Supplemental Digital Content is available in the text.
Abstract
Background:
Neoadjuvant radiotherapy (NRT) enhances breast-conserving surgery outcomes, reducing local recurrence of breast cancer and increasing median survival. However, its effect on postoperative morbidity remains under-studied. We sought to assess the impact of NRT on 30-day postoperative morbidity after mastectomy.
Methods:
We analyzed data from women undergoing mastectomy (with or without immediate reconstruction) using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) 2005–2011 datasets. ACS-NSQIP is a prospective, risk-adjusted, outcomes-based registry. Data included demographic and perioperative factors. Outcomes studied included surgical site (wound and prosthesis/flap complications), systemic (cardiac, respiratory, neurological, urinary, and venous thromboembolism events), and overall morbidity. Logistic regression was used to estimate the unadjusted odds ratio (uOR) and adjusted odds ratio (aOR) between NRT and postoperative 30-day morbidity.
Results:
The study population included 77,902 women, of which 61,039 (78.4%) underwent mastectomy only and 16,863 (21.6%) underwent mastectomy with immediate breast reconstruction. NRT was administered to 266 (0.4%) mastectomy-only and 75 (0.4%) immediate breast reconstruction patients. In the mastectomy-only group, there were no significant differences in the rates of postoperative surgical site morbidity (aOR = 1.41; 95% confidence interval (CI): 0.76–2.63; P = 0.276), systemic morbidity (aOR = 0.72; 95% CI: 0.40–1.26; P = 0.252), and overall morbidity (aOR = 0.85; 95% CI: 0.54–1.33; P = 0.477) between NRT and control groups. Similarly, no significant differences were found for these three outcomes in the immediate breast reconstruction population. Statistical power for every comparison was >80%.
Conclusions:
This study suggests that NRT is not associated with significantly higher 30-day postoperative complications among breast cancer patients undergoing mastectomy with or without immediate breast reconstruction.
INTRODUCTION
Neoadjuvant radiotherapy (NRT) carries substantial survival benefits to cancer patients.1, 2 In breast cancer specifically, NRT was shown to reduce tumor bulk and, when added to neoadjuvant chemotherapy (NCT), was shown to improve oncological outcomes for women with locally advanced disease.3, 4 As compared with NCT alone, the combination of NRT and NCT promoted complete pathological response, clinical remission, and increased median survival.5 This has allowed breast-conserving surgery to be performed with enhanced safety and frequency, with rates reaching 43–92%.6–10 Isolated NRT has also been found to promote favorable long-term survival among women undergoing breast-conserving surgery11, 12 or mastectomy.13
Despite the oncologic benefits of NRT, clinicians may be reluctant to recommend it in certain cases because of concerns about its potential toxicity and negative wound-healing effects.14 In fact, in the adjuvant setting, radiation has been associated with morbid effects on soft tissues, including impaired healing, bacterial invasion, pigmentation irregularities, and fibrous tissue formation with reduced tensile strength.15 In the preoperative setting, however, one study limited to patients with locally advanced rectal cancer has suggested that NRT is less toxic to patients than conventional adjuvant radiotherapy.1
To date, evidence on the postmastectomy impact of NRT, without the confounding effect of concurrent chemotherapy, remains very limited.13, 16 To address this knowledge gap, we evaluated the impact of NRT on 30-day postoperative morbidity while adjusting for concurrent chemotherapy. Specifically, we aimed to evaluate its effect on surgical site, systemic, and overall morbidity rates in breast cancer patients undergoing mastectomy with or without immediate reconstruction.
