Abstract
Background:
Melanoma is a rare neoplasm in the pediatric population. Recent publications suggest a possible increase in incidence over the past few decades. The purpose of this study was to analyze trends in pediatric patients diagnosed with malignant melanoma in British Columbia (BC) in the past 35 years.
Methods:
A retrospective review was performed. All patients in BC diagnosed with melanoma before 18 years of age from 1979 to 2014 were included. Patient demographics, melanoma description, treatment details, and survival data were collected.
Results:
Seventy-eight subjects were identified for the study. Patients were equally distributed by sex. Sixty-one (78%) of the subjects were diagnosed in the postpubertal age (≥12 years old). The most common sites of occurrence were the extremities (n = 33) and the trunk (n = 27), with the location on the trunk showing the highest mortality rate (22%). All patients were surgically treated and some had additional chemotherapy (12) and/or radiotherapy (12). Fatal outcome was recorded in 12 of the 78 subjects, 10 of whom had postpubertal diagnosis. The average time from date of diagnosis to date of death was 9.3 years.
Conclusions:
The incidence of melanoma in the pediatric population remains exceedingly rare: less than 2.5 per million children younger than 18 years. The diagnosis is rarely made before puberty; the incidence is equal in males and females and has not changed over a 35-year time period in BC. Our study shows 85% survival with the majority of patients having had surgical excision only.
INTRODUCTION
Melanoma is a rare diagnosis in the pediatric population accounting for 3% of all pediatric cancers1; approximately 2% of all melanomas are reported in patients under 20 years of age and 0.3–0.4% of these occur in the prepubertal group (less than 12 years of age).2 Due to its rarity and diagnostic challenges, pediatric patients may be expected to present with poorer prognostic features.3 Melanoma may be atypical in children and does not always follow the ABCD (asymmetry, border irregularity, color variability, and diameter > 6 mm) diagnostic criteria that are indicative in adult cases.4 Cordoro et al.5 concluded that 60% of children 0–10 years old and diagnosed with melanoma did not meet the common melanoma detection criteria but most often presented with amelanosis, bleeding, raised papulonodular primary lesions, uniform color, variable diameter, and de novo development.
Risk factors for childhood melanoma include congenital nevi, dysplastic nevi, and familial atypical nevus syndrome.6 Total body nevus count is thought to be an important risk factor.7,8 Children with xeroderma pigmentosum or those receiving immunosuppressive therapy are known to also be at greater risk.9 Regardless of risk factors, primary melanoma arises directly from melanocyte transformation or in precursor lesions.10
Precursor lesions are of great importance to plastic surgeons because they are often the subjects of consultation, especially congenital nevi. The likelihood that a particular nevus will develop into melanoma may be a deciding factor in its removal.
Melanomas in children may have the capacity to grow more rapidly than those in adults and to metastasize widely and precipitate death.4 Benign lesions like Spitz nevi have been confused with melanoma11–13 and may delay correct diagnosis. In any malignancy, prognosis is more encouraging when disease is treated early but less optimistic when patients present with advanced disease.3
This study analyzes melanoma diagnosed in childhood in British Columbia (BC) over a 35-year period. The province’s population-based cancer registry allowed us to document true incidence and outcome. Secondarily, we hoped to increase awareness of the existence of pediatric melanoma to improve early diagnosis, treatment, and prognosis when it does appear.
METHODS
We received approval and a waiver of consent for this retrospective study (UBC C&W Research Ethics Board H14-01234).
Eligibility Criteria
Eligible subjects were younger than 18 years when diagnosed with melanoma in BC from May 1, 1979, to April 30, 2014. The definition of melanoma used in this study was based on the International Classification of Diseases for Oncology codes 8720–8790. Data were collected from 3 sources: the BC Cancer Agency (BCCA) database, the BC Children’s Hospital (BCCH) Discharge Database, and the BCCH Oncology Database.
