a SBP goal less than 120 mmHg had 33% lower risk of CVD and about 32% lower risk of mortality [12]. Nevertheless, critics argue that the suggested benefits of antihypertensive treatment were observed in unusually healthy participants and that these results may not be applicable to wider populations, which may experience multiple comorbidities and functional deficits, in whom quality of life may be impaired and life expectancy may be limited [13]. Some evidence suggests that there is a J-shaped curve, with mortality risk being higher in individuals with the lowest levels of BP [14,15]. We have recently reported evidence that suggested that among octogenarians, either very low and very high BP may be associated with increased mortality risk [16]. Use of antihypertensive medications may be associated with adverse outcomes, such as an increased risk of falls and fractures [17]. These are sometimes referred to as collateral risks of AHT [9]. Consequently, there exists uncertainty concerning the best approach to managing BP in very old people [18].
Recently, there have been improving trends in hypertension awareness, treatment and control in younger populations [19], but it is not known to what extent these trends are shared by individuals aged 80 years and older. Most population surveys sample only very small numbers of very old people, and there may be additional barriers to participation in older age possibly leading to nonresponse. Participation in clinical trials may be limited by stringent inclusion criteria and difficulties with recruitment and retention from high levels of frailty and comorbidity [20].
This research is part of a wider project to evaluate cardiovascular risk management in very old people. The research draws on a database of primary care electronic health records (EHRs) as a sampling frame that can yield a large sample of individuals aged 80 years or older. We included a sample of men and women aged 80 years and over, registered with general practices in the United Kingdom between 2001 and 2014. We aimed to evaluate changes in BP recording, in mean SBP and DBP values, the prevalence of hypertension and changes in the use of antihypertensive medications over this period.
METHODS
Data source
This study employed the Clinical Practice Research Datalink (CPRD) as a data source. The CPRD is one of the world's largest databases of primary care EHRs including approximately 7% of UK general practices, with data collected from 1990 to present. The registered active population of about 7 million is generally representative of the UK population in terms of age and sex [21]. Data collected into CPRD comprise clinical diagnoses, records of BP and other clinical measurements, prescriptions, results of investigations and referrals to specialist services. This study received scientific and ethical approval from the Independent Scientific Advisory Committee for CPRD studies (ISAC Protocol 13_151). The CPRD has broad National Research Ethics Service Committee ethics approval for observational research studies.
Study participants
A stratified random sampling approach was used to select study participants. Individuals who had their 80th, 85th, 90th, 95th and 100th birthdays, whereas registered with CPRD were identified and a random sample of up to 50 000 men and 50 000 women was drawn from each age stratum. An open cohort of 265 225 participants who contributed person time between 2001 and 2014 was included in the present analysis; 231 721 (87%) patients had at least one valid BP measurement during the study period. Participants’ person time was eligible to be included in the analysis, whereas they were aged 80 and over and had a valid SBP and DBP measurement in the year.
Study measures
For each study year between 2001 and 2014, we evaluated the mean value for SBP and DBP readings recorded for each participant. Hypertension was defined as a SBP/DBP greater or equal to 140 (SBP) or 90 (SBP) mmHg or current treatment with antihypertensive medication. Antihypertensive drug prescriptions were identified from prescription records, and these were classified into four classes: A, drugs acting on the renin–angiotensin system, including angiotensin-converting enzyme inhibitors and AT1 blocking drugs; B, beta-blockers; C, calcium channel blockers; and D, thiazide diuretics [22]. A further category of ‘Other’ antihypertensive drugs was defined, including centrally acting drugs, alpha-blockers and vasodilators. Controlled hypertension was defined as both SBP/DBP less than 140/90 mmHg.
Age, smoking, BMI and total serum cholesterol values were selected as covariates. Smoking status was determined from information on smoking status in patients’ clinical records [23]. Patients’ smoking status was classified into the categories ‘nonsmoker’, ‘current smoker’ or ‘ex-smoker’. BMI records were classified into the categories underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (≥30 kg/m2). Total cholesterol (TC) values were grouped into the categories less than 3.0, 3.0–3.9, 4.0–4.9, 5.0–5.9 and at least 6.0 mmol/l. The prevalence of comorbidity at the age of 80 years was determined from analysis of Read medical codes, and where appropriate drug product codes, for diabetes mellitus, coronary heart disease, stroke, cancer, chronic obstructive pulmonary disease, musculoskeletal and connective tissue diseases and nervous system diseases. Frailty status was assessed using a previously published 36-item electronic frailty index (eFI) [24]. The eFI was devised from the cumulative deficit frailty model with the eFI score calculated by the presence or absence of individual deficits as a proportion of the total possible. Categories of fit, mild, moderate and severe frailty were defined following Clegg et al.[24], but quantitative traits (including BP values) were omitted.
