Supplemental Digital Content is available in the text.
Keywords: acute coronary syndrome, biomarkers, myocardial infarction, troponin
Abstract
Background:
High-sensitivity cardiac troponin assays enable myocardial infarction to be ruled out earlier, but the optimal approach is uncertain. We compared the European Society of Cardiology rule-out pathway with a pathway that incorporates lower cardiac troponin concentrations to risk stratify patients.
Methods:
Patients with suspected acute coronary syndrome (n=1218) underwent high-sensitivity cardiac troponin I measurement at presentation and 3 and 6 or 12 hours. We compared the European Society of Cardiology pathway (<99th centile at presentation or at 3 hours if symptoms <6 hours) with a pathway developed in the High-STEACS study (High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome) population (<5 ng/L at presentation or change <3 ng/L and <99th centile at 3 hours). The primary outcome was a comparison of the negative predictive value of both pathways for index type 1 myocardial infarction or type 1 myocardial infarction or cardiac death at 30 days. We evaluated the primary outcome in prespecified subgroups stratified by age, sex, time of symptom onset, and known ischemic heart disease.
Results:
The primary outcome occurred in 15.7% (191 of 1218) patients. In those less than the 99th centile at presentation, the European Society of Cardiology pathway ruled out myocardial infarction in 28.1% (342 of 1218) and 78.9% (961 of 1218) at presentation and 3 hours, respectively, missing 18 index and two 30-day events (negative predictive value, 97.9%; 95% confidence interval, 96.9–98.7). The High-STEACS pathway ruled out 40.7% (496 of 1218) and 74.2% (904 of 1218) at presentation and 3 hours, missing 2 index and two 30-day events (negative predictive value, 99.5%; 95% confidence interval, 99.0–99.9; P<0.001 for comparison). The negative predictive value of the High-STEACS pathway was greater than the European Society of Cardiology pathway overall (P<0.001) and in all subgroups, including those presenting early or known to have ischemic heart disease.
Conclusions:
Use of the High-STEACS pathway incorporating low high-sensitivity cardiac troponin concentrations rules out myocardial infarction in more patients at presentation and misses 5-fold fewer index myocardial infarctions than guideline-approved pathways based exclusively on the 99th centile.
Clinical Trial Registration:
URL: http://clinicaltrials.gov. Unique identifier: NCT01852123.
Editorial, see p 1612
Chest pain is a frequent presenting symptom in patients attending the emergency department, with significant resource implications for healthcare providers.1 Although the majority of patients with chest pain do not have an acute myocardial infarction,2 prompt and accurate exclusion of this diagnosis remains challenging in clinical practice, and unnecessary hospital admissions often result.3–5 Guidelines from the European Society of Cardiology (ESC) support the use of high-sensitivity cardiac troponins and earlier testing to rule out myocardial infarction where concentrations are <99th centile upper reference limit at presentation in those patients with symptoms for >6 hours and at 3 hours in the remainder.6 A similar approach was recommended by the National Institute of Clinical Health and Excellence, although concerns were raised about the generalizability of the studies evaluating the effectiveness of this approach.7
Recent studies have demonstrated that very low cardiac troponin concentrations can help to further risk stratify patients.8–22 Therefore, the latest European guidelines include an additional 1-hour pathway incorporating lower thresholds of cardiac troponin for risk stratification.6 We recently demonstrated in consecutive patients with suspected acute coronary syndrome that a cardiac troponin concentration <5 ng/L at presentation had a negative predictive value (NPV) of 99.6% (95% confidence interval [CI], 99.3–99.8) for myocardial infarction during the index presentation or myocardial infarction or cardiac death at 30 days. Furthermore, patients with cardiac troponin concentrations <5 ng/L had very low rates of adverse cardiac events at 1 year.14
Although it is clear that high-sensitivity cardiac troponins enable myocardial infarction to be ruled out earlier, the optimal approach is uncertain. Therefore, we compared the safety and efficacy of the ESC pathway based on the 99th centile alone with our clinical pathway incorporating low cardiac troponin concentrations to risk stratify patients.
Methods
Study Population
Patients with suspected acute coronary syndrome were recruited from the emergency department of the Royal Infirmary of Edinburgh, a tertiary care hospital in Scotland, between June 1, 2013, and September 30, 2015, into a substudy of the High-STEACS trial (High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome). All patients in whom the attending clinician requested cardiac troponin for suspected acute coronary syndrome were eligible for inclusion. We did not enroll patients with ST-segment–elevation myocardial infarction, those who were unable to provide consent, or those from outside our region to ensure complete follow-up. Blood samples were obtained at presentation and at 6 to 12 hours for high-sensitivity cardiac troponin testing as part of routine clinical care. Patients provided written informed consent for additional sampling at 3 hours with the results of testing at this time point not used to guide patient care. This prespecified analysis was restricted to those patients for whom serial samples were available (Figure I in the online-only Data Supplement). This clinical trial was registered (NCT01852123), approved by the national research ethics committee, and conducted in accordance with the Declaration of Helsinki.
High-Sensitivity Cardiac Troponin I Assay
The Abbott ARCHITECTSTAT high-sensitive cardiac troponin I assay (Abbott Laboratories, Abbott Park, IL) is a 2-step chemiluminescent assay with a limit of detection of 1.2 ng/L and coefficient of variation of <10% at 6 ng/L.23 This assay performance has been independently validated across multiple centers under routine laboratory working conditions, with a reported interlaboratory coefficient of variation of 12.6% at 3.5 ng/L across 33 instruments.14 The upper reference limit 99th centiles were determined in 4590 samples from healthy individuals as 16 ng/L for women and 34 ng/L in men,10 and from December 10, 2013, on, these thresholds were used in clinical practice.
Baseline Characteristics
Patient baseline characteristics, including chest pain characteristics, onset of symptoms, medical history, cardiovascular risk factors, medication, and clinical observations, in addition to investigations including serial 12-lead electrocardiography and cardiac imaging, were obtained from a dedicated case record form, a patient questionnaire, and the electronic patient record (TrakCare, InterSystems, Cambridge, MA). Hyperlipidemia or hypertension was defined as a history of the condition or by the use of lipid-lowering or antihypertensive therapies, respectively. Ischemic heart disease was defined as a history of angina, prior myocardial infarction, or prior coronary revascularization.