Database Descriptions
BCCA is the agency responsible for the BC Cancer Registry, which collects data and provides cancer statistics for the BC population and serves as a source of information for research. BC has legislation in place that makes the reporting of cancer to the BC Cancer Registry mandatory.14 We had access to the data collected up to 2012.
The BCCH Discharge Database records data from charts of patients who have undergone treatment at BCCH. Data were available from 1984 onward.
The BCCH Oncology Database specifically includes only (and all) patients treated in the hospital’s Division of Oncology since 1997; this gave us more detailed information for certain patients.
Eligible patients were identified if they appeared in any of these databases. Their data were compiled for analysis and duplicates were merged.
Data and Analysis
Data collection included patient demographics, details of diagnosis, treatment administered, and outcome. The data were analyzed with descriptive statistics. Statistically significant differences between the incidence of melanoma in prepubertal versus postpubertal age were tested by Pearson chi-square test, and the difference between the subgroups was tested by Fisher exact probability test.
RESULTS
Seventy-five cases were identified from the BCCA database, 20 from the BCCH Discharge Database, and 6 from the BCCH Oncology Database. After eliminating duplicates and ineligible subjects (nonmelanoma), a cohort of 78 subjects remained. Characteristics of the patient population (n = 78) can be found in Table 1. Ethnicity data were not recorded in any of the databases.
Table 1.
Patient Characteristics
The number of new pediatric melanoma cases diagnosed in children aged 0–17 years averaged 1.97 per year during the study period in the province of BC. According to Population BC statistics, the mean population of children 0–17 years in BC during the study period was estimated to be 846,425, making the incidence of melanoma 2.36 per 1,000,000 per year. No gross increase in incidence in pediatric melanoma was observed over the study period with 40 patients diagnosed in the first half and 38 in the second half (Fig. 1). In the same period of time, melanoma was diagnosed in 20,975 of the BC adult population (estimated 3,111,330), accounting for an incidence of 193 per 1,000,000 per year. Therefore, cases of melanoma diagnosed in children represent 0.37% of melanoma incidence overall. Reassuringly, prepubertal cases in this study represent a mere 0.08% of all melanomas diagnosed in BC.
Fig. 1.
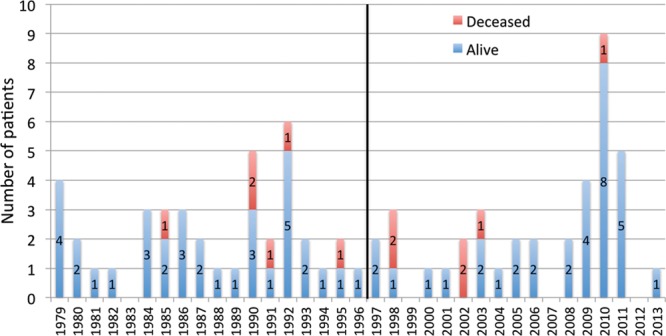
Pediatric patient status (alive or deceased) with reference to the year of diagnosis.
The mean age at diagnosis was 13.3 years, with 17 children (21.8%) diagnosed at age 11 or younger (our proxy for prepubertal age) and 61 children (78.2%) at 12–17 years (postpubertal). The comparison of the incidence of pre- and postpubertal melanoma cases (17 and 61) calculated from the same-age population in BC (547,416 and 299,009, respectively) showed statistically significantly lower incidence in the prepubertal (0.09 per 100,000 per year) versus postpubertal (0.58 per 100,000 per year) cases (P < 0.0001). There were 6 cases of prepubertal melanoma in the first half of the examined time frame (1979–1996) and 11 cases in the second half (1997–2014). In the postpubertal group, 34 cases occurred in the first half and 27 cases occurred in the latter half of the study period. Although the number of prepubertal melanoma almost doubled in the second half of the study period, there was no statistically significant difference in incidence compared with the postpubertal group (P = 0.11).