Analysis
Descriptive statistics were used to describe baseline characteristics. Mean SBP and DBP in each year were determined from 2001 to 2014, and trends were plotted by sex and treatment status. The class of antihypertensive drugs and number of different classes of drugs prescribed were plotted by study year from 2001 to 2014. Linear regression models were fitted to estimate trends in BP values from baseline to the latest measure before study end date or last data collection date or death date. The results were presented as mean change per decade with 95% confidence intervals (95% CIs). The analysis was further explored to study, the treatment effect on BP trends by frailty status. Separate regression models was fitted or frailty status classified as fit (frailty index ≤0.12), mild (frailty index 0.12–0.24), moderate (0.24–0.36) and severe (>0.36). All analyses were adjusted for age, comorbidity, BMI categories, cholesterol categories, classes and number of antihypertensive drugs. Sensitivity analyses were carried out to evaluate whether general practitioners extended their BP recording to patients with lower BPs. In addition, we divided general practices into quartiles of BP recording in 2001 and the SBP and DBP trends were estimated in each quartile of BP recording. Stata version 13.0 was used to conduct all statistical analysis (Stata Corp.; College Station, Texas, USA).
RESULTS
Table 1 presents the characteristics of the study participants. The initial study sample included 265 225 participants (116 401 men and 148 824 women) aged 80 years and over. Mean age at entry to the study was 82.8 years for men and 84.5 years for women, whereas the mean ages at exit from the study were 88.0 and 90.1 years, respectively. There were 16% of men with five or more comorbidities compared with 11% of women. A greater proportion of men were current smokers compared with women. Women were more likely to have higher recorded TC values, with more women having TC levels at least 6 mmol/l. A larger proportion of men than women were overweight (31 vs. 21%). Women generally showed greater frailty than men (4.4% of women vs. 3% of men were severe by eFI).
TABLE 1.
Characteristics of study participants
| Men (116 401) | Women (148 824) | |
| Age at entrya (mean, SD) | 82.8 ± 4.12 | 84.5 ± 5.28 |
| Age at exita (mean, SD) | 88.0 ± 4.9 | 90.1 ± 5.6 |
| Year at entry (mean, SD) | 2005 ± 4 | 2004 ± 4 |
| Year at exit (mean, SD) | 2010 ± 4 | 2010 ± 4 |
| BMI categories (kg/m2) | ||
| <18.5 | 1113 (1.0) | 3401 (2.3) |
| 18.5–25 | 33 546 (28.8) | 37 810 (25.4) |
| 25–30 | 36 201 (31.1) | 31 401 (21.1) |
| ≥30 | 11 038 (9.5) | 15 442 (10.4) |
| Not known | 34 503 (29.6) | 60 770 (40.8) |
| Cholesterol categories (mmol/l) | ||
| <3 | 1272 (1.1) | 376 (0.3) |
| 3–4 | 10 711 (9.2) | 4503 (3.0) |
| 4–5 | 22 091 (19.0) | 16 631 (11.2) |
| 5–6 | 14 622 (12.6) | 21 635 (14.5) |
| ≥6 | 5576 (4.8) | 19 532 (13.1) |
| Not known | 62 129 (53.4) | 86 147 (57.9) |
| Smoking status | ||
| None | 49 438 (42.5) | 94 994 (63.8) |
| Yes | 13 476 (11.6) | 9388 (6.3) |
| Ex | 46 149 (39.7) | 32 360 (21.7) |
| Not known | 7338 (6.3) | 12 082 (8.1) |
| Frailty index categories | ||
| Fit | 55 048 (47.3) | 64 873 (43.6) |
| Mild | 42 593 (36.6) | 54 262 (36.5) |
| Moderate | 15 266 (13.1) | 23 144 (15.6) |
| Severe | 3494 (3.0) | 6545 (4.4) |
Figures are frequencies (column percentage) except where indicated.
a‘Age at entry’ refers to age at start date of the study and ‘age at exit’ refers to age at end date of the study period.
Table 2 presents changes in BP recording and BP management over the study period. The proportion of participants with BP readings in each year increased from 51% in 2001 to 78% in 2014. The proportion of all participants with controlled BP (<140/90 mmHg) increased from 14% in 2001 to 44% in 2014. The mean SBP declined from 149.8 (SD 19.6) mmHg in 2001 to 135.4 (15.9) mmHg in 2014, whereas mean DBP declined from 79.8 (9.4) mmHg in 2001 to 72.7 (8.9) mmHg. The proportion of participants with BP recorded and who were prescribed antihypertensive drugs increased from 64% in 2001 to 76% to 2014. The prevalence of hypertension decreased slightly from 89% in 2001 to 85% in 2014. Individuals with a SBP less than 120 mmHg and on treatment increased from 2.9 to 10.6% over the study period.
TABLE 2.