METHODS
Population and Methods
All women who underwent mastectomy with or without reconstruction from 2005 to 2011 were identified using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, a prospective, risk-adjusted, outcomes-based registry, which enables multi-institutional investigations involving a sizable and diverse patient population. ACS-NSQIP participants include over 250 university and private hospitals in six nations. The database records extensive demographic information, perioperative risk factors, and postoperative complications.17
Procedures were identified using the following current procedural terminology (CPT) codes: 19160, 19162, 19180, 19182, 19200, 19220, 19240, 19301, 19302, 19303, 19304, 19305, 19306, and 19307 for mastectomy (either partial or total), and 19340, 19342, 19357, 19361, 19364, 19366, 19367, 19368, and 19369 for breast reconstruction (see table, Supplemental Digital Content 1, which provides a description of the CPT codes utilized for this study; http://links.lww.com/PRSGO/A359). Patients receiving only mastectomy CPT codes define the mastectomy-only population. Patients receiving two or more CPT codes simultaneously (one for mastectomy and another for breast reconstruction) define the immediate reconstruction population.
Study Design
The ACS-NSQIP database collects morbidity events occurring within 30 postoperative days. The study outcomes were defined as follows: surgical site morbidity included superficial and deep incisional surgical site infection (SSI), organ space SSI, wound dehiscence, and prosthesis/flap failure. Systemic morbidity was defined by the occurrence of any of the following: pneumonia, unplanned intubation, pulmonary embolism, >48 hours on the ventilator, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke/cerebrovascular accident, coma >24 hours, cardiac arrest, myocardial infarction, deep vein thrombosis requiring treatment, sepsis, septic shock, and return to the operating room within 30 days. Postoperative overall morbidity included all the aforementioned surgical site and systemic complications. The risk factor of interest was NRT, defined in the ACS-NSQIP database as any radiotherapy preceding the index surgery by 90 days or less.
For the outcomes of interest (surgical site, systemic, and overall morbidity), unadjusted odds ratio (uOR) and adjusted odds ratio (aOR) reflecting events occurring within 30 postoperative days were estimated using univariate and multivariable logistic regression to compare patients receiving NRT with those not receiving NRT in both the mastectomy-only and the immediate breast reconstruction populations. We used a model-wise approach,18 and adjusted extensively for clinically and statistically relevant confounders (variables with P < 0.1 in univariate analysis), which included: age, body mass index (BMI), smoking status, preoperative chemotherapy, work relative value unit, operation year, inpatient status, type of anesthetic method, American Society of Anesthesiologist (ASA) classification, wound classification, previous cardiovascular morbidity, previous respiratory morbidity, previous renal morbidity, previous hematology/oncology morbidity, previous hepatobiliary morbidity, previous central nervous system morbidity, diabetic status, alcohol consumption (more than two drinks per day in 2 weeks before admission), history of previous operation within 30 days of the surgery, operation time, race/ethnicity, and breast reconstruction technique (this variable was included only in the immediate breast reconstruction analysis). Two variables were excluded from the multivariable model due to >10% missing data: preoperative anemia (16.9% missing data) and intraoperative transfusions (34.6% missing data).
Statistical Analysis
Continuous variables, such as age, are presented as “mean ± SD”, and categorical variables, such as gender, are presented as the number of patients and its corresponding proportion with respect to the exposure groups [n (%)]. P-values for continuous variables correspond to a Kruskal–Wallis rank sum test of the null, assuming the location parameters of the distribution of the variable are the same in each exposure group. P-values for categorical variables correspond to a Fisher’s exact test for testing the null of independence of rows and columns in a contingency table with fixed marginals. (see table, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A359).
Data management and analyses were done using STATA/SE12. Power calculations were computed using PS Power and Sample Size Program.19 In accordance with the Johns Hopkins guidelines (which follow the US Code of Federal Regulations for the Protection of Human Subjects), institutional review board approval was not needed or sought for our analysis because we received deidentified data only.
RESULTS
We identified 85,851 women who underwent mastectomy with or without immediate breast reconstruction. After excluding 7,949 patients with missing NRT data, a total of 77,902 patients with complete data constituted the study population: 61,039 mastectomy-only and 16,863 immediate breast reconstruction patients. A total of 266 (0.4%) mastectomy-only patients and 75 (0.4%) immediate breast reconstruction patients received NRT (Fig. 1). The population demographic characteristics are shown in table, Supplemental Digital Content 2, which displays general and preoperative patient characteristics; http://links.lww.com/PRSGO/A360.