There was no difference in incidence by sex (M = 38, F = 40) within the study group. The mean pediatric male population over the study period in BC was 435,766 and female 410,659, so there was also no difference found in population-based melanoma incidence (P = 0.71). Similarly, the sex distribution was balanced in the prepubertal (M = 9, F = 8) and the postpubertal group (M = 29, F = 32). The M:F ratio according to the sites of primary occurrence was extremities 13:20, trunk 14:13, face and scalp 8:2, and ocular 2:5, with no statistically significant difference (P = 0.10).
Of the 12 deaths that occurred in the study population, all were attributed to melanoma and all but 1 occurred in children diagnosed at 11 years of age or older (Fig. 2). Ten were a result of cutaneous melanoma, 1 of ocular melanoma, and 1 was a result of primary melanoma of the central nervous system (CNS). The latter case was a prepubertal patient with neurocutaneous melanosis (but with only a CNS component), which transformed into melanoma and a fatal outcome 3 months after diagnosis. Excluding this patient, the mean time between the date of diagnosis and the date of death was 9 years and 4 months, with a minimum of 1 year and 6 months and a maximum of 19 years and 11 months. With this lag time, assuming the death rate remains the same, we may predict up to 2 further fatalities among the cases diagnosed within the past 11 years. The locations of melanoma were extremities (n = 33), trunk (n = 27), face/scalp (n = 10), ocular (n = 7), and CNS (n = 1; Fig. 3). Of the cutaneous lesions, melanoma occurring on the trunk resulted in the highest mortality rate (22.2%; Fig. 4).
Fig. 2.
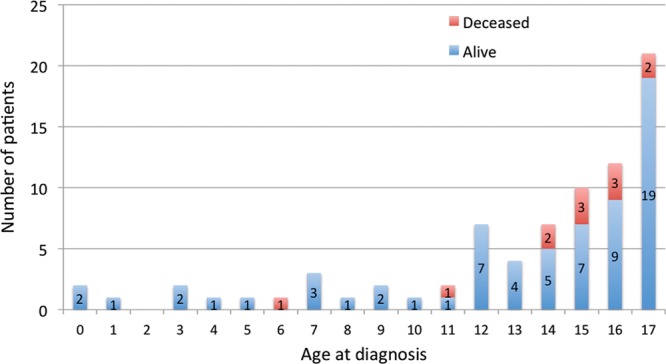
Current patient status (alive or deceased) with reference to their age at diagnosis with melanoma.
Fig. 3.
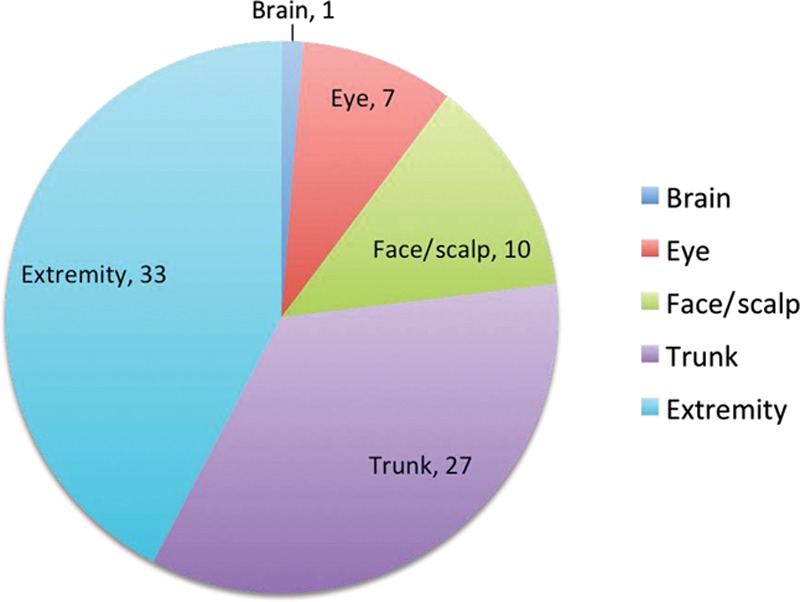
Sites of primary occurrence.