Diagnostic Adjudication
The final diagnosis was adjudicated for all patients by 2 independent physicians (A.R.C. and A.A.), with consensus from a third physician (J.A. or N.L.M.) when there was discrepancy after review of all clinical information, both noninvasive and invasive investigations, and outcomes from presentation to 30 days. Patients were classified as having type 1 myocardial infarction, type 2 myocardial infarction, or myocardial injury in accordance with the third universal definition of myocardial infarction as previously reported.6,14 Any cardiac troponin I concentration above the sex-specific 99th centile upper reference limit was considered evidence of myocardial necrosis. Type 1 myocardial infarction was defined as myocardial necrosis in the context of a presentation with symptoms suggestive of acute coronary syndrome or evidence of myocardial ischemia. Patients with symptoms or signs of myocardial ischemia resulting from increased oxygen demand or decreased supply (eg, tachyarrhythmia, hypotension, or anemia) secondary to an alternative pathology and myocardial necrosis were classified as having type 2 myocardial infarction. Myocardial injury was defined as evidence of myocardial necrosis in the absence of any clinical features of myocardial ischemia. Agreement for a diagnosis of type 1 myocardial infarction was very good (κ= 0.82; 95% CI, 0.75–0.89).
Clinical Outcomes
The primary outcome was a composite of index type 1 myocardial infarction or type 1 myocardial infarction or cardiac death at 30 days. We used regional and national registries in addition to individual patient follow-up at 30 days to ensure that follow-up was complete for the entire study population. All subsequent events were adjudicated with the same approach used for the index presentation. TrakCare software application (InterSystems Corp, Cambridge, MA) is a regional electronic patient record system that provides data on all hospital admissions to both tertiary or secondary care hospitals in southeast Scotland. All in-hospital and community deaths are recorded in a comprehensive national database, the General Register of Scotland. Cardiac death was defined as any death caused by myocardial infarction, arrhythmia, or heart failure.
Clinical Pathways
We compared the safety and efficacy of 2 pathways to rule out the composite outcome of index myocardial infarction and myocardial infarction or cardiac death at 30 days (Figure 1). The ESC pathway rules out myocardial infarction when cardiac troponin concentrations are <99th centile at presentation in patients with symptoms for >6 hours. In patients with symptoms for <6 hours, a second troponin measurement is performed 3 hours from presentation, with myocardial infarction ruled out if cardiac troponin remains <99th centile or is >99th centile without a significant change in concentration.6 Previously published guidance from the ESC Working Group on Acute Cardiac Care recommends use of a change in cardiac troponin concentration >50% of the 99th centile upper reference limit at 3 hours.24
Figure 1.
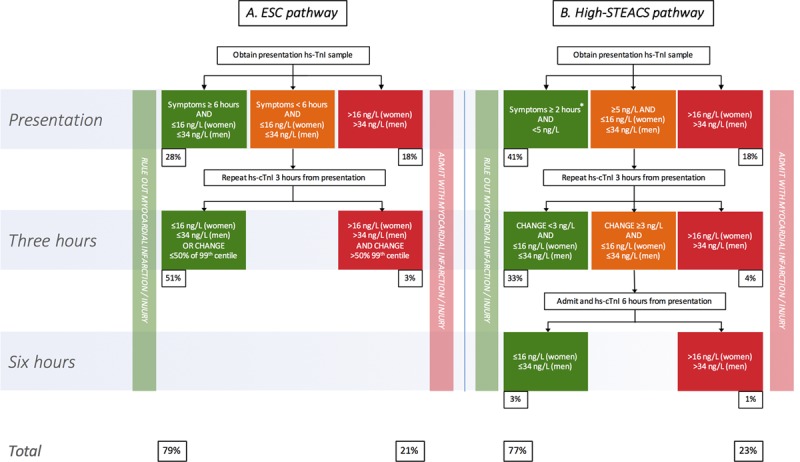
Summary of the European Society of Cardiology (ESC; A) and High-STEACS (High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome; B) rule-out pathways for myocardial infarction. Percentages indicate number of patients ruled in or out at a given time point as a proportion of the analysis population (n=1218). *In the High-STEACS pathway, patients with cardiac troponin concentrations <5 ng/L who present within 2 hours of symptom onset are retested at 3 hours. hs-cTnI indicates high-sensitivity cardiac troponin I (www.highsteacs.com).
We compared the ESC pathway with the High-STEACS pathway, based on our previous observations, that uses a risk stratification threshold of 5 ng/L at presentation (see www.highsteacs.com for further information and a web-based pathway app).14,25 This threshold has since been externally validated in separate populations, with a recent a multicenter study across 5 independent cohorts finding that a troponin concentration of <5 ng/L had an NPV of 99.2% (95% CI, 98.8–99.5).15 In our pathway, patients with cardiac troponin concentrations <5 ng/L at presentation are considered low risk, and myocardial infarction is ruled out without further testing unless they present early with symptom onset <2 hours from presentation; in that case, cardiac troponin is retested 3 hours after presentation.14 Patients with cardiac troponin concentrations ≥5 ng/L at presentation are retested at 3 hours. Myocardial infarction is ruled out at 3 hours if cardiac troponin concentrations are unchanged and remain <99th centile on retesting. A change in cardiac troponin concentration was defined as an increase or decrease ≥3 ng/L at 3 hours because this is the lowest measurable concentration within the normal reference range that exceeds analytical variation of the assay.26 This change in cardiac troponin concentration was internally and externally validated with data from the APACE (Advantageous Predictors of Acute Coronary Syndromes Evaluation) cohort (Table I in the online-only Data Supplement).