Fig. 1.
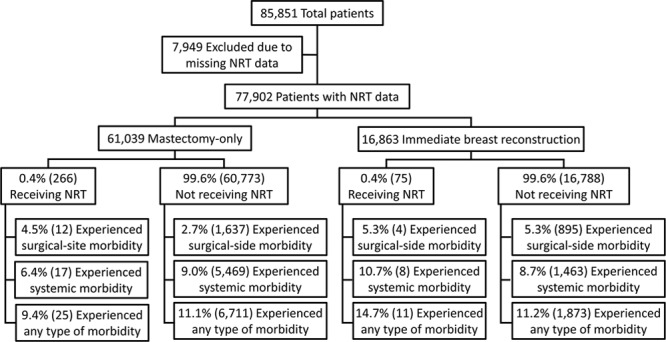
Patient selection algorithm, including surgical site, systemic, and overall morbidity rates stratified by NRT status.
Results are presented separately for the mastectomy-only and immediate breast reconstruction groups. For all outcomes, complication rates of the exposed group (those receiving NRT) were compared with the unexposed group (those not receiving NRT; see table, Supplemental Digital Content 3, which displays percentages of patients by NRT status who experienced morbidity; http://links.lww.com/PRSGO/A361).
Surgical Site Morbidity
Mastectomy-only Group (No Reconstruction)
Among 266 patients receiving NRT, 12 (4.5%) experienced a surgical site complication, as opposed to 1,637 (2.7%) patients not receiving NRT (Fig. 1). Univariate regression demonstrated that NRT was not associated with significantly higher local complication rates in the mastectomy-only group (uOR = 1.71; 95% CI: 0.95–3.05; P = 0.071). Similarly, after adjustment using multivariable regression, NRT was not associated with higher local complication rates in the group undergoing mastectomy alone (aOR = 1.41; 95% CI: 0.76–2.63, P = 0.276; see table, Supplemental Digital Content 2, http://links.lww.com/PRSGO/A360).
Immediate Reconstruction Group (Mastectomy with Concurrent Reconstruction)
Among 75 patients in the immediate breast reconstruction group who received NRT, four (5.3%) experienced local morbidity. Comparably, of 16,788 patients in the immediate breast reconstruction group who did not receive NRT, 895 (5.3%) experienced surgical site morbidity. Univariate regression showed that NRT was not associated with higher surgical site complication rates in the immediate breast reconstruction group (uOR = 1.00; 95% CI: 0.36–2.75, P = 0.999). Moreover, after extensive adjustment using multivariable regression, NRT was not associated with higher surgical site morbidity rates in this group (aOR = 1.05; 95% CI: 0.37–2.96, P = 0.934; see table, Supplemental Digital Content 2, http://links.lww.com/PRSGO/A360).
Additionally, we identified the predictors of 30-day surgical site morbidity among women undergoing mastectomy with or without immediate reconstruction (see table, Supplemental Digital Content 4, which displays predictors of 30-day surgical site morbidity among women undergoing mastectomy with or without immediate reconstruction; http://links.lww.com/PRSGO/A362). These included increasing BMI; smoking; work relative value unit; inpatient status; anesthesia type; ASA class ≥2; any degree of wound contamination; previous cardiovascular, respiratory, and hematology/oncology morbidity; diabetes (on insulin or oral treatment); increasing operative time; and Asian and African American race.