Fig. 4.
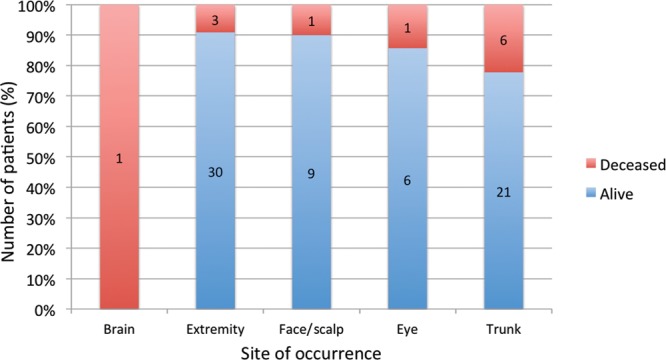
Patient status with reference to site of occurrence.
The most common histotypes reported at diagnosis in this population are outlined in Figure 5. Ninety percent of the superficial spreading melanomas occurred in the postpubertal age group. In 36 cases, melanoma histotype was unspecified (not otherwise specified [NOS]); we did not have the ability to request review of the pathological slides. One case of malignant transformation within a congenital nevus was documented, but we cannot assume that there are no congenital nevi within the NOS group. Unfortunately, the clinical provenance of melanoma was not included comprehensively in any of the databases.
Fig. 5.
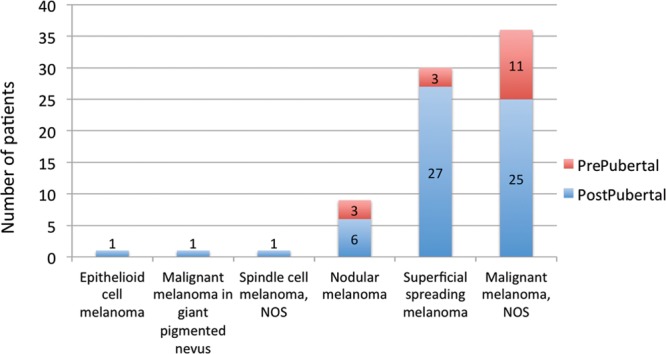
Classification of melanoma at diagnosis, according to International Classification of Diseases for Oncology, 3rd edition.
Treatment beyond excisional biopsy included chemotherapy, radiotherapy, further surgery, or a combination thereof (Fig. 6). Eight patients treated with chemotherapy and 7 treated with radiotherapy eventually died.
Fig. 6.
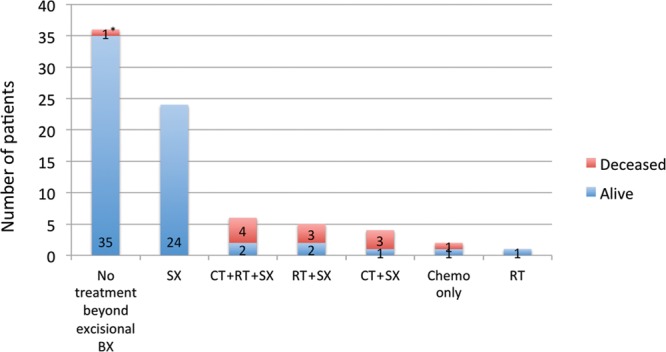
Treatment beyond excisional biopsy. CT, chemotherapy; RT, radiotherapy; SX, further surgery). *This patient was a 6-year-old child with neurocutaneous melanosis, which transformed into malignant melanoma of the CNS resulting in a fatal outcome 3 months after the diagnosis.
Staging at diagnosis, sentinel node biopsy, and excisional biopsy margin data were limited or unavailable for analysis.
The distribution of melanoma across different regions of BC does not seem representative of the relative population size in each location. The incidence in children aged 0 to 17 years per region ranged from 0.8 to 28.4 per million (Fig. 7).
Fig. 7.