Statistical Analysis
Baseline characteristics are summarized as mean (standard deviation) or median (interquartile range) as appropriate. Patients with maximal cardiac troponin concentrations ≤99th centile were compared with those >99th centile with the use of a χ2 test or the Wilcoxon rank-sum test. The primary outcome was the NPV of each pathway using the composite end point of index type 1 myocardial infarction or subsequent type 1 myocardial infarction or cardiac death at 30 days. Because we estimated that the NPV would approach 100%, we used a bayesian approach with a Jeffreys prior (β distribution with both shape parameters equal to 0.5) because this is more robust when CIs approach 0 or 1.27 We derived a weighted generalized score statistic to compare the NPV of the ESC and the High-STEACS pathway, as previously described.28 We evaluated the NPV in prespecified subgroups stratified by time of symptom onset (<3, <6, or ≥6 hours), age (<65 or ≥65 years), sex, and history of ischemic heart disease. We determined absolute (hs-TnI3hr−hs-TnI0hr) and relative ([(hs-TnI3hr−hs-TnI0hr)/ hs-TnI0hr]×100, where hs-TnI0hr is high-sensitivity troponin I at presentation and hs-TnI3hr is high-sensitivity troponin I at 3 hours) change in cardiac troponin concentration from presentation to 3 hours, and we determined sensitivity, specificity and positive predictive value (PPV) with 95% CIs using a bayesian approach as for the NPV. In a sensitivity analysis, we evaluated the NPV for a primary outcome encompassing type 1 or type 2 myocardial infarction, myocardial injury, or myocardial infarction or cardiac death at 30 days. To ensure that our findings were generalizable to those centers that do not apply sex-specific diagnostic thresholds, we evaluated the performance of both pathways using a single 99th centile upper reference limit for men and women of 26 ng/L. A further sensitivity analysis evaluated the NPV in patients without evidence of myocardial ischemia (defined as ≥2 mm ST-segment–elevation depression or new T-wave inversion) on the presenting ECG who were considered intermediate or low risk with a GRACE (Global Registry of Acute Coronary Events) score of <140.6 We evaluated pathway efficacy by determining the number of patients ruled out at 0 and 3 hours as a proportion of the total study population, with comparison by the McNemar test for paired proportions. A 2-sided value of P<0.05 was considered statistically significant. All analyses were performed with R (version 3.2.2).
Results
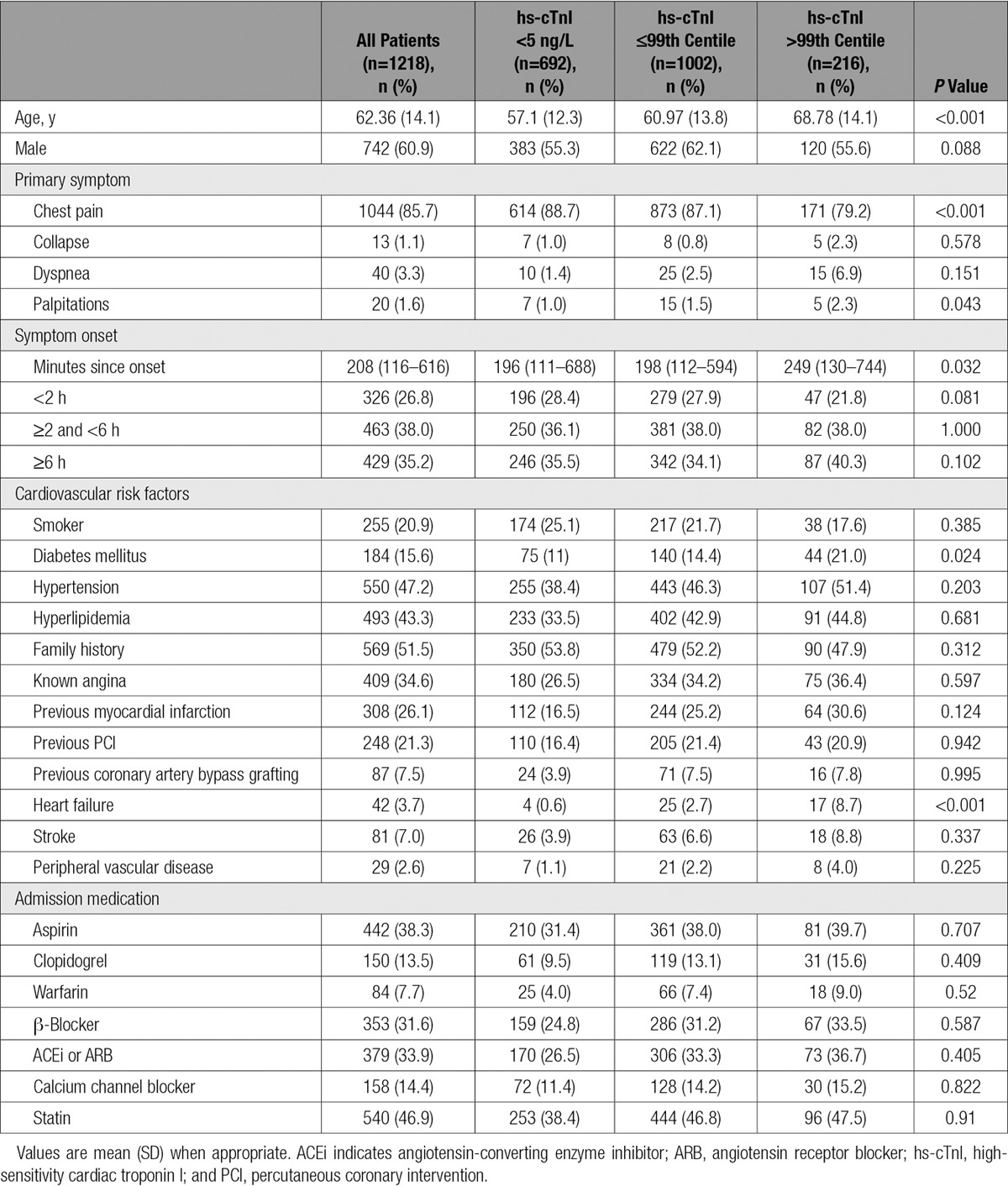
We identified 1218 patients with suspected acute coronary syndrome who met our inclusion and exclusion criteria (62.4±14.1 years of age; 61% male; Table 1 and Figure I in the online-only Data Supplement). The adjudicated diagnosis was type 1 myocardial infarction in 15.5% (189 of 1218), type 2 myocardial infarction in 5.5% (67 of 1218), and myocardial injury in 2.1% (26 of 1218). There were 6 subsequent type 1 myocardial infarcts and 6 cardiac deaths at 30 days. At presentation, 216 patients had troponin concentrations >99th centile, with 11.9% (145 of 1218) with type 1 myocardial infarction, 3.8% (46 of 1218) with type 2 myocardial infarction, and 2.1% (25 of 1218) with myocardial injury.
