Supplemental Digital Content is available in the text.
Abstract
Background:
Breast capsular contracture remains an elusive problem faced by plastic surgeons and is the leading long-term complication after breast implantation. Follistatin (Fst) is a protein with known anti-inflammatory and antifibrotic properties and has the potential to limit the severity of diseases associated with inflammation and fibrosis such as capsular contracture. The aim of this study was to examine the effect of Fst288 on capsular fibrosis around silicone implants in a mouse model.
Methods:
BALB/c mice were implanted subcutaneously with untreated silicone implants (baseline control). In the experimental group, immediately after silicone implant insertion, the implant pocket received either a single injection of 1 µg Fst288 or normal saline (internal control). The animals were killed at 3, 5, 7, 14, 28, and 90 days after surgery, and serum, implants, and the surrounding tissue were removed for histological and immunohistochemical analyses.
Results:
Fst288 treatment resulted in significant decreases in capsule thickness at 28 days (P < 0.05) and 3 months (P < 0.001), decreased collagen production at 14 days (P < 0.05) and 3 months (P < 0.01), decreased angiogenesis at 3 months (P < 0.001), decreased α-smooth muscle actin levels at 3 months (P < 0.05), and a decrease in the number of CD45+ cells at days 5 (P < 0.05) and 7 (P < 0.01), respectively, when compared with control implants.
Conclusions:
A single injection of Fst288 at the time of silicone implant insertion into the mice results in a significant reduction in pericapsular inflammation and capsular fibrosis.
BACKGROUND
Breast capsular contracture remains an elusive problem faced by plastic surgeons and is the leading long-term complication after breast implantation and a major cause of patient dissatisfaction.1–3 Capsular contracture causes the breast to harden and change in shape after breast reconstruction and augmentation using a prosthesis.4,5
Capsular contracture is a multifactorial process with many risk factors6 including breast feeding, pregnancy, increased age, the duration of implants in situ, trauma, thicker capsules, hematoma, infection, radiation therapy, foreign body responses, gel bleed, and seroma formation.7–15
Excessive inflammation and the resulting fibrosis are widely regarded as important causes of capsular contracture,16–20 suggesting that treatments that control the inflammatory response may also reduce capsular contracture rates.16 Various growth factors, capsular characteristics, and inflammatory mediators are implicated in capsular contracture, including excessive myofibroblast activity and collagen deposition, increased capsule thickness, and increased levels of transforming growth factor β (TGFβ), and interleukin (IL) 6, 8, and 17.6,18,21–31 Subclinical infections where bacteria remain dormant within a protective biofilm have also been implicated in capsule formation and contracture32–34 (see figure, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A390).
More recently, proteins, members of the TGFβ family, have been identified as proinflammatory and profibrotic agents and controllers of the inflammatory response (see figure, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A390). These proteins, activin A and B, respond rapidly to a lipopolysaccharide inflammatory challenge35 and are causative agents in bleomycin-induced fibrosis.36 Fst, a naturally occurring protein, binds irreversibly to activin A and B, leading to lysosomal degradation of these proteins.37 The capacity of Fst to bind activins and attenuate their inflammatory and fibrotic actions has established Fst as a potential therapeutic agent for limiting inflammation and fibrosis associated with capsular contracture36,37 (see figure, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A390). We propose that administration of Fst at the time of breast implant insertion has the possibility of decreasing the inflammatory response and thereby reducing pericapsular fibrosis by reducing extracellular matrix (ECM) formation.38–40 The aim of this study was to explore the anti-inflammatory and antifibrotic effects of Fst on the pathophysiological processes that occur after the implantation of silicone implants.
MATERIALS AND METHODS
Animal Studies
All experiments were approved by the Monash Medical Centre Animal Ethics Committee and observed the Australian Code of Practice for the Care and the Use of Animals for Scientific Purposes. The experiments used 8-week-old male BALB/c mice housed in the B-Block Animal House, Monash Medical Centre (MMCA/2012/36).
Silicone Implants
A 3 × 3 cm sheet of smooth silicone (Mentor Corp, Santa Barbara, CA) was sterilized with 80% alcohol. Five-millimeter discs (n = 224) weighing 8 mg were created using 5-mm biopsy punches (Kai Medical, Solingen, Germany).
Surgical Technique
Mice were anesthetized using an intraperitoneal injection of ketamine (100 mg/mL)/xylazine (20 mg/mL) mixture (Sigma-Aldrich, St. Louis, MO) (0.1 ml/10 g). Under anesthetic, each mouse was weighed, shaved, skin prepped with 80% (vol/vol) ethanol, and injected subcutaneously with the long-acting analgesic carprofen (Sigma-Aldrich, St. Louis, MO) (3 mg/kg). Using an aseptic technique, 2 equidistant 5-mm incisions were made through the skin on each flank (4 incisions in total) using fine tenotomy scissors. Incisions were placed approximately 2 cm apart vertically and 3 cm apart horizontally. A subcutaneous pocket ~6 mm in diameter was created using blunt dissection along a plane deep to the dermis. Each pocket received one 5-mm silicone implant. For mice to be euthanized at 14 days or less, incisions were closed using 6/0 nylon suture (Ethicon, Somerville, N.J.). For mice to be euthanized after 14 days, incisions were closed using 6/0 Vicryl suture (Ethicon, Somerville, N.J.). After surgery, mice were placed on a warming pad, allowed to recover from the anesthetic, and then returned to their cages where they were monitored daily with free access to food and water.