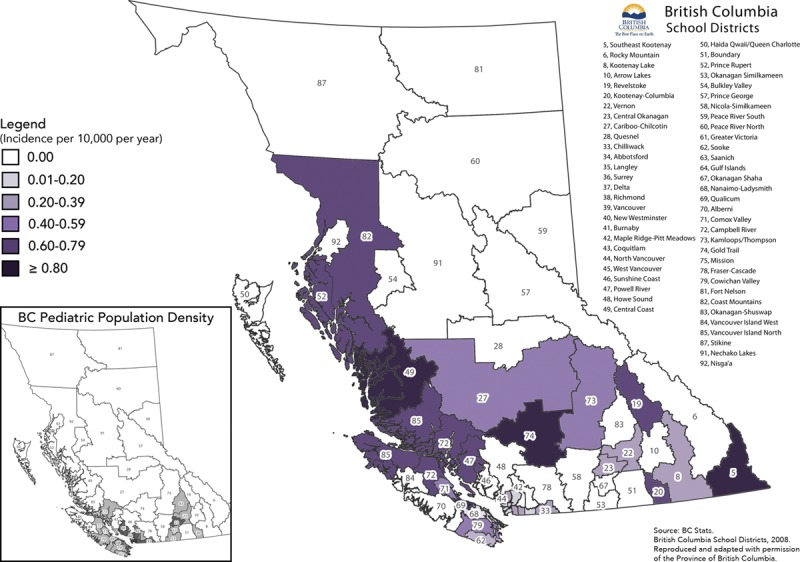
Distribution of pediatric melanoma in BC. Source: BC Stats. British Columbia School Districts, 2008. Reproduced and adapted with permission of the Province of British Columbia. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
DISCUSSION
This is the first review of pediatric melanoma in BC. Reviews of this kind performed in other geographic locations (the United States,3,15 Sweden,16 and Australia17) have shown increases over time in the incidence of pediatric melanoma. Over a 35-year time period in BC, we found no trend toward increasing incidence and also found that the number of cases remained exceedingly small. Many surgeons have referred patients with worrisome skin lesions, but it may be reassuring to note that in our population, melanoma arising in childhood (1 in ~425,000 children) is an order of magnitude less frequent than the rarest of rare inborn errors of metabolism.
Similarly, we could not determine a relationship between the calendar year of patient’s diagnosis and his or her current status. The 11 diagnoses that eventually resulted in death occurred evenly in the first and second half of the 35-year time span. There is a lag time averaging 9 years from diagnosis to death, which prevents us from forming conclusions about improvement in prognosis.
Contrary to results found in the literature,16,18,19 our population showed no significant sex-related trends in incidence. Although 20 of 33 cases (60%) occurring on the extremities (upper and lower limbs) presented in female patients, this was not statistically significant. However, the trend of these data may agree with the findings by Strouse et al.3 who attribute this to the practice of sunbathing or indoor tanning popular among young females.
In the time period examined, pediatric cases of melanoma represent 0.37% of the melanoma cases overall with the prepubertal cases making up only 0.08%. These values are lower than others published in the literature (2% and 0.3–0.4%, respectively).2,20–24 This could be attributable to our study location having a greater distance from the equator or compared with countries with similar latitudes, a population with greater geographic or ethnic variability. Increased ultraviolet exposure is a well-established risk factor for developing melanoma in adults25; all patients in this study resided within the latitude range 48.42–54.52, and this area would be expected to experience less ultraviolet exposure than a population examined in the United States or Australia but not Sweden. In a pediatric population, particularly the prepubertal group, it may seem obvious that environmental exposures may be less of a contributing risk factor, although childhood may also be the time of most frequent outdoor activity. In the development of benign nevi, sunlight has a prominent role, but their formation is also under genetic control.26,27 A recent twin study in adults suggests that total body nevus count may be the best predictor of melanoma risk (the nevus count on 1 arm was used to estimate the total body nevus count) and can be used to estimate melanoma risk in general practice.31 We do not have the nevus count data of our patient population, nor do we have conclusive evidence that nevus count related to age in pediatric patients is correlated with pediatric melanoma. This may be a focus for further study.