Table 1.
Baseline Demographics Stratified by Cardiac Troponin Concentration at Presentation
ESC Pathway
The ESC pathway ruled out 28.1% (342 of 1218) of patients at presentation and 78.9% (961 of 1218) of all patients by 3 hours. However, this approach missed 18 index type 1 myocardial infarctions (4 on presentation, 14 at 3 hours) and 2 subsequent myocardial infarctions within 30 days, for an overall NPV of 97.9% (95% CI, 96.9–98.7; Table 2 and Figure 2). The sensitivity of this pathway is 89.3% (95% CI, 84.9–93.5), and a summary of the missed events is provided in Table II in the online-only Data Supplement.
Table 2.
Diagnostic Performance of ESC and High-STEACS Pathways for the Primary Outcome at 3 Hours
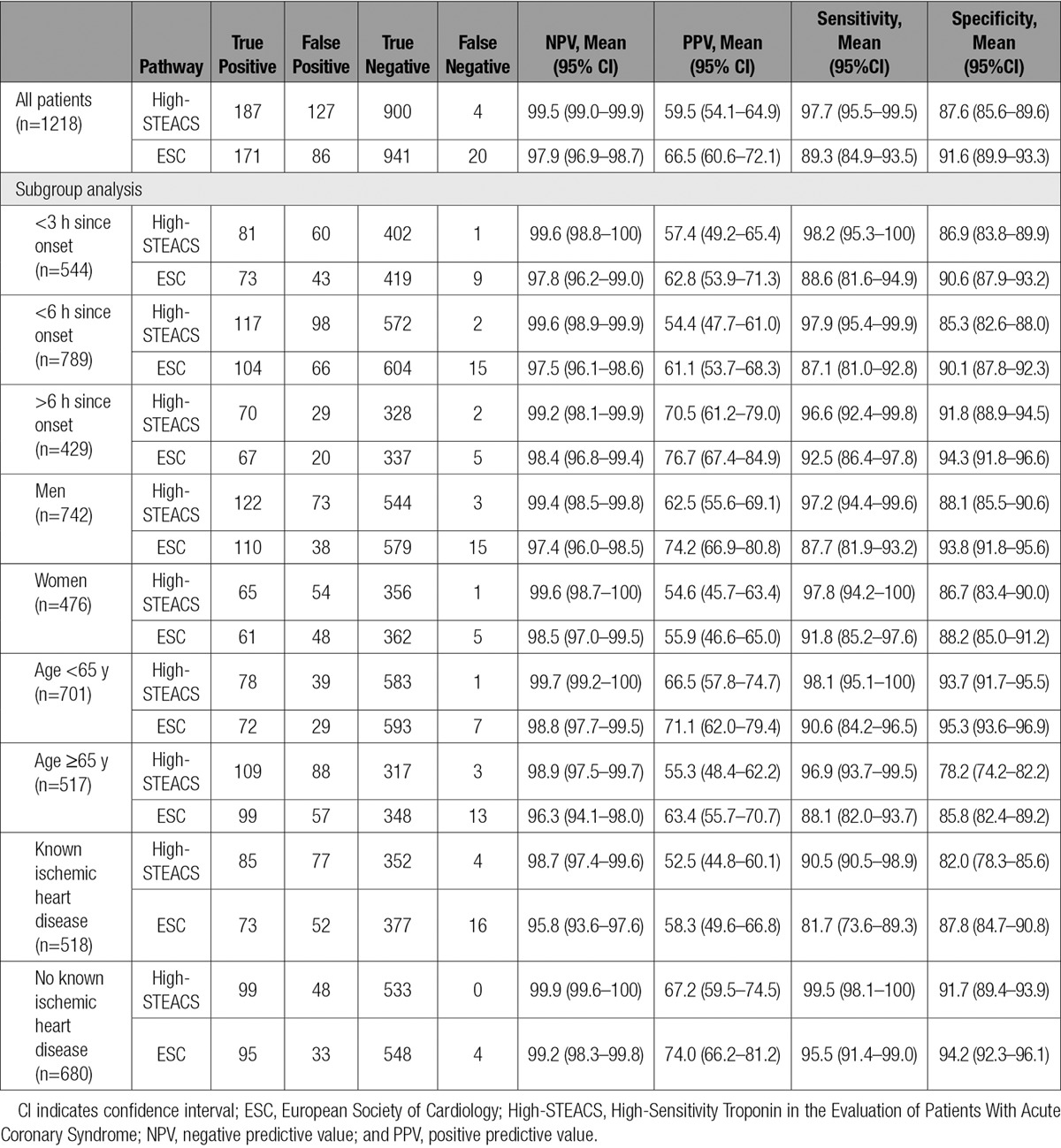
Figure 2.
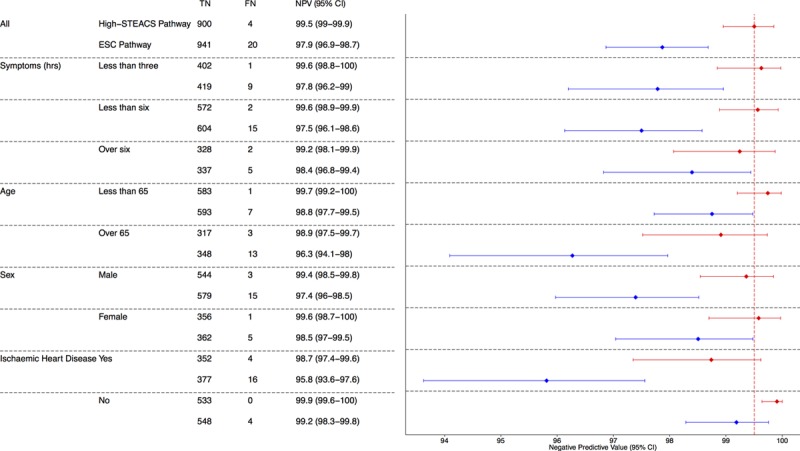
Negative predictive value (NPV) for index type 1 myocardial infarction or for myocardial infarction or cardiac death at 30 days of conventional and High-STEACS (High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome) pathways. Forest plot of the NPV and 95% confidence intervals (CIs) of the High-STEACS pathway (red) and the European Society of Cardiology (ESC) pathway (blue) stratified by prespecified subgroups. Numbers are true negative (TN), false negative (FN), and NPV (95% CIs). The vertical dashed line (red) highlights the central estimate of the NPV of the High-STEACS pathway in the total population.