Experiment 1—Baseline Group
One untreated 5-mm silicone implant was placed in each subcutaneous pocket. Forty-nine mice were used in this experiment, and implants were removed and assessed at days 0, 3, 5, 7, 14, and 28 and at 3 months (n = 7 mice/group).
Experiment 2—Fst288-treated Group
One untreated 5-mm silicone implant was placed into each subcutaneous pocket. After the incision had been closed to prevent leakage, the pockets on 1 flank received 1 µg of Fst288 (Hudson Institute for Medical Research, Monash University, Melbourne, Australia) in 0.02 mL saline (50 µg/mL), whereas on the opposite flank, the controls received only 0.02 mL of saline into the pocket. Forty-two mice were used in this experiment, and implants were removed and assessed at days 3, 5, 7, 14, and 28, and at 3 months (n = 7 mice/group).
Serum and Tissue Collection
Baseline Group: At days 0, 3, 5, 7, 14, and 28, and at 3 months postinsertion, mice (n = 7/group) were weighed and anesthetized using ketamine/xylazine (0.1 mL/10 g) as described previously. A blood sample was taken by cardiac puncture (26 gauge needle), and mice were euthanized by cervical dislocation. Each implant was removed inside a 1.5 cm × 1.5 cm block of tissue containing the tissue layers from epidermis to subcutaneous muscle. One specimen from each flank was fixed in 10% (vol/vol) buffered formalin for histology, and the remaining 2 specimens from opposite flanks were snap-frozen on dry ice and stored at -80°C for protein extraction and enzyme-linked immunosorbent assay (ELISA) analysis. Blood samples were allowed to clot and were centrifuged at 3,200 rpm for 30 minutes, and the serum was isolated and stored at -80°C for analysis.
Fst288-Treated Group: These mice (n = 7/group) were treated as for the baseline group described above, at days 3, 5, 7, 14, and 28, and at 3 months postinsertion.
Tissue Processing and Histological Analysis
Formalin-fixed tissue with silicone implants in situ were placed in cassettes and embedded in wax using standard procedures (Histology Facility, Hudson Institute of Medical Research, Monash Medical Centre). Tissue blocks were sectioned transversely to produce 5-μm sections that were stained with hematoxylin and eosin for standard histological analysis.20 Masson trichrome stain was used to distinguish collagen and elastin.29,41,42
Immunohistochemistry Analysis
CD45 (leukocyte common antigen), a marker commonly used to detect inflammation, was used.43 To detect CD45, 5-µm sections were placed on Menzel-Glaser Polysine slides (Thermo Scientific, Waltham, Mass.) and stained using an automated system (DAKO Autostainer Plus, Dako, CO) at room temperature.
α-smooth muscle actin (α-SMA), a common marker used to detect differentiated myofibroblasts,20 was used to identify these cells. α-SMA was detected in 5-µm sections that were attached to Menzel-Glaser Polysine slides (Thermo Scientific) and stained at room temperature using an automated system (DAKO Autostainer Plus).
Histological Measurements
To prevent bias, all histological assessments were undertaken without the assessor being aware of from which treatment group the slides were obtained. Histological sections were scanned using Aperio ScanScope AT-Turbo Scanner (Aperio, Calif.), and the scanned images were analyzed using Aperio imagescope software (Aperio, Calif.). Systematic random sampling was used to determine the microscopic fields to be assessed, and 30 random fields were analyzed per sample.
ECM formation: Collagen content was measured by calculating the percentage of Masson trichrome–positive tissue (stained blue) within the peri-implant capsular tissue per high-powered field at 40× magnification.20
Capsule thickness: Capsule thickness (micrometer) was calculated by direct visual measurement of the distance between the outer layer of the capsule and the inner layer of the capsule using the ruler tool in Aperio imagescope software at 40× magnification.44
Angiogenesis: The number of blood vessels/section was counted under direct view using Masson trichrome stain at 40× magnification and averaged.45
Serum Cytokine Analysis
Serum samples were assayed for Fst, activin A, and IL-6 using standard ELISA (Monash Institute of Medical Research) as outlined below.
Immunoassays
Activin A assay—Activin A was measured using a specific ELISA46 according to the manufacturer’s instructions (Oxford Bio-Innovations, Cherwell, Oxfordshire, UK) with some modifications as described by O’Connor et al.47
Fst288 assay—Fst288 was measured using a discontinuous radioimmunoassay, which detects “total” Fst288.47 Immune complex precipitation was achieved using a goat anti-rabbit immunoglobulin secondary antibody.
IL-6 assay—Mouse serum and homogenate samples were diluted in the ratio 1:3 with 10% fetal calf serum in phosphate buffer solution. Duplicates (50 µL) were assayed using the BD OptEIA reagents and protocol supplied by Labsystems (Helsinki, Finland) and the data processed using GenesisLite EIA software (Labsystems, Helsinki, Finland).47
Tissue Cytokine Analysis
Frozen tissue samples were homogenized in 1% (vol/vol) protease inhibitor (Calbiochem, San Diego, CA) using a Janke & Kunkel Ultraturrax T25 homogenizer (IKA Labortechnik, Staufen, Germany). Homogenates were then centrifuged at 4°C to remove debris and assayed for Fst, activin A, and IL-6 using the techniques outlined above.47
Statistical Analysis
For all results, Student’s t test was used to compare the means (Graph Pad, San Diego, CA). Results are expressed as mean ± standard error of the mean. All P values less than 0.05 were considered to indicate a significant difference between groups.