Systemic Morbidity
Mastectomy-only Group (No Reconstruction)
Of 266 mastectomy-only patients who received NRT, 17 (6.4%) experienced a systemic morbidity. In contrast, of 60,773 mastectomy-only patients with no NRT, 5,469 (9.0%) experienced a systemic complication (Fig. 1). Univariate regression showed that NRT was not associated with higher systemic morbidity rates in the mastectomy-only group (uOR = 0.69; 95% CI: 0.42–1.13, P = 0.140). Correspondingly, after extensive adjustment using multivariable regression, NRT was not associated with higher systemic morbidity rates in this group (aOR = 0.72; 95% CI: 0.40–1.26, P = 0.252; see table, Supplemental Digital Content 3, http://links.lww.com/PRSGO/A361).
Immediate Reconstruction Group (Mastectomy with Concurrent Reconstruction)
Of 75 patients who received NRT, eight (10.7%) experienced systemic morbidity. On the other hand, of 16,788 patients who did not receive NRT, 1,463 (8.7%) experienced a systemic complication. Univariate regression showed that NRT was not associated with higher systemic morbidity rates in the immediate breast reconstruction group (uOR = 1.25; 95% CI: 0.60–2.61, P = 0.551). Additionally, after adjustment using multivariable regression, NRT was not associated with higher systemic morbidity rates in this group (aOR = 1.14; 95% CI: 0.47–2.73, P = 0.773; see table, Supplemental Digital Content 3, http://links.lww.com/PRSGO/A361).
Independent predictors of 30-day systemic morbidity among women undergoing mastectomy with or without immediate reconstruction included increasing age, NCT, BMI, smoking, work relative value unit, operation year, ASA classes 2 and 3, contaminated or dirty/infected wounds, previous respiratory or hematology/oncology morbidity, increasing operative time, and Asian race (see table, Supplemental Digital Content 5, which displays predictors of 30-day systematic morbidity among women undergoing mastectomy with or without immediate reconstruction; http://links.lww.com/PRSGO/A363).
Overall Morbidity
Mastectomy-only Group (No Reconstruction)
Out of 266 mastectomy-only patients that received NRT, 25 (9.4%) experienced morbidity; and out of 60,773 mastectomy-only patients who did not receive NRT, 6,711 (11.1%) experienced morbidity (Fig. 1). Univariate logistic regression showed that NRT was not significantly associated with higher overall morbidity in the mastectomy-only group (uOR = 0.84; 95% CI: 0.55–1.26; P = 0.394). Similarly, after adjustment using multivariable regression, NRT was not associated with higher overall morbidity in this group (aOR = 0.85; 95% CI: 0.54–1.33; P = 0.477; see table, Supplemental Digital Content 4, http://links.lww.com/PRSGO/A362).
Immediate Reconstruction Group (Mastectomy with Concurrent Reconstruction)
Of 75 patients who received NRT, 11 (14.7%) experienced morbidity. In contrast, of 16,788 patients who did not receive NRT, 1,873 (11.2%) experienced morbidity (Fig. 1). Univariate regression showed that NRT was not associated with higher overall morbidity in the immediate breast reconstruction group (uOR = 1.36; 95% CI: 0.72–2.59; P = 0.338). Analogously, after adjustment using multivariable regression, NRT did not show significant association with higher overall morbidity in this group (aOR = 1.54; 95% CI: 0.78–3.04; P = 0.215; see table, Supplemental Digital Content 4, http://links.lww.com/PRSGO/A362).
Finally, we identified the predictors of overall 30-day postoperative morbidity among women undergoing mastectomy with or without immediate reconstruction (see table, Supplemental Digital Content 6, which displays overall 30-day postoperative morbidity among women undergoing mastectomy with or without immediate reconstruction; http://links.lww.com/PRSGO/A364). These predictors included age, NCT, increasing BMI, smoking, work relative value index, operation year, ASA class 3, any degree of wound contamination, previous respiratory morbidity, diabetes on insulin treatment, increasing operative time, and Asian and African American race.