High-STEACS Pathway
In comparison, the High-STEACS pathway ruled out 40.7% of patients (496 of 1218) at presentation and 74.2% of all patients (904 of 1218) by 3 hours. There were 2 missed index type 1 myocardial infarctions (none at presentation, 2 at 3 hours) and 2 recurrent events, for an overall NPV of 99.5% (95% CI, 99.0–99.9; Table 2 and Figure 2). All events missed by the High-STEACS pathway were also missed by the ESC pathway. The sensitivity of the High-STEACS pathway was 97.7% (95% CI, 95.5–99.5). A summary of missed events is provided in Table III in the online-only Data Supplement.
The High-STEACS pathway identifies more patients potentially suitable for discharge at presentation after a single cardiac troponin measurement compared with the ESC pathway (40.7% [95% CI, 38.0–43.5] versus 28.1% [95% CI, 25.6–30.7], respectively; P<0.001; Figure 1). At 3 hours, the High-STEACS pathway ruled our fewer patients than the ESC pathway (74.2% [95% CI, 71.7–76.6] versus 78.9% [95% CI, 76.5–81.1]; P<0.001). In the 57 patients ruled out at 3 hours by the ESC pathway but not the High-STEACS pathway, there were 13 missed index myocardial infarctions (22.8%).
The NPV of the High-STEACS pathway was greater than that for the ESC pathway overall (99.5% [95% CI, 99.0–99.9] versus (97.9% [95% CI, 96.9–98.9]; P<0.001) and for all prespecified subgroups (Table 2 and Figure 2). In the subgroup of patients who presented within 3 hours of symptom onset, there were more false negatives and the NPV was lower with the ESC pathway (9 false negatives; NPV, 97.8%; 95% CI, 96.2–99.0) than with the High-STEACS pathway (1 false-negative; NPV, 99.6%; 95% CI, 98.8–100.0). Similar differences were apparent in those patients presenting within 6 hours of symptom onset (ESC versus High-STEACS: NPV, 97.5% [95% CI, 96.1–98.6] versus 99.6% [95% CI, 98.9–99.9]). In men, the NPV of the ESC pathway was lower than the NPV of the High-STEACS pathway (97.4% [95% CI, 96.0–98.5] versus 99.4% [95% CI, 98.5–99.8]), although both pathways performed similarly in women. The lowest NPV for both the ESC pathway and High-STEACS pathway was in the subgroup of patients known to have ischemic heart disease (95.8% [95% CI, 93.6–97.6] and 98.7% [95% CI, 97.4–99.6], respectively).
In patients with an index type 1 myocardial infarction missed by the ESC pathway, the median change in cardiac troponin concentration between presentation and 3 hours was 5.5 ng/L (interquartile range, 4.0–13.3 ng/L). The majority of these patients (16 of 18) were not ruled out at 3 hours by the High-STEACS pathway because the change in cardiac troponin concentration was ≥3 ng/L (Table IV in the online-only Data Supplement), and further testing at 6 hours is recommended. In an external validation cohort of 2533 patients with suspected acute coronary syndrome (Table I in the online-only Data Supplement), a change in cardiac troponin concentration <3 ng/L at 3 hours ruled out 69.9% of those patients who required retesting (514 of 735) and missed no patients with an index diagnosis of type 1 myocardial infarction (Table V in the online-only Data Supplement).
The specificity and PPV for the ESC pathway were greater than for the High-STEACS pathway at 3 hours (specificity of 91.6% [95% CI, 89.9–93.3] and PPV of 66.5% [95% CI, 60.6–72.1] versus specificity of 87.6% [95% CI, 85.6–89.6] and PPV of 59.5% [95% CI, 54.1–64.9]; Table 2). However, the overall specificity and PPV of the High-STEACS pathway were comparable when patients requiring additional testing at 6 hours were included (specificity, 91.4% [95% CI, 89.7–93.1]; PPV, 67.9% [95% CI, 62.3–73.3]; Table VI in the online-only Data Supplement).
Sensitivity Analyses
In a sensitivity analysis, we evaluated both pathways using a single 99th centile upper reference limit for men and women of 26 ng/L. The performance of both pathways was similar, with an NPV of 97.7% (95% CI, 96.6–98.5) for the ESC pathway (20 missed index type 1 myocardial infarctions and 2 missed events at 30 days) and 99.4% (95% CI, 98.8–99.8) for the High-STEACS pathway (3 missed index type 1 myocardial infarctions and 2 missed 30-day events). The ESC pathway missed a similar proportion of men and women (10 men, 12 women).
We performed a further sensitivity analysis excluding patients with evidence of myocardial ischemia on the ECG or with a GRACE score >140 (n=224), of whom 71 had an index type 1 myocardial infarction. The diagnostic accuracy of both pathways improved. The ESC pathway still missed 13 index and 1 subsequent event (NPV, 98.3%; 95% CI, 97.3–99.0), whereas the High-STEACS pathway missed only 1 index and 1 subsequent event (NPV, 99.7%; 95% CI, 99.2–99.9; P<0.001).
We evaluated the diagnostic performance of both pathways for a composite end point incorporating an index diagnosis of type 1 or type 2 myocardial infarction, myocardial injury, or myocardial infarction or cardiac death at 30 days. The High-STEACS pathway missed an additional 5 events, whereas the ESC pathway missed an additional 9 events (High-STEACS: NPV, 99.0% [95% CI, 98.2–99.5], 9 false negatives: 2 index type 1 and 5 index type 2 myocardial infarctions, 2 type 1 myocardial infarctions at 30 days; ESC: NPV, 96.9% [95% CI, 95.8–97.9], 29 false negatives: 18 index type 1 and 9 index type 2 myocardial infarctions, 2 type 1 myocardial infarctions at 30 days).