RESULTS
Capsule Thickness Assessed by Histological Techniques
There were no significant differences in implant capsule thickness between the left and right side at any of the time points in the baseline group (Fig. 1A). However, significant reductions in capsule thickness were observed between the Fst288-treated and control groups (Fig. 2) (33.9 ± 2.1 µm, n = 7 versus 44.4 ± 3.4 µm, n = 6 and 40.2 ± 2.9 µm, n = 7 versus 69.5 ± 3.0 µm, n = 6) at days 28 (P = 0.0195) and 90 (P < 0.0001), respectively (Fig. 1B).
Fig. 1.
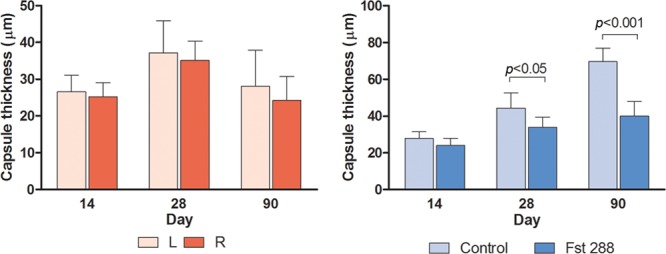
Data obtained using Masson trichrome staining to facilitate measurement of capsule thickness (µm) at days 14, 28, and 90 in (A) baseline group and (B) Fst implant group. Results are expressed as mean ± standard error of the mean.
Fig. 2.
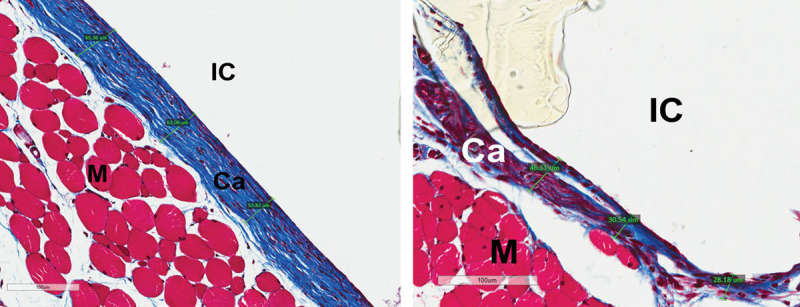
Masson trichrome staining showing capsule thickness (µm) at (A) 3 months, mouse 4, control (20×) and (B) 3 months, mouse 4, Fst 288 (20×). Ca, capsule; IC, implant cavity; M, muscle.
ECM Formation
A significant difference was observed in ECM formation around the implants from left and right sides at day 28 in the baseline group (35.7 ± 5%, n = 7 versus 15.2 ± 3.5%, n = 7) with a mean difference of 20.6 ± 6.6% (P = 0.0085, Fig. 4A). In the Fst implant experiment, there was a significant reduction in the percentage of Masson trichrome–positive tissue at day 14 in the Fst288-treated group compared with the control group (17.9 ± 2.6%, n = 7 versus 28.9 ± 3.6%, n = 7) and at 3 months (39.8 ± 1.8%, n = 7 versus 57.8 ± 3.9%, n = 7) (Fig. 3). The mean differences were 11.1 ± 4.5% (a 38% reduction, P = 0.029) and 17.9 ± 4.3% (a 34% reduction, P < 0.0013), respectively (Fig. 4B).
Fig. 4.
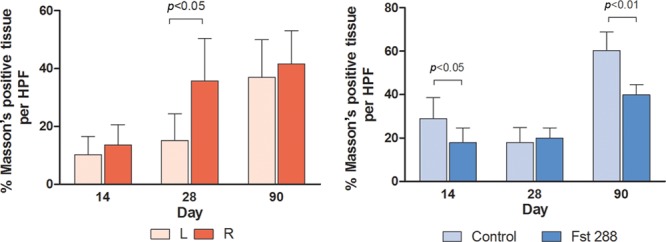
ECM formation (percentage Masson positive tissue per HPF) at days 14, 28, and 90 in (A) baseline group and (B) Fst implant group. Results are expressed as mean ± standard error of the mean. HPF, high-powered field.
Fig. 3.
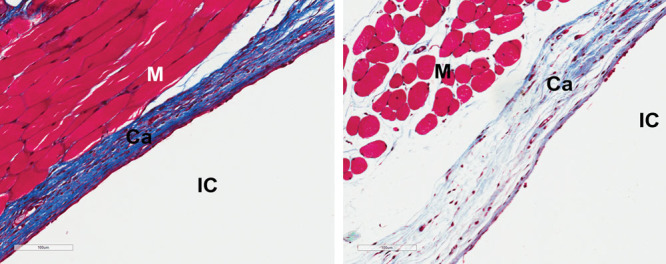
Masson trichrome staining showing ECM production (collagen stained blue) in (A) 3 month, mouse 5, control (20×) and (B) 3 months, mouse 5, Fst 288 (20×). Ca, capsule; IC, implant cavity; M, muscle.
Angiogenesis
There were no significant differences detected in the measurements of angiogenesis between the left and right sides across any of the time points in the baseline group (Fig. 5A). There was, however, a significant reduction in the average number of blood vessels in the Fst288-treated group compared with the control group at 3 months (0.29 ± 0.03, n = 7 versus 0.57 ± 0.04, n = 6). This represents a 50% reduction in angiogenesis (mean difference of 0.28 ± 0.06, P = 0.0005, Fig. 5B).
Fig. 5.