Twelve children died from melanoma over the time period. Of the 10 deaths that were attributed to cutaneous melanoma, 9 occurred in what we have assigned as the postpubertal age group; however, because the overall numbers of diagnoses were higher postpubertally, the mortality rate was not statistically significantly different. We chose 11 years as a proxy indicator of the end of prepuberty, but we cannot confirm that the patient diagnosed at age 11, and therefore included in our prepubertal group, was in fact prepubertal physiologically. Significantly, there have been no deaths from cutaneous melanoma diagnosed before age 11.
Malignant melanoma is second only to thyroid cancer in the types of adult cancers seen in children.29 Melanoma in the postpubertal age group tends to act like adult melanoma30 and may be more likely to metastasize, resulting in poorer prognosis and increased fatality. We cannot conclude that treatment in the younger group may be more curative, but we also cannot rule this out. Excision of a suspicious nevus is recommended regardless of relationship to puberty, but after puberty, hormonal changes are thought to enhance development of melanoma.31 We were unable to glean from any of the databases the denominator of cases arising from congenital nevi.
The increased incidence of melanoma in certain geographical areas may not be truly representative due to the small population size. Our study was unable to conclude anything about the relationship between patients and geographical location or the ethnicity of patient or regional populations.
Of the 18 patients who received radiation and/or chemotherapy, 11 (61%) died. It is likely that the patients who were given these forms of treatment presented with a more advanced form of the disease and had a poorer prognosis even at the time of diagnosis. The small number of patients who received chemotherapy all received individually different protocols; no conclusions can be drawn about effectiveness of treatment.
As an illustrative case of prepubertal melanoma and course of treatment, 1 of the patients in this study was diagnosed and treated for melanoma at the age of 3. The male subject presented with 2 congenital nevi at birth: 1 on the left scalp (dime sized and light brown in color with dark middle area) and another on the right inguinal region (light brown in color). At 6 months, the scalp lesion began to grow in height. Trauma to the area would occasionally cause bleeding and the wounds that resulted would often have healing delay. He was seen by a plastic surgeon in his community at the age of 20 months who felt the lesion was likely an atypical hemangioma and that there was a low risk of malignancy. As time went on and ulceration became more persistent, the possibility of neoplasia was entertained. At the age of 3 years and 9 months, the lesion was biopsied with narrow margins by the same plastic surgeon and the pathologist thought it was a spitz nevus with unusual features but could not rule out melanoma. A review of the slide by a quaternary dermatopathologist changed its diagnosis to melanoma, nodular type, 4.5-mm depth, and Clark level IV with ulceration. A referral to a BCCH pediatric plastic surgeon was made and wide local excision (2 cm) with sentinel lymph node biopsy (jugular chain) and skin graft reconstruction was undertaken within 9 days of referral. The wide excision pathology reported no residual lesion in the specimen, and all 3 nodes were negative for metastatic melanoma. PET-CT and CT scan of lungs showed no evidence of distant disease, and a follow-up PET-CT 2 years later also was negative. The congenital nevus of the inguinal region was also completely excised with narrow margins but showed no evidence of malignancy. The patient was followed up by an oncologist but given no ancillary treatment and had no further recurrence of the melanoma as of the age of 6 when he moved from the province. In this case, treatment was provided by the appropriate specialists and administered in a timely manner once enough suspicion was acquired, but there was a significant lag time to initial biopsy that may benefit from education about how these malignancies may present so differently in young children.
Prompt treatment has been shown to be important in melanoma in general. Because pediatric melanoma may not always present the same way as it does in adults, it is important to be on the lookout for unusual presentations beyond the ABCD diagnostic criteria that are used in an adult clinical setting. Specific ABCD diagnostic characteristics have even been suggested for melanoma in children: Amelanotic; Bleeding, Bump; Color uniformity; De novo, any Diameter.5 Suspicion of melanoma warrants referral to a specialist for biopsy; in younger children, a pediatric specialist may have easier access to pediatric anesthesia and imaging, if required, and other specialist follow-up.