Discussion
In patients with suspected acute coronary syndrome, we describe a clinical pathway using low cardiac troponin concentrations within the reference range to risk stratify patients. This approach identifies more patients as low risk at presentation and has a better overall NPV than guideline-approved pathways based solely on the 99th centile. Implementation of this pathway has the potential to improve the efficiency and safety of early rule-out approaches for patients with suspected acute coronary syndrome.
We make a number of important and clinically relevant observations. First, we demonstrate that the High-STEACS pathway misses fewer patients with an index diagnosis of myocardial infarction or myocardial infarction or cardiac death events at 30 days than the pathway approved by the ESC (4 versus 20 missed events). Second, the NPV of our pathway is 99.5% and better than that of the existing ESC pathway across all prespecified subgroups. In particular, the ESC pathway was less effective in men, those with a history of ischemic heart disease, and those presenting early after the onset of symptoms. Third, in patients without an elevated troponin concentration at presentation, the High-STEACS pathway identified half as low risk with a single measurement compared with a third identified with the established pathway. This is despite being safer and missing fewer patients with an index myocardial infarction.
The ESC guideline recommends the use of high-sensitivity cardiac troponin assays, and its central algorithm advises that the 99th centile be used as the threshold to rule in and rule out myocardial infarction at presentation and at 3 hours.6 However, the 99th centile may not be the optimal threshold to rule out myocardial infarction, and our observations suggest that this threshold does not provide an acceptable NPV or sensitivity (97.9% [95% CI, 96.9–98.7] and 89.3% [95% CI, 84.9–93.5], respectively). The performance of the ESC pathway is improved by inclusion of a risk stratification threshold and recognition that changes in cardiac troponin concentration within the reference range are important. In a large external validation cohort, we report that more than two thirds of patients with troponin concentrations above our risk stratification threshold at presentation can be safely ruled out at 3 hours if troponin concentrations are unchanged (<3 ng/L), with no missed diagnosis of type 1 myocardial infarction.
Our findings are consistent with a recently published evaluation of the ESC pathway that reported a NPV of 99.0% (95% CI, 98.1–99.5) and sensitivity of 93.2% (95% CI, 87.5–96.8) in a pooled analysis of 5 international cohorts.22 It is important to note that this analysis included lower-risk patients without ischemia on the ECG. In practice, risk stratification and early rule-out pathways are likely to be applied only to patients without overt myocardial ischemia on the ECG.25 However, interpretation of the ECG may be subjective and dependent on clinician experience; therefore, we included all patients in our evaluation to ensure that our safety estimates were conservative. Likewise, many clinicians use risk stratification tools to identify patients suitable for early discharge. Although the ESC guidelines do not advocate use of GRACE score for this purpose, it is widely used and is recommended to guide further investigation in patients in whom myocardial infarction has been ruled out. When we restricted our analysis to patients with no significant ST-segment depression or T-wave inversion on the ECG and GRACE scores of <140, we observed a modest improvement in the NPV of the ESC pathway (98.3%; 95% CI, 97.3–99.0), although even in this lower-risk group, the ESC pathway was inferior to the High-STEACS pathway (NPV, 99.7%; 95% CI, 99.2–99.9). Although the inclusion of all patients in the primary analysis ensures that our safety estimates are conservative, it is important to highlight that in clinical practice, careful clinical assessment and risk assessment are mandatory for all diagnostic pathways. In implementing our pathway, we recommend that patients with overt myocardial ischemia on the ECG at presentation be admitted for further assessment (Figure II in the online-only Data Supplement).
Pickering and colleagues22 used a single diagnostic threshold for myocardial infarction (26 ng/L) in both men and women, although a sensitivity analysis showed that the performance of the ESC pathway was similar with the use of sex-specific thresholds. In our analysis, we observed a reduction in the performance of the ESC pathway in men evaluated with the same assay with sex-specific thresholds (34 ng/L in men, 16 ng/L in women; 15 missed events and 5 missed events, respectively). In our sensitivity analysis, use of a single diagnostic threshold of 26 ng/L in men and women did not improve the overall performance of the ESC pathway. In contrast, the safety of the High-STEACS pathway was robust across both sexes and all prespecified subgroups of patients. Although the use of sex-specific thresholds in pathways that rely on the 99th centile remains contentious in clinical practice, risk stratification thresholds are not influenced by sex14; therefore, a single threshold can be applied equally to risk stratify men and women at presentation.
The efficacy of early rule-out pathways is also an important consideration. We demonstrate that the High-STEACS pathway ruled out a higher proportion of patients than the ESC pathway at presentation (40.7% versus 28.1%; P<0.001). Although our pathway rules out fewer patients at 3 hours (74.2% versus 78.9%; P<0.001), of the additional 57 patients ruled out by the ESC pathway, 1 in 5 (22.8%) was incorrectly ruled out and had an index diagnosis of type 1 myocardial infarction identified on subsequent testing. By identifying those patients with a change in cardiac troponin concentration (≥3 ng/L) from presentation to 3 hours and undertaking further testing, the High-STEACS pathway would not miss any of these events. This highlights the value of high-sensitivity cardiac troponin assays, which permit the identification of small but important changes in troponin concentration within the normal reference range and allow refinement in the risk stratification of patients with suspected acute coronary syndrome. The only disadvantage of our pathway is that in prioritizing safety, the specificity and PPV for a diagnosis of myocardial infarction are lower than for the ESC pathway at 3 hours (4% and 7%, respectively). Specificity is also important, but in our view, it need not be prioritized in early rule-out pathways. In patients we identify who require hospital admission, the diagnosis of myocardial infarction is best determined by demonstrating a rise and fall in cardiac troponin concentration over 6 to 12 hours.
The latest ESC guidelines have introduced a 1-hour pathway that incorporates a risk stratification step using high-sensitivity cardiac troponin concentrations within the reference range.6 This approach shows promise and has been validated with both high-sensitivity troponin I and high-sensitivity troponin T assays, with an NPV of 99.6% (95% CI, 98.4–100) and 99.1% (95% CI, 98.2–99.7), respectively.18,19 However, to the best of our knowledge, no previous studies have directly compared pathways that use a risk stratification step with low cardiac troponin concentrations with those based exclusively on the 99th centile. Further studies are needed to compare the efficacy and safety of retesting at 1 and 3 hours in pathways that incorporate a risk stratification threshold.