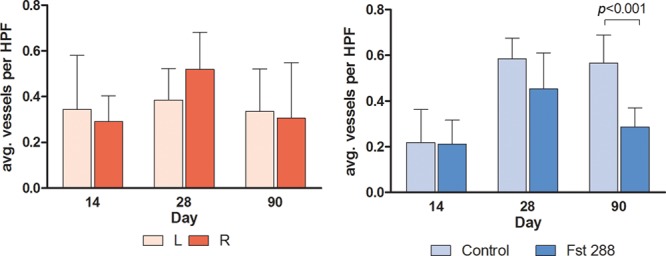
Angiogenesis (average vessels per HPF) at days 14, 28, and 90 in (A) baseline group and (B) Fst implant group. Results are expressed as mean ± standard error of the mean. HPF, high-powered field.
Immunohistochemistry
α-SMA
There were no significant differences in α-SMA–positive tissue between left and right sides in the baseline group (Fig. 6). However, a significant reduction in the percentage of α-SMA–positive tissue was observed between the Fst288-treated group and controls (9.8 ± 1.3%, n = 7 versus 17.0 ± 1.9%, n = 6). This equates to a 43% reduction in α-SMA–positive tissue around the Fst288-treated silicone implants (mean difference of 7.2 ± 2.3%, P = 0.01, Fig. 6).
Fig. 6.
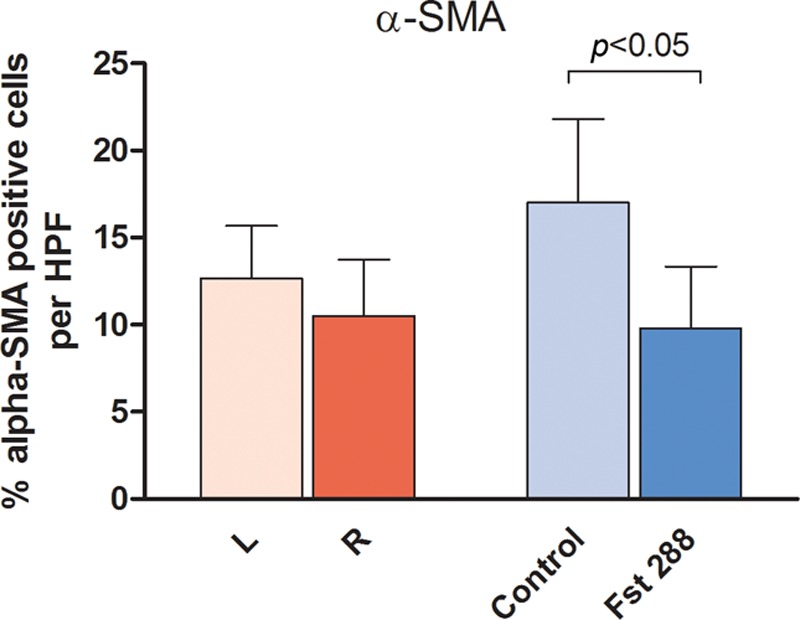
Immunohistochemistry analysis of α-SMA staining at 3 months (percentage of α-SMA–positive tissue per HPF) (A) baseline group and (B) Fst implant group. Results are expressed as mean ± standard error of the mean. HPF, high-powered field.
CD45
CD45-positive cell numbers in the peri-implant tissue were similar between left and right sides in the baseline group at each time point (Fig. 7A). However, a significant reduction was seen in the percentage of CD45-positive cells between the Fst288-treated groups and controls at days 5 and 7 (34.2 ± 3.9%, n = 7 versus 49.7 ± 3.8%, n = 7 and 5.2 ± 0.8%, n = 7 versus 12.3 ± 1.9%, n = 7, respectively). This represents a 31% reduction in CD45-positive cells in the Fst288-treated silicone implants at day 5 (mean difference of 15.44 ± 5.420%, P = 0.0147, Fig. 7B) and a 58% reduction in CD45-positive cells in the Fst288-treated silicone implants at day 7 (mean difference of 15.44 ± 5.420%, P = 0.0042, Fig. 7B).
Fig. 7.
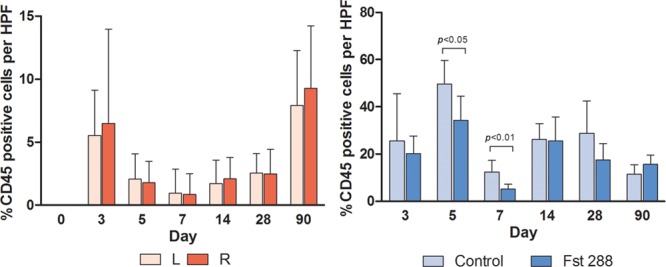
Immunohistochemistry analysis of CD45 staining (percentage of CD45-positive cells per high-powered field) in (A) baseline group and (B) Fst implant group. Results are expressed as mean ± standard error of the mean.
Tissue Cytokines and Fst288
No significant differences in IL-6 levels were observed in the baseline groups across all time points (Fig. 8A). IL-6 levels in the control group (130.4 ± 14.4 pg/mL, n = 7) were significantly lower than the levels in those receiving Fst288 (239.6 ± 33.5 pg/mL, n = 7) at day 5 (P < 0.05, Fig. 8B). There were no other significant differences in peri-implant tissue levels between the control and Fst288-treated implants across the time points (Fig. 8B). There were no significant differences in tissue activin A levels across any of the baseline time points or in the Fst implant experiment (Fig. 8C, D). There were also no significant differences in tissue Fst288 levels across any of the baseline time points (Fig. 8E). However, at day 3, Fst288 levels were significantly higher in the Fst288-treated implants (62.5 ± 10.8 ng/mL, n = 7) compared with those in the opposite controls (28.6 ± 3.2 ng/mL, n = 7), with a mean difference of 33.9 ± 11.3 ng/mL (119% increase, P = 0.0109, Fig. 8F).