Changes in mean blood pressure values and blood pressure management over time
| Year | No. of participants | Age (mean ± SD) | BP recorded (Freq.%) | Mean SBP (mmHg) | Mean DBP (mmHg) | Treated, N (%) | Hypertensiona (Freq.%) | BP controlledb (Freq.%) | BP controlledc (Freq.%) |
| 2001 | 98 214 | 86.1 ± 4.8 | 50 058 (51.2) | 149.8 ± 19.6 | 79.8 ± 9.4 | 32 034 (64.0) | 44 644 (89.2) | 7096 (14.2) | 1445 (2.9) |
| 2002 | 111 017 | 86.1 ± 4.8 | 62 497 (56.5) | 148.9 ± 19.5 | 79.2 ± 9.5 | 41 522 (66.4) | 55 587 (88.9) | 10 219 (16.4) | 2104 (3.4) |
| 2003 | 119 338 | 86.1 ± 4.8 | 76 120 (63.9) | 147.3 ± 19.3 | 78.4 ± 9.4 | 51 698 (67.9) | 67 265 (88.4) | 14 418 (18.9) | 3114 (4.1) |
| 2004 | 126 676 | 86.1 ± 4.8 | 94 085 (74.4) | 145.1 ± 18.4 | 77.5 ± 9.2 | 64 840 (68.9) | 82 411 (87.6) | 20 702 (22.0) | 4474 (4.8) |
| 2005 | 128 448 | 86.3 ± 4.8 | 97 131 (75.7) | 143.2 ± 17.7 | 76.6 ± 9.1 | 69 301 (71.4) | 84 970 (87.5) | 24 607 (25.3) | 5310 (5.5) |
| 2006 | 128 438 | 86.4 ± 4.8 | 98 927 (77.1) | 141.7 ± 17.3 | 75.8 ± 9.1 | 72 206 (73.0) | 86 334 (87.3) | 28 561 (28.9) | 6204 (6.3) |
| 2007 | 127 931 | 86.6 ± 4.8 | 100 901 (78.9) | 140.7 ± 17.0 | 75.3 ± 8.9 | 74 656 (74.0) | 87 863 (87.1) | 31 618 (31.3) | 6922 (6.9) |
| 2008 | 126 891 | 86.7 ± 4.8 | 100 557 (79.3) | 139.7 ± 16.7 | 74.8 ± 9.0 | 75 432 (75.0) | 87 430 (87.0) | 34 093 (33.9) | 7677 (7.6) |
| 2009 | 124 795 | 86.8 ± 4.8 | 98 907 (79.3) | 138.9 ± 16.6 | 74.3 ± 9.0 | 75 009 (75.8) | 86 058 (87.0) | 35 568 (36.0) | 8051 (8.1) |
| 2010 | 117 366 | 87.1 ± 4.8 | 93 464 (79.7) | 138.2 ± 16.5 | 74.0 ± 9.0 | 71 226 (76.2) | 81 134 (86.8) | 35 003 (37.5) | 8138 (8.7) |
| 2011 | 108 893 | 87.4 ± 4.8 | 87 176 (80.1) | 137.5 ± 16.4 | 73.6 ± 8.9 | 66 414 (76.2) | 75 236 (86.3) | 33 702 (38.7) | 8091 (9.3) |
| 2012 | 99 785 | 87.6 ± 4.9 | 79 415 (79.6) | 136.9 ± 16.3 | 73.4 ± 8.9 | 60 514 (76.2) | 68 393 (86.1) | 32 042 (40.4) | 7925 (10.0) |
| 2013 | 86 367 | 87.8 ± 5.0 | 68 896 (79.8) | 136.1 ± 15.9 | 73.0 ± 8.8 | 52 302 (75.9) | 58 771 (85.3) | 28 999 (42.1) | 7094 (10.3) |
| 2014 | 71 003 | 87.8 ± 5.2 | 54 973 (77.5) | 135.4 ± 15.9 | 72.7 ± 8.9 | 41 831 (76.1) | 46 792 (85.1) | 24 110 (43.9) | 5848 (10.6) |
aHypertension (BP > 140/90 mmHg or treated for hypertension).
bBP controlled (BP < 140/90 mmHg and treated for hypertension as percentage of all participants with BP recorded in year).
cBP controlled (SBP < 120 mmHg and treated for hypertension as percentage of all participants with BP recorded in year).
Figure 1 presents changes in BP treatment during the study period from 2001 to 2014. Fig. 1a shows trends over time in the number of different classes of antihypertensive drugs prescribed. There was evidence of intensification of AHT over time, with a decline in the proportion receiving no AHT and an increase in the proportion prescribed multiple classes of antihypertensive (AHT) drugs. The proportion of patients receiving no treatment declined from 36 to 24%. The proportion receiving monotherapy (a single class of antihypertensive drug) decreased from 33 to 31%. The proportion receiving drugs from two different AHT classes increased from 22 to 30%; from three different AHT classes from 8 to 12%; and four or five different AHT classes from 2 to 3%. Additional analysis revealed similar trends in men and women and in 5-year age groups over the age of 80 years. Figure 1b presents changes in the use of different classes of antihypertensive drugs from 2001 to 2014. The proportion of participants prescribed drugs acting on the renin–angiotensin system (A) increased from 24% in 2001 to 48% in 2014, whereas the proportion of patients prescribed thiazide diuretics (D) declined from 30% to 19%. There were modest increases in the proportions prescribed beta-blockers (B) and calcium-channel blockers (C).