LIMITATIONS
This was a retrospective study limited by the scope of data collected in the BCCA database. Overall death rate might be underrepresented due to the lag time between diagnosis and eventual death (mean of 9 years and 4 months). Applying the mean expected death rate over the past 11 years of data, we may expect another 2 fatalities in our cohort. Pathologic diagnosis of melanoma in children is sometimes controversial, and many specimens are sent for second pathology opinions. We do not have access to data that reveal the extent of any controversial diagnoses.
CONCLUSIONS
Melanoma in British Columbian children remains exceedingly rare with an incidence of 2.36 per million per year in ages 17 and under. Within the group, incidence is higher in those aged 12 years and over, and in the 35-year study period, no children with cutaneous melanoma died of disease diagnosed before the age of 11. We cannot make conclusions about the risk of melanoma within congenital cutaneous lesions, or their lifetime risk, though considering the prevalence of congenital nevi in the pediatric population and the low rate of melanoma diagnosis, urgent removal of clinically stable congenital nevus may be indicated only rarely. We found no trends showing potential association with ethnicity or latitude. We found no trend in incidence, patient factors, or prognosis over the 35 years of our study.
The presentation of melanoma in younger patients may be atypical and not high on the list of differential diagnoses of unusual lesions in children, even to plastic surgeons. One of the goals of this study was to bring not only awareness of the existence of pediatric melanoma to primary healthcare providers but also reassurance that the diagnosis remains rare.
ACKNOWLEDGEMENT
The authors would like to thank Iris Liu for her work on the British Columbia School District Image adaptation (Figure 7).
Footnotes
This article has been presented as an oral presentation at the American Federation for Medical Research Western Regional Meeting in Carmel, California (January 2015) and at the Canadian Society of Plastic Surgeons 69th Annual Meeting (June 2015).
Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Han D, Zager JS, Han G, et al. The unique clinical characteristics of melanoma diagnosed in children. Ann Surg Oncol. 2012;19:3888–3895.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari A, Bono A, Baldi M, et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649–654.. [DOI] [PubMed] [Google Scholar]
- 3.Strouse JJ, Fears TR, Tucker MA, et al. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741.. [DOI] [PubMed] [Google Scholar]
- 4.Mones JM, Ackerman AB. Melanomas in prepubescent children: review comprehensively, critique historically, criteria diagnostically, and course biologically. Am J Dermatopathol. 2003;25:223–238.. [DOI] [PubMed] [Google Scholar]
- 5.Cordoro KM, Gupta D, Frieden IJ, et al. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol. 2013;68:913–925.. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Wendtner MH, Berking C, Baumert J, et al. Cutaneous melanoma in childhood and adolescence: an analysis of 36 patients. J Am Acad Dermatol. 2002;46:874–879.. [DOI] [PubMed] [Google Scholar]
- 7.Garbe C, Büttner P, Weiss J, et al. Associated factors in the prevalence of more than 50 common melanocytic nevi, atypical melanocytic nevi, and actinic lentigines: multicenter case-control study of the Central Malignant Melanoma Registry of the German Dermatological Society. J Invest Dermatol. 1994;102:700–705.. [DOI] [PubMed] [Google Scholar]
- 8.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44.. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–250.. [DOI] [PubMed] [Google Scholar]
- 10.Mills O, Messina JL. Pediatric melanoma: a review. Cancer Control. 2009;16:225–233.. [DOI] [PubMed] [Google Scholar]