Fig. 8.
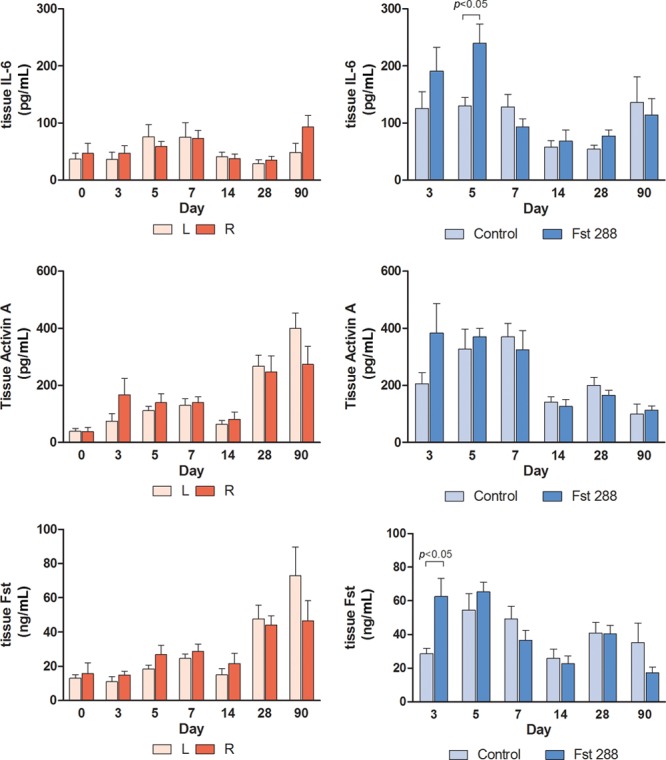
Tissue levels of (A) IL-6 baseline group, (B) IL-6 Fst implant group, (C) activin A, baseline group, (D) activin A, Fst implant group, (E) Fst288, baseline group, and (F) Fst288, Fst implant group. Data expressed as mean ± standard error of the mean.
Serum Cytokines and Fst288
Serum activin A levels were significantly higher (48.6%) in the Fst implant group (49.3 ± 3.9 pg/mL, n = 7) compared with those in the baseline group (25.4 ± 3.7 pg/mL, n = 7) at day 7 (mean difference 23.9 ± 5.4 pg/mL, P < 0.01; see figure, Supplemental Digital Content 2, http://links.lww.com/PRSGO/A391).
Serum IL-6 levels in the Fst implant group (2.6 ± 2.1 pg/mL, n = 7) decreased significantly (79.7%) compared with those in the baseline group (12.9 ± 3.6 pg/mL, n = 7) at day 3 (mean difference 10.3 pg/mL, P < 0.05; see figure, Supplemental Digital Content 2, http://links.lww.com/PRSGO/A391).
Surgical Complications/Side Effects
No mouse in this study exhibited anesthetic or surgical complications or reactions to Fst288 or any other agents used. This included any infection, hematoma, wound dehiscence, excessive intraoperative bleeding, systemic unwellness, diarrhea, and weight loss.
DISCUSSION
Inflammation, fibrosis, and capsular contracture still remain a very significant problem faced by plastic surgeons. Our data show that treatment with Fst288 decreases capsular fibrosis in a mouse model and offers a potentially useful treatment to minimize inflammation and fibrosis. Selection of Fst288 for this study was based on the evidence that Fst288 binds activins, inhibiting their proinflammatory/profibrotic actions.37,40,48–52
This study established that a single injection of 1 µg Fst288 around silicone implants produced a significant increase in the peri-implant tissue levels of Fst288, peaking at day 3. Tissue IL-6 levels were unexpectedly higher in the Fst288-treated group at day 5 but had no effect on activin A tissue levels. These findings differed from other studies in which Fst288 treatment decreased tissue levels of IL-6 and activin A.36,37,48,49,53–55 In those studies, IL-6 and activin A levels were measured directly from wound or scar tissue, whereas our study measured tissue levels in the capsule and a 1.5 cm × 1.5 cm block of tissue surrounding the capsule. Our tissue levels may not, therefore, be an accurate reflection of the peri-implant tissue levels of IL-6, activin A, and Fst288. Furthermore, the Fst288 and activin A assays are unable to distinguish between bound and unbound activin A and Fst288, which may be an explanation.
In our study, mice receiving Fst288 treatment had significantly decreased serum IL-6 levels at day 3 post surgery, whereas in all other mice serum Fst288 remained stable across all time points. However, the increased serum activin A levels observed at day 7 post surgery in the Fst-treated group are consistent with those reported by Dohi et al56 in their studies of activin A and Fst levels in a mouse model of inflammatory bowel disease.