FIGURE 1.
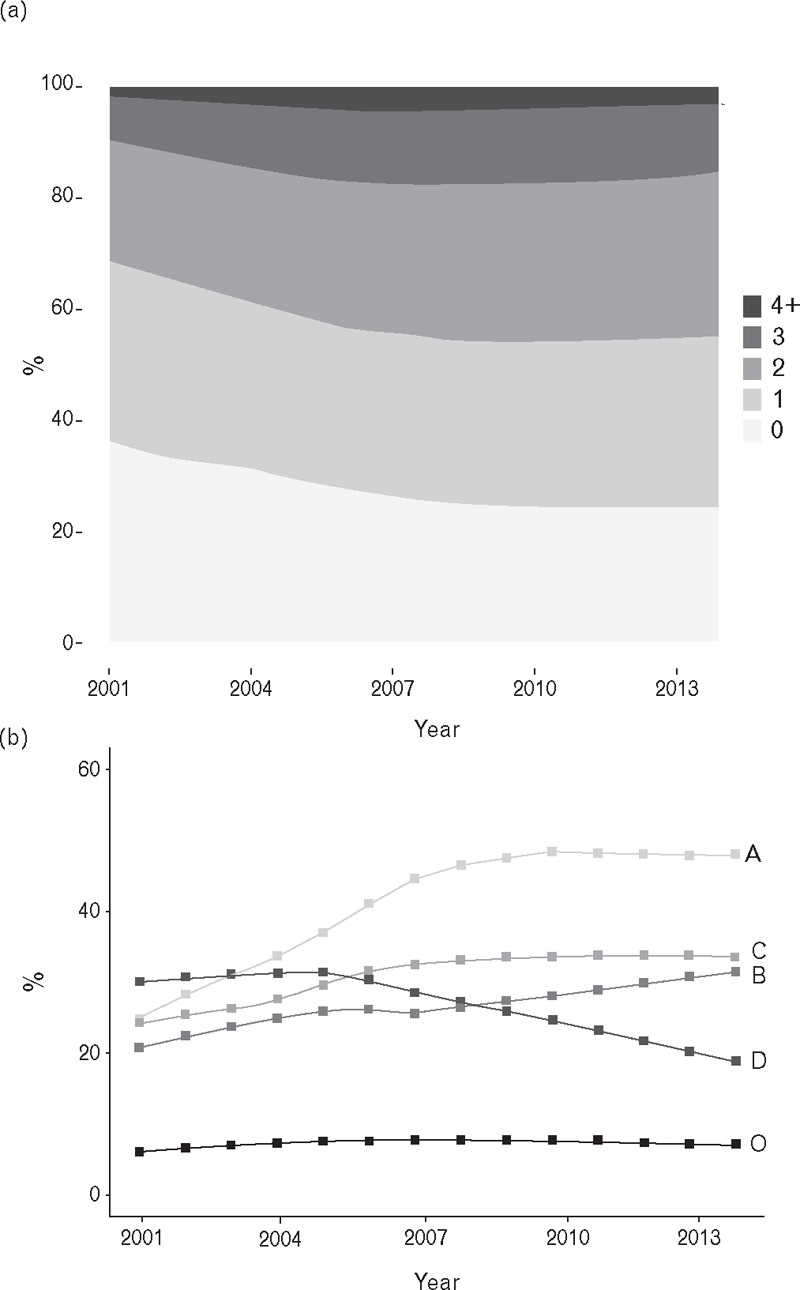
Use of antihypertensive medications by year of study. (a) Numbers of different classes of antihypertensive drugs prescribed. (b) Trends in utilization of different classes of antihypertensive drugs including A, drugs acting on the renin–angiotensin system; B, beta-blockers; C, calcium channel blockers; D, thiazide diuretics and O, other.
Figure 2 presents trends in mean SBP and DBP for men and women by antihypertensive treatment status. SBP and DBP decreased over time in both men and women and in those receiving AHT and those not. Between 2001 and 2014, mean SBP decreased from 148 to 134 mmHg in treated men and from 143 to 132 mmHg in untreated men. Similar reductions were observed in women treated with antihypertensive drugs and those not treated (Fig. 2). Women generally showed higher BP values than men, in both the treated and untreated groups. The gradient of decrease appeared to be slightly greater in treated participants, especially among women (Fig. 2). In subgroup analyses, this association was similar in each 5-year age groups from 80 to 99 years.
FIGURE 2.