DISCUSSION
Our results indicate that NRT is not significantly associated with higher postoperative 30-day morbidity in women undergoing mastectomy (either partial or total) or in women who have immediate breast reconstruction. This suggests that the oncologic advantages of NRT may be garnered without significantly increasing postoperative complication rates. NRT has not only increased the safety, frequency, and efficacy of breast-conserving surgery, but also represents an essential therapeutic modality for large, operable, early breast cancers that are not amenable to breast-conserving surgery, inoperable locally advanced disease, inflammatory breast cancer, breast cancer with partial response to NCT, and patients with tumor progression under NCT.4
Our findings support previous studies investigating the postoperative effects of NRT in other cancers.20–24 In these studies, there was no significant increase in postoperative adverse events associated with NRT.20–24 Gérard et al conducted a randomized clinical trial to assess the impact of NRT before radical surgery for rectal cancer, and found that patients receiving NRT had significantly decreased local recurrence rates with acceptable side effects.20 The Swedish Rectal Cancer Trial and the total mesorectal excision trial (TME trial) reported similar findings, and added the benefit of improved tumor-specific survival.21, 22 Furthermore, The Polish Colorectal group reported that a short-course NRT for rectal cancer has low toxicity and high compliance rates, which may be considered a significant benefit.23 Along these lines, Lebwohl et al reported that treatment interruption was higher in patients receiving adjuvant radiation therapy than in patients receiving NRT for rectal cancer.24
Specifically in regard with breast surgery, McCarthy et al reported that radiation is not significantly associated with increased complications after alloplastic (expander or implant) reconstruction based on an analysis of 1,170 cases, though this study did not distinguish premastectomy from postmastectomy radiation therapy.25 Furthermore, Weintraub and Kahn conducted a retrospective study of 120 breast cancer patients comparing those undergoing radiation before or during reconstruction with controls. They reported similar rates of infection, wound dehiscence, and other complications for both groups.26
It is worth noting that smaller, single-institution studies have reported increased postoperative morbidity in the setting of NRT.27–31 Chang et al found that among 41 patients, those who underwent NRT before skin-sparing mastectomy had a significantly higher rate of native skin flap compromise compared with those not receiving NRT.27 Hultman and Chaiza had similar conclusions based on a study of 37 patients, where NRT patients had increased risks of complications after skin-sparing mastectomy.28 Selber et al noted a significantly higher incidence of seroma among 500 transverse rectus abdominis myocutaneous flap breast reconstructions in patients with NRT.29 Krueger et al prospectively studied 81 patients undergoing mastectomy with reconstruction and concluded that NRT increased reconstruction failure and complication rates.30 Finally, Ascherman et al found that radiation therapy before implant placement (either NRT or postmastectomy radiation) was a significant risk factor for complications among 104 breast cancer patients.31
The discrepancies between our findings and the contradictory results listed previously may be explained by the magnitude of the differences on complication rates between the NRT and non-NRT groups reported by those studies. The limited literature suggesting significantly higher morbidity among NRT compared with those not receiving NRT reports highly variable complication rates ranging from 14.3% to >40%.27–31 The smaller the true difference in complication rates, the greater the power required to detect it. Therefore, using the lowest difference available in the literature (14.3% higher rate among radiotherapy group vs control),31 we estimate that our study provides adequate power (>80%) to identify any complication rate in the above range if such differences actually exist.27–31 In the case of mastectomy-only, given the large sample size of this group, we estimate over 90% power to detect the difference of 14.3% reported in the literature.31 Furthermore, we performed extensive multivariable analyses, adjusting for 22 demographic and perioperative factors along with previous comorbidities and risk factors. Moreover, a relevant advantage of our study is that the ACS-NSQIP personnel perform systematic, prospective collection of multi-institutional data across the United States (over 250 university and private hospitals); this enabled our analyses to estimate morbidity rates that closely resemble those of the US population rather than the single-institution data analyzed by previous studies.27–31
Despite these strengths, our study carries some limitations. The ACS-NSQIP follows patients for a period of 30 postoperative days, which is an adequate length of time to analyze the primary end points of our study (short-term postoperative surgical site, systemic, and overall morbidity), but would not permit the evaluation of long-term morbidity (eg, radiation-induced fibrosis and long-term reconstruction failure rates). Moreover, because the ACS-NSQIP database does not contain data regarding specific tumor characteristics, neoadjuvant chemotherapeutic agents used, dose of radiation, or breast-specific outcomes relevant to plastic surgery (eg, capsular contracture and esthetic outcomes), these factors could not be included in our multivariable models. Finally, the ACS-NSQIP database reports on radiotherapy administered 90 days before the operation, and our study cohorts were defined based on this criterion. Although this definition may underestimate the number of patients who received NRT, we believe that this limitation is reduced considering that the current practice trend is for patients to undergo surgery 6 weeks post-NRT completion.5 Nevertheless, for the cohort receiving NRT, we were unable to determine the exact timing of radiotherapy (ie, NRT was delivered in the 90 days preceding the surgery, but this could range from 1–12 weeks before the surgery).