Our study clearly demonstrates that a 1-µg subcutaneous Fst288 injection around the implant at the time of insertion resulted in significant decreases of 38% and 34% in collagen production at 14 days and 3 months after implant insertion, respectively. This was associated with a significant decrease in capsule thickness at 28 days and 3 months, in angiogenesis at 3 months, in α-SMA levels at 3 months, and in CD45+ cells at days 5 and 7. These findings are supported by other studies where Fst288 showed both anti-inflammatory and antifibrotic properties.40,48,50–52,57,58
No significant difference was seen in any of the above parameters between implants on the opposite flanks in the baseline group, except for day 28 where there was a significant difference of 58% in collagen production between 2 groups of untreated implants. In this day 28 group, an increase in serum IL-6 suggested the presence of a subclinical systemic inflammatory response although there were no obvious signs of infection or surgical complications. These data highlight the complexity of capsule formation and fibrosis7–15 and suggest that other factors such as subclinical infection that were not assessed in our study may be involved in the process.32–34
The findings of reduced angiogenesis in the Fst-treated implants was not consistent with previous studies in which exogenous Fst288 increased angiogenesis.59–61 However, Chelenski et al62 reported that an epidermal growth factor–like module of the Fst domain decreased angiogenesis in vitro.
Our findings, supported by other studies, suggest that targeting the inflammatory response during wound healing may prevent or modulate the fibrotic response during wound healing and thus may benefit the treatment of fibrotic conditions.63 Excessive inflammation leading to excess collagen deposition is widely postulated as an important cause of capsular contracture, such as in the presence of a low-grade infection. If inflammation can be controlled, capsular contracture rates may be significantly reduced, with an associated improvement in surgical outcomes.16,18–20
Several animal studies that mimic the fibrotic process support our results. These studies include the use of radiation and fibrin glue that resulted in a successful reproducible animal model of capsular contracture.6,64 Others have demonstrated the link between inflammation and capsular contracture by measuring elevated serum hyaluronan levels, which correlated with an increased Baker grade.65–67 Other research groups modulated the effects of the inflammatory pathway to reduce fibrosis and contracture using pirfenidone that inhibits TGFβ synthesis, enalapril that negates the fibrotic and inflammatory effects of angiotensin II, tumor necrosis factor–stimulated gene 6 overexpression (which provides an anti-inflammatory effect via modulation of hyaluronan), and the anti-inflammatory medications zafirlukast and montelukast.20,44,68–70 Although these studies have been performed using only small numbers of animals, they strongly suggest a link between inflammation and capsular contracture.
Although our data do not show a significant effect on tissue levels of activin A and IL-6, they provide direction for future studies, for example, a dose–response evaluation of Fst288 and/or use of multiple injections of Fst288 to elicit a greater tissue response. Other future studies may use larger implants in bigger animals to more closely mimic human breast implantation, investigate the effect of Fst288 on the presence of implant biofilm, and to refine Fst288 and activin assays to distinguish free activin A and Fst288. To the authors’ knowledge, there are no published studies where Fst has been administered to humans. Our results suggest that treatment with Fst288 may not only be important in prevention of capsular contracture but would also be a modulator of fibrosis in other models of fibrotic and inflammatory diseases such as keloid scars, hypertrophic scars, Dupuytren’s contracture, and scars following a burn injury.52
CONCLUSIONS
An injection of Fst288 at the time of silicone implant insertion into the implant pocket results in a significant decrease in leukocyte infiltration, capsular angiogenesis, myofibroblast proliferation, and a significant reduction in capsular fibrosis. Clinical studies are needed now to demonstrate the efficacy of Fst288 as a therapeutic tool to combat problematic fibrotic conditions such as capsular contracture.
ACKNOWLEDGMENTS
The authors thank associate professor Craig Harrison and Sue Hayward (The Hudson Institute, Melbourne) for help with the Fst and activin A assays and the cytokine analyses. In addition, the authors would like to acknowledge Oxford Brookes University for the reagents provided for the activin A assay and Helen Ludlow and Nigel Groome for the supply of reagents for the activin assays. They are also grateful for the help provided by Lesley Wiadrowski and Camilla Cohen (Monash Institute of Medical Research and Department of Anatomy, Monash University, Melbourne, respectively) with histology processing and photography. The authors would also like to thank Dr. Mulyoto Pangestu (Department of Obstetrics and Gyaencology, Monash University, Melbourne) for his help with logistical matters and ordering of products and all the staff at B-block animal house (Monash Medical Centre, Melbourne) for taking care of the mice used in this study.
Supplementary Material
Footnotes
Drs. Temple-Smith, Kretser, and Southwick contributed equally to study design, analysis, drafting, revision, and research supervision.