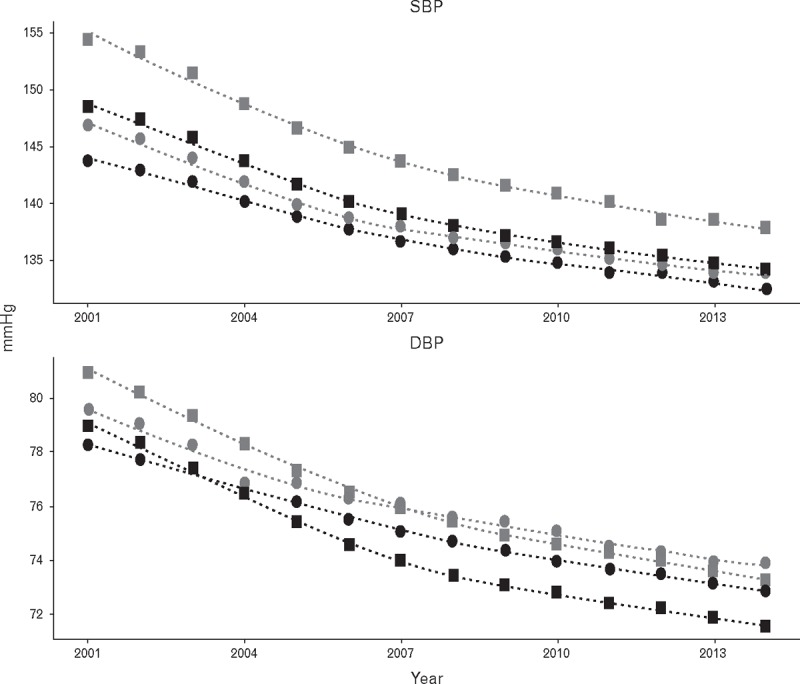
Trends in mean SBP and DBP in people aged 80 years or over from 2001 to 2014. Squares, women; circles, men; light symbols, treated with antihypertensive drugs; and dark symbols, not treated with antihypertensive drugs.
Table 3 shows the results of linear regression analyses exploring the association of SBP and DBP with year of study. In patients who were classified as ‘fit’ (free of frailty), SBP declined by approximately 12 mmHg/decade (12.4 mmHg 95% CI 13.0–11.9) in those who treated and by 8.5 (9.1–7.8) mmHg/decade in those who were not treated for hypertension. Adjustment for covariates (smoking, BMI, cholesterol and multimorbidity) had negligible effect on these estimates. Trends were generally similar in men and women, but estimates were slightly higher in women. Declines in BP were smaller in magnitude as level of frailty increased; in participants with ‘severe’ frailty the decline in SBP was about half that observed in ‘fit’ participants, and there was no difference in estimate between treated and untreated participants at this level of frailty. Similar patterns of association were observed for DBP but changes in DBP were of smaller magnitude.
TABLE 3.
Trend in mean SBP and DBP between 2001 and 2014
| SBP | DBP | |||
| Frailty status | No antihypertensive medicationa | Treated with antihypertensive medicationa,b | No antihypertensive medicationa | Treated with antihypertensive medicationa,b |
| All | ||||
| Fit | −8.5 (−9.1 to −7.8) | −12.4 (−13.0 to −11.9) | −3.3 (−3.7 to −2.9) | −5.1 (−5.4 to −4.8) |
| Mild | −8.8 (−9.3 to −8.2) | −10.4 (−10.7 to −10.1) | −3.7 (−4.0 to −3.4) | −4.2 (−4.4 to −4.0) |
| Moderate | −7.7 (−8.4 to −7.0) | −8.7 (−9.1 to −8.3 | −3.2 (−3.6 to −2.9) | −3.4 (−3.6 to −3.2) |
| Severe | −7.1 (−8.2 to −6.1) | −6.6 (−7.2 to −6.0) | −2.9 (−3.5 to −2.4) | −2.8 (−3.2 to −2.5) |
| Men | ||||
| Fit | −8.0 (−8.9 to −7.1) | −10.6 (−11.3 to −9.9) | −3.2 (−3.7 to −2.7) | −4.4 (−4.8 to −4.0) |
| Mild | −8.1 (−8.9 to −7.3) | −8.6 (−9.0 to −8.1) | −3.6 (−4.0 to −3.2) | −3.7 (−3.9 to −3.4) |
| Moderate | −7.3 (−8.3 to −6.3) | −7.2 (−7.8 to −6.6) | −3.1 (−3.6 to −2.5) | −2.9 (−3.2 to −2.6) |
| Severe | −5.8 (−7.6 to −4.0) | −4.1 (−5.1 to −3.0) | −2.4 (−3.3 to −1.5) | −2.1 (−2.6 to −1.6) |
| Women | ||||
| Fit | −8.8 (−9.8 to −7.7) | −14.1 (−14.8 to −13.4) | −3.2 (−3.7 to −2.7) | −5.7 (−6.1 to −5.3) |
| Mild | −9.2 (−9.9 to −8.4) | −11.9 (−12.4 to −11.5) | −3.6 (−4.0 to −3.2) | −4.7 (−4.9 to −4.4) |
| Moderate | −8.0 (−8.9 to −7.0) | −9.8 (−10.3 to −9.2) | −3.3 (−3.7 to −2.8) | −3.7 (−4.0 to −3.5) |
| Severe | −7.8 (−9.2 to −6.5) | −7.9 (−8.7 to −7.1) | −3.3 (−3.9 to −2.6) | −3.2 (−3.6 to −2.8) |
Figures represent the mean change in blood pressure per decade (mmHg). Figures are estimated change in SBP or DBP per decade.