- 11.Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, ‘Spitzoid melanoma’ and risk assessment. Mod Pathol. 2006;19(suppl 2):S21–S33.. [DOI] [PubMed] [Google Scholar]
- 12.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–520.. [DOI] [PubMed] [Google Scholar]
- 13.Barnhill RL, Flotte TJ, Fleischli M, et al. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer. 1995;76:1833–1845.. [DOI] [PubMed] [Google Scholar]
- 14.von Tigerstrom B, Ries NM. Cancer surveillance in Canada: analysis of legal and policy frameworks and tools for reform. Health Law J. 2009;17:1–49.. [PubMed] [Google Scholar]
- 15.Austin MT, Xing Y, Hayes-Jordan AA, et al. Melanoma incidence rises for children and adolescents: an epidemiologic review of pediatric melanoma in the United States. J Pediatr Surg. 2013;48:2207–2213.. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson P, Boeryd B, Sander B, et al. Increasing incidence of cutaneous malignant melanoma in children and adolescents 12-19 years of age in Sweden 1973-92. Acta Derm Venereol. 1998;78:289–292.. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman D, Valery P, McWhirter W, et al. Incidence of cutaneous childhood melanoma in Queensland, Australia. Int J Cancer. 1995;63:765–768.. [DOI] [PubMed] [Google Scholar]
- 18.Averbook BJ, Lee SJ, Delman KA, et al. Pediatric melanoma: analysis of an international registry. Cancer. 2013;119:4012–4019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berk DR, LaBuz E, Dadras SS, et al. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults--the Stanford experience 1995–2008. Pediatr Dermatol 2010;27:244–254.. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Maldonado R, Orozco-Covarrubias ML. Malignant melanoma in children. A review. Arch Dermatol. 1997;133:363–371.. [PubMed] [Google Scholar]
- 21.Bader JL, Li FP, Olmstead PM, et al. Childhood malignant melanoma. Incidence and etiology. Am J Pediatr Hematol Oncol. 1985;7:341–345.. [PubMed] [Google Scholar]
- 22.Boddie AW, Jr, Smith JL, Jr, McBride CM. Malignant melanoma in children and young adults: effect of diagnostic criteria on staging and end results. South Med J. 1978;71:1074–1078.. [DOI] [PubMed] [Google Scholar]
- 23.Conti EM, Cercato MC, Gatta G, et al. ; EUROCARE Working Group. Childhood melanoma in Europe since 1978: a population-based survival study. Eur J Cancer. 2001;37:780–784.. [DOI] [PubMed] [Google Scholar]
- 24.Saenz NC, Saenz-Badillos J, Busam K, et al. Childhood melanoma survival. Cancer. 1999;85:750–754.. [DOI] [PubMed] [Google Scholar]
- 25.Bauer J, Garbe C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297–306.. [DOI] [PubMed] [Google Scholar]
- 26.Bataille V, Snieder H, MacGregor AJ, et al. Genetics of risk factors for melanoma: an adult twin study of nevi and freckles. J Natl Cancer Inst. 2000;92:457–463.. [DOI] [PubMed] [Google Scholar]
- 27.Wachsmuth RC, Turner F, Barrett JH, et al. The effect of sun exposure in determining nevus density in UK adolescent twins. J Invest Dermatol. 2005;124:56–62.. [DOI] [PubMed] [Google Scholar]
- 28.Ribero S, Zugna D, Osella-Abate S, et al. Prediction of high naevus count in a healthy U.K. population to estimate melanoma risk. Br J Dermatol. 2016;174:312–318.. [DOI] [PubMed] [Google Scholar]
- 29.Rao BN, Hayes FA, Pratt CB, et al. Malignant melanoma in children: its management and prognosis. J Pediatr Surg. 1990;25:198–203.. [DOI] [PubMed] [Google Scholar]
- 30.Coit DG, Ernstoff MS, Busam KJ. Is pediatric melanoma always malignant? Cancer. 2013;119:3910–3913.. [DOI] [PubMed] [Google Scholar]
- 31.Pack GT, Gerber DM, Scharnagel IM. End results in the treatment of malignant melanoma; a report of 1190 cases. Ann Surg. 1952;136:905–911.. [DOI] [PMC free article] [PubMed] [Google Scholar]