CONCLUSIONS
Our study suggests that NRT does not significantly increase 30-day postoperative morbidity in the setting of mastectomy. However, several important questions remain. Future randomized prospective studies are needed to compare locoregional control and survival between NRT and adjuvant radiotherapy. Additionally, these studies should aim to validate our short-term morbidity findings and to explore the long-term morbidity differences between these two treatment modalities. If validated in RCTs, oncologists and breast surgeons should consider including the use of NRT into their management plan to harvest its advantages in the context of limited additional risk of complications.
ACKNOWLEDGMENTS
The coauthors acknowledge the following institutional support: The Johns Hopkins Department of Plastic Surgery receives research support from LifeCell Corp. (Branchburg, NJ). The Johns Hopkins Department of Plastic Surgery receives educational support from LifeCell Corp. (Branchburg, NJ), Sientra Corp. (Santa Barbara, CA), and TEI Biosciences (Boston, MA), which make products that are used in plastic surgery reconstruction procedures.
Supplementary Material
Footnotes
Presented at the American Society for Reconstructive Microsurgery 2014 Annual Meeting, Kauai, HI, January 8–14, 2014, and at the Plastic Surgery Research Council 59th Annual Meeting, New York City, NY, March 7–9, 2014.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Popek S, Tsikitis VL. Neoadjuvant vs adjuvant pelvic radiotherapy for locally advanced rectal cancer: which is superior? World J Gastroenterol. 2011;17:848–854.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon N, Zhuge Y, Cheung M, et al. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol. 2010;17:791–803.. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18:1927–1934.. [DOI] [PubMed] [Google Scholar]
- 4.Bourgier C, Calvo FA, Marsiglia H, et al. Overview of preoperative radiochemotherapy in breast cancer: past or future? Clin Transl Oncol. 2011;13:446–450.. [DOI] [PubMed] [Google Scholar]
- 5.Tansley P, Ramsey K, Wong S, et al. New treatment sequence protocol to reconstruct locally advanced breast cancer. ANZ J Surg. 2013;83:630–635.. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarthy AB, Kelley MC, McLaren B, et al. Neoadjuvant concurrent paclitaxel and radiation in stage II/III breast cancer. Clin Cancer Res. 2006;12:1570–1576.. [DOI] [PubMed] [Google Scholar]
- 7.Bollet MA, Sigal-Zafrani B, Gambotti L, et al. ; Institut Curie Breast Cancer Study Group. Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: results of a phase II study. Eur J Cancer. 2006;42:2286–2295.. [DOI] [PubMed] [Google Scholar]
- 8.Matuschek C, Bölke E, Roth SL, et al. Long-term outcome after neoadjuvant radiochemotherapy in locally advanced noninflammatory breast cancer and predictive factors for a pathologic complete remission: results of a multivariate analysis. Strahlenther Onkol. 2012;188:777–781.. [DOI] [PubMed] [Google Scholar]
- 9.Bondiau PY, Courdi A, Bahadoran P, et al. Phase 1 clinical trial of stereotactic body radiation therapy concomitant with neoadjuvant chemotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2013;85:1193–1199.. [DOI] [PubMed] [Google Scholar]
- 10.Touboul E, Buffat L, Lefranc JP, et al. Possibility of conservative local treatment after combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer. Int J Radiat Oncol Biol Phys. 1996;34:1019–1028.. [DOI] [PubMed] [Google Scholar]