aAdjusted for age, BMI categories, total cholesterol category, smoking and multimorbidity.
bAdditional adjustment for number of antihypertensive drug classes and class of drug prescribed.
Sensitivity analysis showed that in the lowest quartile of practices, 41% of fewer patients had BP recorded during 2001, whereas in the highest quartile 64% or more patients had BP recorded during 2001. Estimated trends in SBP and DBP were similar for general practices in each quartile of BP recording.
DISCUSSION
Main findings
Patients aged 80 years and over experienced significant intensification of BP management between 2001 and 2014. The proportion of all patients aged 80 years and over treated with antihypertensive drugs has increased from two-thirds to three-quarters, whereas the proportion treated with two or more classes of antihypertensive drugs has increased from approximately one-third to nearly one half. Consequently, about 90% of all individuals aged 80 years and over may be classed as hypertensive according to the criterion of BP at least 140/90 mmHg or treated with antihypertensive drugs. During the same period, there has been a substantial decline in SBP and DBP of between 10 and 12 mmHg/decade for SBP and 3 to 5 mmHg/decade for DBP. This trend was apparent both in participants treated with antihypertensive drugs and in those untreated. This has been accompanied be a rapid increase in the proportion of all participants whose BP meets the criterion of less than 140/90 mmHg for acceptable BP control.
Comparison with other studies
There is now increasing evidence of benefit from BP lowering over the age of 75 or 80 years. The findings of the HYVET trial [25] have now been supported by recent publication of the results of the SPRINT [12]. A European Society of Hypertension–European Union Geriatric Medicine Society Working Group [26] also endorsed the need for more active BP management in very old people. Some observational studies suggest that mortality may be higher at low BP values, but a negative association of BP with frailty category could account for this association [27–30].
Trends in BP among individuals aged 80 years and older are comparable with data reported for adults of all ages from the Health Survey for England [19]. Mean SBP among men in England is reported to have decreased from 134 mmHg in 1994 to 129 mmHg in 2011, and for women from 131 to 122 mmHg [19]. In the US National Health and Nutrition Examination Survey (NHANES), participants aged 80 years and over showed a decrease in mean SBP from 147 mmHg in 1988–1994 to 140 mmHg in 2005–2010. In the Tromso study, SBP in middle age decreased by 10.6 mmHg in women and 4.5 mmHg in men between 1979 and 2008. They suggested that this was mainly due to improved life style factors such as a reduction in smoking and salt intake and an improvement in diet [31]. The present report reveals comparable decreases in SBP and DBP but shows that changes are broadly similar in men and women, in participants treated for hypertension as well as those not, and in subgroups of age over 80 years. NHANES data report the use of multiple classes of antihypertensive drugs increasing from 37 to 48% between 2001 and 2010 [32–34], with increasing utilization of all classes of antihypertensive drugs. This is generally consistent with the recommendations of guidelines for the general population and similar to our finding that monotherapy for treatment of hypertension has decreased over the study period [35,36]. In the present study, women tended to have higher recorded BP values than men, irrespective of treatment status. A similar pattern of sex disparity in BP measurement was noted in a Spanish study [37]. This finding appears to support the view that men who reach older ages may in some respects be healthier than their female counterparts [38,39].
The prevalence of hypertension was modestly higher in the present CPRD cohort compared with the sample of community dwelling adults aged 80 years and over in the NHANES study, with a prevalence of 76.5% [32] in the United States. A similar prevalence of hypertension (74%) was reported in the Framingham heart study among individuals aged 80 and over in the community [40]. A Spanish study, which included over 300 patients aged 80 years and over, reported the prevalence of hypertension to be 72.8% [41].
Previous studies have related increased hypertension treatment practices with a decline in mean SBP and DBP [19,33,42], but only one previous study had focused on older adults aged 80 years and over in the United States. The results suggested that there was an increase in awareness, treatment and control of hypertension from 1988 to 2010 [32]. Our results showing a decrease in both SBP and DBP in the untreated group are consistent with the findings from the Tromsø study, which showed a trend of BP reduction in the entire population, suggesting a whole population change potentially related to factors other than treatment [31].