One of the limitations of these studies, including our own, is that they are observational in nature and enroll selected patients rather than all consecutive patients. Indeed, because no patients were discharged on the basis of pathway decisions, the true efficacy and safety of this approach are unknown. The ESC pathway recommends repeat testing in patients who present within 6 hours of symptom onset. Although the inclusion of patients who present early is a strength of our study, fewer patients may be ruled out at presentation by the ESC pathway as a consequence. At present, clinicians do not have evidence from prospective randomized controlled trials to inform their practice.29 Therefore, we are conducting a multicenter, stepped-wedge, cluster randomized trial to determine the efficacy and safety of our pathway (Figure II in the online-only Data Supplement) in unselected consecutive patients across Scotland (URL: http://clinicaltrials.gov. Unique identifier: NCT03005158). The outcome of this trial will help to inform our practice and provide an evidence base for future recommendations on the use of high-sensitivity cardiac troponins to risk stratify patients with suspected acute coronary syndrome.
Conclusions
The High-STEACS pathway, incorporating low cardiac troponin concentrations to risk stratify patients, rules out more patients on presentation and misses fewer index or recurrent myocardial infarctions than guideline-approved pathways based exclusively on the 99th centile. Implementation of this pathway has the potential to improve the efficiency and safety of early rule-out approaches for patients with suspected acute coronary syndrome.
Sources of Funding
This research was funded by the British Heart Foundation (SP/12/10/29922 and PG/15/51/31596) and by an NHS Scotland Health Informatics Challenge Grant (HICG/1/40) from the Chief Scientists Office. Abbott Laboratories provided the troponin I assay reagents, calibrators, and controls without charge. Drs Mills and Newby are supported by the Butler Senior Research Fellowship (FS/16/14/32023) and Chair (CH/09/002) awards from the British Heart Foundation. Dr Chapman is supported by a project grant and a fellowship (PG/15/51/31596, FS/16/75/32533) from the British Heart Foundation. Dr Anand is supported by a Research Fellowship from Chest Heart and Stroke Scotland (15/A163). Dr Newby is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). Drs Twerenbold and Reichlin have received research grants from the Swiss National Science Foundation (P300PB-167803 and PASMP3-136995, respectively). Drs Reichlin and Mueller have received research grants from the Swiss Heart Foundation. Dr Reichlin has received research grants from the University of Basel, the Professor Max Cloetta Foundation, and the Department of Internal Medicine, University Hospital Basel. Dr Mueller has received research grants from the Swiss National Science Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Alere, AstraZeneca, Beckman Coulter, BG Medicine, Biomerieux, BRAHMS, Critical Diagnostics, Nanosphere, Roche, Siemens, Singulex, Sphingotec, and 8sense. The validation cohort (APACE Study) was supported by research grants from the Swiss National Science Foundation, the European Union, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, BRAHMS, Biomerieux, Beckman Coulter, Nanosphere, Roche, Singulex, 8sense, and Siemens.
Supplementary Material
Disclosures
Drs Chapman, Anand, and Shah have received honoraria from Abbott Diagnostics. Dr Mills has acted as a consultant for Abbott Diagnostics, Beckman-Coulter, Roche, and Singulex. Dr Twerenbold has received speaker/consulting honoraria from Roche and BRAHMS. Dr Reichlin has received speaker honoraria from BRAHMS and Roche. Dr Mueller has received speaker/consulting honoraria from Abbott, Alere, AstraZeneca, Biomerieux, BMS, Boehringer Ingelheim, BRAHMS, Cardiorentis, Duke University, Eli Lilly, Novartis, Radiometer, Roche, Sanofi, Siemens, and Singulex. All other authors report no conflicts.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.025021/-/DC1.
Circulation is available at http://circ.ahajournals.org.
Clinical Perspective
What Is New?
The European Society of Cardiology recommends high-sensitivity cardiac troponin assays to rule out myocardial infarction using a 3-hour pathway based on the 99th centile.
In this study of 1218 patients, the European Society of Cardiology pathway had a negative predictive value of 97.9%, missing 18 index and 2 recurrent myocardial infarctions.
We propose a new pathway using a high-sensitivity cardiac troponin I assay that incorporates a risk stratification threshold of <5 ng/L at presentation and no change (<3 ng/L) at 3 hours.
This pathway had a higher negative predictive value than the European Society of Cardiology pathway at 99.5%, missing 2 index and 2 recurrent events.
What Are the Clinical Implications?
The 99th centile is not the optimal threshold to rule out myocardial infarction at presentation or at 3 hours, and pathways based exclusively on this threshold may miss patients with myocardial infarction.
Use of the High-STEACS (High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome) pathway, incorporating a risk stratification threshold at presentation and recognizing small but important changes in cardiac troponin within the normal reference range on serial testing, will minimize the risk of missed events.
It is important to note that the use of risk stratification thresholds identifies more patients as low risk at presentation, permitting a higher proportion of patients to be safely discharged.