- 11.Calitchi E, Kirova YM, Otmezguine Y, et al. Long-term results of neoadjuvant radiation therapy for breast cancer. Int J Cancer. 2001;96:253–259.. [DOI] [PubMed] [Google Scholar]
- 12.Darai E, Mosseri V, Hamelin JP, et al. [Conservative surgery after radiotherapy with preoperative doses in the treatment of breast cancer]. Presse Med. 1991;20:2144–2148.. [PubMed] [Google Scholar]
- 13.Semiglazov VF, Topuzov EE, Bavli JL, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol. 1994;5:591–595.. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J. Radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:406–407.. [DOI] [PubMed] [Google Scholar]
- 15.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997;42:99–106.. [DOI] [PubMed] [Google Scholar]
- 16.Roth SL, Audretsch W, Bojar H, et al. Retrospective study of neoadjuvant versus adjuvant radiochemotherapy in locally advanced noninflammatory breast cancer: survival advantage in cT2 category by neoadjuvant radiochemotherapy. Strahlenther Onkol. 2010;186:299–306.. [DOI] [PubMed] [Google Scholar]
- 17.Khuri SF, Henderson WG, Daley J, et al. ; Principal Investigators of the Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery Study. Ann Surg. 2008;248:329–336.. [DOI] [PubMed] [Google Scholar]
- 18.Sarhane KA, Flores JM, Cooney CM, et al. Preoperative anemia and postoperative outcomes in immediate breast reconstructive surgery: a critical analysis of 10,958 patients from the ACS-NSQIP database. Plast Reconstr Surg Glob Open. 2013;1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont WD, Plummer WD., Jr. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601.. [DOI] [PubMed] [Google Scholar]
- 20.Gérard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg. 1988;208:606–614.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlberg M, Glimelius B, Påhlman L. Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Ann Surg. 1999;229:493–497.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Gijn W, Marijnen CA, Nagtegaal ID, et al. ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582.. [DOI] [PubMed] [Google Scholar]
- 23.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223.. [DOI] [PubMed] [Google Scholar]
- 24.Lebwohl B, Ballas L, Cao Y, et al. Treatment interruption and discontinuation in radiotherapy for rectal cancer. Cancer Invest. 2010;28:289–294.. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892.. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub JL, Kahn DM. The timing of implant exchange in the development of capsular contracture after breast reconstruction. Eplasty. 2008;8:e31. [PMC free article] [PubMed] [Google Scholar]
- 27.Chang EI, Ly DP, Wey PD. Comparison of aesthetic breast reconstruction after skin-sparing or conventional mastectomy in patients receiving preoperative radiation therapy. Ann Plast Surg. 2007;59:78–81.. [DOI] [PubMed] [Google Scholar]
- 28.Hultman CS, Daiza S. Skin-sparing mastectomy flap complications after breast reconstruction: review of incidence, management, and outcome. Ann Plast Surg. 2003;50:249–255.; discussion 255. [DOI] [PubMed] [Google Scholar]
- 29.Selber JC, Kurichi JE, Vega SJ, et al. Risk factors and complications in free TRAM flap breast reconstruction. Ann Plast Surg. 2006;56:492–497.. [DOI] [PubMed] [Google Scholar]
- 30.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713–721.. [DOI] [PubMed] [Google Scholar]
- 31.Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117:359–365.. [DOI] [PubMed] [Google Scholar]