The decrease in BP in the untreated groups may be explained by public health initiatives undertaken in recent years to reduce and manage comorbidities by emphasizing the benefits of weight loss, salt reduction, smoking cessation, healthy diet and exercise. There has been a major reduction in salt consumption in the United Kingdom; salt intake in United Kingdom has decreased by 1.4 g/day in 2000 to 8.1 g/day in 2011 [43]. This reduction in BP observed among this elderly study population may be the result of the initiatives set by National Service Framework for Older People that promoted the health and well being of the elderly by programmes led by the National Health Service (NHS) with the help of the councils. This encouraged increased physical activity, healthy eating, immunization programmes for influenza, access to smoking cessation programmes and other initiatives including providing support in terms of benefits for those facing poverty [44]. It may also be that antihypertensive drug therapy has been introduced at lower levels of BP over time.
Strengths and limitations
This study drew on a large, longitudinal and nationally representative data resource. This allowed us to present data for a very large sample of older adults, stratified by sex and frailty status. We acknowledge several limitations of the data. The number of general practices providing data changed over time and both general practices and patients entered and left the study over time, and this could have contributed to changes in case-mix over the study period, but analyses were adjusted for case-mix. BP readings were recorded in clinical practice, and recordings were not made using standardized methods. As BP, measurements were carried out in clinical settings, ‘white coat hypertension’ might have contributed to higher BP readings [45]. BP measurement methods may have changed over time, with automated devices gradually replacing traditional mercury sphygmomanometers, although this may have not biased the data. Antihypertensive drug utilization estimates were based on prescription records and not on prescriptions dispensed or drugs taken. Confounding by indication may be important in observational data, and this may explain why BP values were higher for treated than untreated participants.
In conclusion, there has been an increase in intensification of BP treatment in the population aged 80 years and over, with an increasing proportion now treated with multiple classes of antihypertensive drugs. A substantial decline in BP was observed over the past two decades. As a result, the proportion of patients achieving the criterion of BP less than 140/90 mmHg has increased, which may lead to improved cardiovascular outcomes [46]. It is well recognized that treatment and control of hypertension in the elderly should be placed in the context of increased comorbidity or frailty among older people [47]. Further research is needed to understand in more detail the health outcomes of intensified BP management in people over 80 years of age, including the impact on their wellbeing.
ACKNOWLEDGEMENTS
The work was supported by the Dunhill Medical Trust (grant number: R392/1114). M.C.G. and A.D. were supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas’ NHS Foundation Trust and King's College London.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: AHT, antihypertensive therapy; BP, blood pressure; CPRD, Clinical Practice Research Datalink; CVD, cardiovascular diseases; eFI, electronic frailty index; FI, frailty index; GP, general practitioner; EHR, electronic health record; HSE, Health Survey for England; HYVET, Hypertension in the Very Elderly Trial; NHANES, National Health and Nutrition Examination Survey; NHS, National Health Services; NRES, National Research Ethics Service Committee; SPRINT, SBP Intervention Trial; US, United States
REFERENCES
- 1.Murray CJL, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013; 381:997–1020. [DOI] [PubMed] [Google Scholar]
- 2.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014; 383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez BL, Labarthe DR, Huang B, Lopez-Gomez J. Rise of blood pressure with age. New evidence of population differences. Hypertension 1994; 24:779–785. [DOI] [PubMed] [Google Scholar]
- 4.British Heart Foundation. Coronary heart disease statistics: a compendium of health statistics. Oxford: Department of Public Health, University of Oxford; 2012. [Google Scholar]
- 5.Office of National Statistics. Population ageing in the United Kingdom, its constituent countries and the European Union. 2012; Available from: http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/dcp171776_258607.pdf. [02/03/2012; cited; 12]. [Google Scholar]
- 6.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26. [DOI] [PubMed] [Google Scholar]
- 7.WHO. A global brief on hypertension: silent killer, global public health crisis. Geneva: WHO; 2013. [Google Scholar]
- 8.Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013; 42:338–345. [DOI] [PubMed] [Google Scholar]
- 9.Buford TW. Hypertension and aging. Ageing Res Rev 2016; 26:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 11.Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chobanian AV. SPRINT results in older patients: How low to go? JAMA 2016; 315:2669–2670. [DOI] [PubMed] [Google Scholar]