Disclosure: Dr. Frenkiel has no financial or commercial interests to disclose. The following scholarships to support the research were received by Dr. Frenkiel: NHMRC Postgraduate Scholarship and Avant doctor in training Scholarship. Funding support for this research was provided by the Advanced Plastic Surgery Education Foundation. Dr. Temple-Smith is a shareholder in Paranta Biosciences. Dr. Kretser is a director and shareholder in Paranta Biosciences based on his isolation of follistatin and elucidation of its mode of action as an anti-inflammatory and antifibrotic agent. Dr. Southwick is a shareholder in Paranta Biosciences. Paranta Biosciences is a company developing follistatin as a therapeutic. Paranta Biosciences has not supported this research in any way nor will it stand to benefit directly from this research. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
References
- 1.Barr S, Bayat A. Breast surgery review article: breast implant surface development: Perspectives on development and manufacture. Aesthet Surg J. 2011;31:56–67.. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Woods JE, O’Fallon WM, et al. Complications leading to surgery after breast implantation. N Engl J Med. 1997;336:677–682.. [DOI] [PubMed] [Google Scholar]
- 3.Siggelkow W, Klosterhalfen B, Klinge U, et al. Analysis of local complications following explantation of silicone breast implants. Breast. 2004;13:122–128.. [DOI] [PubMed] [Google Scholar]
- 4.Kjoller K, Holmich LR, Jacobsen PH, et al. Capsular contracture after cosmetic breast implant surgery in Denmark. Ann Plast Surg. 2001;47:359–366.. [DOI] [PubMed] [Google Scholar]
- 5.Kjoller K, Holmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg. 2002;48:229–237.. [DOI] [PubMed] [Google Scholar]
- 6.Zimman OA. A rabbit model for capsular contracture: development and clinical implications. Plast Reconstr Surg. 2007;119:1955–1956.. [DOI] [PubMed] [Google Scholar]
- 7.Siggelkow W, Faridi A, Spiritus K, et al. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003;24:1101–1109.. [DOI] [PubMed] [Google Scholar]
- 8.Biggs TM, Yarish RS. Augmentation mammaplasty: a comparative analysis. Plast Reconstr Surg. 1990;85:368–372.. [DOI] [PubMed] [Google Scholar]
- 9.Rohrich RJ, Kenkel JM, Adams WP. Preventing capsular contracture in breast augmentation: in search of the Holy Grail. Plast Reconstr Surg. 1999;103:1759–1760.. [DOI] [PubMed] [Google Scholar]
- 10.Luke JL, Kalasinsky VF, Turnicky RP, et al. Pathological and biophysical findings associated with silicone breast implants: a study of capsular tissues from 86 cases. Plast Reconstr Surg. 1997;100:1558–1565.. [DOI] [PubMed] [Google Scholar]
- 11.Chandler PJ, Jr, Kasper CS. Frequency and distribution of talc contamination in patients with silicone gel-filled breast implants. Ann Plast Surg. 2003;51:358–360.. [DOI] [PubMed] [Google Scholar]
- 12.Kasper CS. Histologic features of breast capsules reflect surface configuration and composition of silicone bag implants. Am J Clin Pathol. 1994;102:655–659.. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt BR. Capsular contracture: hard breasts, soft data. Clin Plast Surg. 1988;15:521–532.. [PubMed] [Google Scholar]
- 14.Moyer HR, Ghazi BH, Losken A. The influence of silicone gel bleed on capsular contracture: a generational study. Plast Reconstr Surg. 2012;130:793–800.. [DOI] [PubMed] [Google Scholar]
- 15.Wagner H, Beller FK, Pfautsch M. Electron and light microscopy examination of capsules around breast implants. Plast Reconstr Surg. 1977;60:49–55. [DOI] [PubMed] [Google Scholar]
- 16.Scuderi N, Mazzocchi M, Fioramonti P, et al. The effects of zafirlukast on capsular contracture: preliminary report. Aesthet Plast Surg. 2006;30:513–520.. [DOI] [PubMed] [Google Scholar]
- 17.Araco A, Gravante G, Araco F, et al. Capsular contracture: results of 3002 patients with aesthetic breast augmentation. Plast Reconstr Surg. 2006;118:1499–1500.; author reply 1500–2. [DOI] [PubMed] [Google Scholar]
- 18.Araco A, Caruso R, Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819.. [DOI] [PubMed] [Google Scholar]
- 19.Poeppl N, Schreml S, Lichtenegger F, et al. Does the surface structure of implants have an impact on the formation of a capsular contracture? Aesthet Plast Surg. 2007;31:133–139.. [DOI] [PubMed] [Google Scholar]
- 20.Gancedo M, Ruiz-Corro L, Salazar-Montes A, et al. Pirfenidone prevents capsular contracture after mammary implantation. Aesthet Plast Surg. 2008;32:32–40.. [DOI] [PubMed] [Google Scholar]
- 21.Gayou RM. A histological comparison of contracted and non-contracted capsules around silicone breast implants. Plast Reconstr Surg. 1979;63:700–707.. [DOI] [PubMed] [Google Scholar]
- 22.D’Andrea F, Nicoletti GF, Grella E, et al. Modification of cysteinyl leukotriene receptor expression in capsular contracture: preliminary results. Ann Plast Surg. 2007;58:212–214. [DOI] [PubMed] [Google Scholar]
- 23.McCoy BJ, Person P, Cohen IK. Collagen production and types in fibrous capsules around breast implants. Plast Reconstr Surg. 1984;73:924–927.. [DOI] [PubMed] [Google Scholar]
- 24.Lossing C, Hansson HA. Peptide growth factors and myofibroblasts in capsules around human breast implants. Plast Reconstr Surg. 1993;91:1277–1286. [DOI] [PubMed] [Google Scholar]
- 25.Wolfram D, Rabensteiner E, Grundtman C, et al. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast Reconstr Surg. 2012;129:327e–337e.. [DOI] [PubMed] [Google Scholar]
- 26.Marques M, Brown SA, Cordeiro ND, et al. Effects of fibrin, thrombin, and blood on breast capsule formation in a preclinical model. Aesthet Surg J. 2011;31:302–309.. [DOI] [PubMed] [Google Scholar]