References
- 1.Makam AN, Nguyen OK. Use of cardiac biomarker testing in the emergency department. JAMA Intern Med. 2015;175:67–75. doi: 10.1001/jamainternmed.2014.5830. doi: 10.1001/jamainternmed.2014.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhelev Z, Hyde C, Youngman E, Rogers M, Fleming S, Slade T, Coelho H, Jones-Hughes T, Nikolaou V. Diagnostic accuracy of single baseline measurement of Elecsys troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta-analysis. BMJ. 2015;350:h15. doi: 10.1136/bmj.h15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91:229–230. doi: 10.1136/hrt.2003.027599. doi: 10.1136/hrt.2003.027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A Chest Pain Guideline Development Group. NICE guidance: chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974–978. doi: 10.1136/hrt.2009.190066. doi: 10.1136/hrt.2009.190066. [DOI] [PubMed] [Google Scholar]
- 5.Goodacre S, Thokala P, Carroll C, Stevens JW, Leaviss J, Al Khalaf M, Collinson P, Morris F, Evans P, Wang J. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess. 2013;17:v-vi–1. doi: 10.3310/hta17010. doi: 10.3310/hta17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 7.NICE. London, UK: National Institute for Health and Care Excellence; 2014. Myocardial infarction (acute): early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays): DG15. https://www.nice.org.uk/guidance/dg15. Accessed July 1, 2016. [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [Google Scholar]
- 9.Mills NL, Churchhouse AM, Lee KK, Anand A, Gamble D, Shah AS, Paterson E, MacLeod M, Graham C, Walker S, Denvir MA, Fox KA, Newby DE. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA. 2011;305:1210–1216. doi: 10.1001/jama.2011.338. doi: 10.1001/jama.2011.338. [DOI] [PubMed] [Google Scholar]
- 10.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, Collinson PO, Apple FS, Gray AJ, Fox KA, Newby DE, Mills NL. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, Wibberley C, Nuttall M, Mackway-Jones K. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–1339. doi: 10.1016/j.jacc.2011.06.026. doi: 10.1016/j.jacc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Rubini Giménez M, Hoeller R, Reichlin T, Zellweger C, Twerenbold R, Reiter M, Moehring B, Wildi K, Mosimann T, Mueller M, Meller B, Hochgruber T, Ziller R, Sou SM, Murray K, Sakarikos K, Ernst S, Gea J, Campodarve I, Vilaplana C, Haaf P, Steuer S, Minners J, Osswald S, Mueller C. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol. 2013;168:3896–3901. doi: 10.1016/j.ijcard.2013.06.049. doi: 10.1016/j.ijcard.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 13.Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol. 2014;63:2569–2578. doi: 10.1016/j.jacc.2014.03.017. doi: 10.1016/j.jacc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, Chapman AR, Langdon T, Sandeman D, Vaswani A, Strachan FE, Ferry A, Stirzaker AG, Reid A, Gray AJ, Collinson PO, McAllister DA, Apple FS, Newby DE, Mills NL High-STEACS investigators. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlton E, Greenslade J, Cullen L, Body R, Than M, Pickering JW, Aldous S, Carley S, Hammett C, Kendall J, Keevil B, Lord S, Parsonage W, Greaves K. Evaluation of high-sensitivity cardiac troponin I levels in patients with suspected acute coronary syndrome. JAMA Cardiol. 2016;1:405–412. doi: 10.1001/jamacardio.2016.1309. doi: 10.1001/jamacardio.2016.1309. [DOI] [PubMed] [Google Scholar]
- 16.Thelin J, Melander O, Öhlin B. Early rule-out of acute coronary syndrome using undetectable levels of high sensitivity troponin T. Eur Heart J Acute Cardiovasc Care. 2015;4:403–409. doi: 10.1177/2048872614554107. doi: 10.1177/2048872614554107. [DOI] [PubMed] [Google Scholar]
- 17.Body R, Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, de Filippi CR, Nowak R, Panteghini M, Jernberg T, Plebani M, Verschuren F, French JK, Christenson R, Weiser S, Bendig G, Dilba P, Lindahl B TRAPID-AMI Investigators. The use of very low concentrations of high-sensitivity troponin T to rule out acute myocardial infarction using a single blood test. Acad Emerg Med. 2016;23:1004–1013. doi: 10.1111/acem.13012. doi: 10.1111/acem.13012. [DOI] [PubMed] [Google Scholar]
- 18.Rubini Gimenez M, Twerenbold R, Jaeger C, Schindler C, Puelacher C, Wildi K, Reichlin T, Haaf P, Merk S, Honegger U, Wagener M, Druey S, Schumacher C, Krivoshei L, Hillinger P, Herrmann T, Campodarve I, Rentsch K, Bassetti S, Osswald S, Mueller C. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015;128:861–870.e4. doi: 10.1016/j.amjmed.2015.01.046. doi: 10.1016/j.amjmed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 19.Mueller C, Giannitsis E, Christ M, Ordóñez-Llanos J, deFilippi C, McCord J, Body R, Panteghini M, Jernberg T, Plebani M, Verschuren F, French J, Christenson R, Weiser S, Bendig G, Dilba P, Lindahl B TRAPID-AMI Investigators. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68:76–87.e4. doi: 10.1016/j.annemergmed.2015.11.013. doi: 10.1016/j.annemergmed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 21.Reichlin T, Twerenbold R, Wildi K, Rubini Gimenez M, Bergsma N, Haaf P, Druey S, Puelacher C, Moehring B, Freese M, Stelzig C, Krivoshei L, Hillinger P, Jäger C, Herrmann T, Kreutzinger P, Radosavac M, Weidmann ZM, Pershyna K, Honegger U, Wagener M, Vuillomenet T, Campodarve I, Bingisser R, Miró Ò, Rentsch K, Bassetti S, Osswald S, Mueller C. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ. 2015;187:E243–E252. doi: 10.1503/cmaj.141349. doi: 10.1503/cmaj.141349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering JW, Greenslade JH, Cullen L, Flaws D, Parsonage W, George P, Worster A, Kavsak PA, Than MP. Validation of presentation and 3 h high-sensitivity troponin to rule-in and rule-out acute myocardial infarction. Heart. 2016;102:1270–1278. doi: 10.1136/heartjnl-2015-308505. doi: 10.1136/heartjnl-2015-308505. [DOI] [PubMed] [Google Scholar]
- 23.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur. Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 25.Shah AS, Anand A, Chapman AR, Newby DE, Mills NL High-STEACS Investigators. Measurement of cardiac troponin for exclusion of myocardial infarction: authors’ reply. Lancet. 2016;387:2289–2291. doi: 10.1016/S0140-6736(16)30517-7. doi: 10.1016/S0140-6736(16)30517-7. [DOI] [PubMed] [Google Scholar]
- 26.Kavsak PA, Don-Wauchope AC, Hill SA, Worster A. Acceptable analytical variation may exceed high-sensitivity cardiac troponin I cutoffs in early rule-out and rule-in acute myocardial infarction algorithms. Clin Chem. 2016;62:887–889. doi: 10.1373/clinchem.2016.255448. doi: 10.1373/clinchem.2016.255448. [DOI] [PubMed] [Google Scholar]
- 27.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–117. [Google Scholar]
- 28.Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics. 2000;56:345–351. doi: 10.1111/j.0006-341x.2000.00345.x. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe AS. TRAPID or trapped? Ann Emerg Med. 2016;68:88–91. doi: 10.1016/j.annemergmed.2016.01.009. doi: 10.1016/j.annemergmed.2016.01.009. [DOI] [PubMed] [Google Scholar]