- 14.Hamada S, Gulliford MC. Mortality in individuals aged 80 and older with type 2 diabetes mellitus in relation to glycosylated hemoglobin, blood pressure, and total cholesterol. J Am Geriatr Soc 2016; 64:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okumiya K, Matsubayashi K, Wada T, Fujisawa M, Osaki Y, Doi Y, et al. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. J Am Geriatr Soc 1999; 47:1415–1421. [DOI] [PubMed] [Google Scholar]
- 16.Dregan A, Ravindrarajah R, Hazra N, Hamada S, Jackson SHD, Gulliford MC. Longitudinal trends in hypertension management and mortality among octogenarians: Prospective cohort study. Hypertension 2016; 68:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsitz LA, Habtemariam D, Gagnon M, Iloputaife I, Sorond F, Tchalla AE, et al. Reexamining the effect of antihypertensive medications on falls in old age. Hypertension 2015; 66:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491. [DOI] [PubMed] [Google Scholar]
- 19.Falaschetti E, Mindell J, Knott C, Poulter N. Hypertension management in England: a serial cross-sectional study from 1994 to 2011. Lancet 2014; 383:1912–1919. [DOI] [PubMed] [Google Scholar]
- 20.Mody L, Miller DK, McGloin JM, Freeman M, Marcantonio ER, Magaziner J, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc 2008; 56:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, vanStaa T, Grundy E, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014; 36:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sever P. New hypertension guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J Renin Angiotensin Aldosterone Syst 2006; 7:61–63. [DOI] [PubMed] [Google Scholar]
- 23.Booth HP, Prevost AT, Gulliford MC. Validity of smoking prevalence estimates from primary care electronic health records compared with national population survey data for England, 2007 to 2011. Pharmacoepidemiol Drug Saf 2013; 22:1357–1361. [DOI] [PubMed] [Google Scholar]
- 24.Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 26.Benetos A, Bulpitt CJ, Petrovic M, Ungar A, Agabiti Rosei E, Cherubini A, et al. An expert opinion from the European Society of Hypertension–European Union Geriatric Medicine Society Working Group on the management of hypertension in very old, frail subjects. Hypertension 2016; 67:820–825. [DOI] [PubMed] [Google Scholar]
- 27.Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tinetti ME, Han L, Lee DH, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mossello E, Pieraccioli M, Nesti N, Bulgaresi M, Lorenzi C, Caleri V, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med 2015; 175:578–585. [DOI] [PubMed] [Google Scholar]
- 30.Benetos A, Labat C, Rossignol P, Fay R, Rolland Y, Valbusa F, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE study. JAMA Intern Med 2015; 175:989–995. [DOI] [PubMed] [Google Scholar]
- 31.Hopstock LA, Bonaa KH, Eggen AE, Grimsgaard S, Jacobsen BK, Lochen ML, et al. Longitudinal and secular trends in blood pressure among women and men in birth cohorts born between 1905 and 1977: the Tromso study 1979 to 2008. Hypertension 2015; 66:496–501. [DOI] [PubMed] [Google Scholar]
- 32.Bromfield SG, Bowling CB, Tanner RM, Peralta CA, Odden MC, Oparil S, et al. Trends in hypertension prevalence, awareness, treatment, and control among US adults 80 years and older, 1988–2010. J Clin Hypertens 2014; 16:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation 2012; 126:2105–2114. [DOI] [PubMed] [Google Scholar]
- 34.Psaty BM, Manolio TA, Smith NL, Heckbert SR, Gottdiener JS, Burke GL, et al. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the Cardiovascular Health Study. Arch Intern Med 2002; 162:2325–2332. [DOI] [PubMed] [Google Scholar]
- 35.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 36.No authors listed. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Roca GC, Llisterri JL, Prieto-Diaz MA, Alonso-Moreno FJ, Escobar-Cervantes C, Pallares-Carratala V, et al. Blood pressure control and management of very elderly patients with hypertension in primary care settings in Spain. Hypertens Res 2014; 37:166–171. [DOI] [PubMed] [Google Scholar]
- 38.Hazra NC, Dregan A, Jackson S, Gulliford MC. Differences in health at age 100 according to sex: population-based cohort study of centenarians using electronic health records. J Am Geriatr Soc 2015; 63:1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaupel JW. Biodemography of human ageing. Nature 2010; 464:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA 2005; 294:466–472. [DOI] [PubMed] [Google Scholar]
- 41.Aguado A, López F, Miravet S, Oriol P, Fuentes MI, Henares B, et al. Hypertension in the very old; prevalence, awareness, treatment and control: a cross-sectional population-based study in a Spanish municipality. BMC Geriatr 2009; 9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarganas G, Knopf H, Grams D, Neuhauser HK. Trends in antihypertensive medication use and blood pressure control among adults with hypertension in Germany. Am J Hypertens 2016; 29:104–113. [DOI] [PubMed] [Google Scholar]
- 43.Floyd CN. Hypertension – state of the art 2015. Clin Med 2016; 16:52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of Health. National service framework for older people. London: Department of Health; 2001. [Google Scholar]
- 45.Tanner RM, Shimbo D, Seals SR, Reynolds K, Bowling CB, Ogedegbe G, et al. White-coat effect among older adults: data from the Jackson Heart Study. J Clin Hypertens 2016; 18:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387:957–967. [DOI] [PubMed] [Google Scholar]
- 47.Berlowitz DR. Hypertension control in the elderly: too much of a good thing? J Clin Hypertens 2014; 16:265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]