- 27.Coleman DJ, Sharpe DT, Naylor IL, et al. The role of the contractile fibroblast in the capsules around tissue expanders and implants. Br J Plast Surg. 1993;46:547–556.. [DOI] [PubMed] [Google Scholar]
- 28.Kamel M, Protzner K, Fornasier V, et al. The peri-implant breast capsule: an immunophenotypic study of capsules taken at explantation surgery. J Biomed Mater Res. 2001;58:88–96. [DOI] [PubMed] [Google Scholar]
- 29.Adams WP, Jr, Haydon MS, Raniere J, Jr, et al. A rabbit model for capsular contracture: development and clinical implications. Plast Reconstr Surg. 2006;117:1214–1219.; discussion 20–1. [DOI] [PubMed] [Google Scholar]
- 30.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284.. [DOI] [PubMed] [Google Scholar]
- 31.Baker JL, Jr, Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg. 1981;68:905–912. [DOI] [PubMed] [Google Scholar]
- 32.Bayston R, Penny SR. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28.. [DOI] [PubMed] [Google Scholar]
- 33.Galland RB, Heine KJ, Trachtenberg LS, et al. Reduction of surgical wound infection rates in contaminated wounds treated with antiseptics combined with systemic antibiotics: an experimental study. Surgery. 1982;91:329–332.. [PubMed] [Google Scholar]
- 34.Ahn CY, Ko CY, Wagar EA, et al. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98:1225–1229. [DOI] [PubMed] [Google Scholar]
- 35.Jones KL, Mansell A, Patella S, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104:16239–16244.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki F, Kojima I. Therapeutic potential of follistatin to promote tissue regeneration and prevent tissue fibrosis. Endocr J. 2007;54:849–854.. [DOI] [PubMed] [Google Scholar]
- 37.de Kretser DM, O’Hehir RE, Hardy CL, et al. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106.. [DOI] [PubMed] [Google Scholar]
- 38.de Kretser DM, O’Hehir RE, Hardy CL, et al. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106. [DOI] [PubMed] [Google Scholar]
- 39.Munz B, Smola H, Engelhardt F, et al. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 1999;18:5205–5215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wankell M, Munz B, Hubner G, et al. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001;20:5361–5372.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert AW, Kiama SG, Seifert MG, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clugston PA, Perry LC, Hammond DC, et al. A rat model for capsular contracture: the effects of surface texturing. Ann Plast Surg. 1994;33:595–599. [DOI] [PubMed] [Google Scholar]
- 43.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. [DOI] [PubMed] [Google Scholar]
- 44.Spano A, Palmieri B, Taidelli TP, et al. Reduction of capsular thickness around silicone breast implants by zafirlukast in rats. Eur Surg Res. 2008;41:8–14.. [DOI] [PubMed] [Google Scholar]
- 45.Domkowski PW, Biswas SS, Steenbergen C, et al. Histological evidence of angiogenesis 9 months after transmyocardial laser revascularization. Circulation. 2001;103:469–471. [DOI] [PubMed] [Google Scholar]
- 46.Groome NP, Tsigou A, Cranfield M, et al. Enzyme immunoassays for inhibins, activins and follistatins. Mol Cell Endocrinol. 2001;180:73–77.. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor AE, McFarlane JR, Hayward S, et al. Serum activin A and follistatin concentrations during human pregnancy: a cross-sectional and longitudinal study. Hum Reprod. 1999;14:827–832. [DOI] [PubMed] [Google Scholar]
- 48.Hubner G, Hu Q, Smola H, et al. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol. 1996;173:490–498.. [DOI] [PubMed] [Google Scholar]
- 49.Matsuse T, Ikegami A, Ohga E, et al. Expression of immunoreactive activin A protein in remodeling lesions associated with interstitial pulmonary fibrosis. Am J Pathol. 1996;148:707–713.. [PMC free article] [PubMed] [Google Scholar]
- 50.De Bleser PJ, Niki T, Xu G, et al. Localization and cellular sources of activins in normal and fibrotic rat liver. Hepatology. 1997;26:905–912.. [DOI] [PubMed] [Google Scholar]
- 51.Cruise BA, Xu P, Hall AK. Wounds increase activin in skin and a vasoactive neuropeptide in sensory ganglia. Dev Biol. 2004;271:1–10. [DOI] [PubMed] [Google Scholar]
- 52.McLean CA, Cleland H, Moncrieff NJ, et al. Temporal expression of activin in acute burn wounds—from inflammatory cells to fibroblasts. Burns. 2008;34:50–55.. [DOI] [PubMed] [Google Scholar]
- 53.de Kretser DM, Hedger MP, Phillips DJ. Activin A and follistatin: their role in the acute phase reaction and inflammation. J Endocrinol. 1999;161:195–198.. [DOI] [PubMed] [Google Scholar]
- 54.Jones KL, De Kretser DM, Phillips DJ. Effect of heparin administration to sheep on the release profiles of circulating activin A and follistatin. J Endocrinol. 2004;181:307–314.. [DOI] [PubMed] [Google Scholar]
- 55.Jones KL, Brauman JN, Groome NP, et al. Activin A release into the circulation is an early event in systemic inflammation and precedes the release of follistatin. Endocrinology. 2000;141:1905–1908.. [DOI] [PubMed] [Google Scholar]
- 56.Dohi T, Ejima C, Kato R, et al. Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology. 2005;128:411–423.. [DOI] [PubMed] [Google Scholar]
- 57.Ohga E, Matsuse T, Teramoto S, et al. Effects of activin A on proliferation and differentiation of human lung fibroblasts. Biochem Biophys Res Commun. 1996;228:391–396.. [DOI] [PubMed] [Google Scholar]
- 58.Patella S, Phillips DJ, Tchongue J, et al. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137–G144.. [DOI] [PubMed] [Google Scholar]
- 59.Kozian DH, Ziche M, Augustin HG. The activin-binding protein follistatin regulates autocrine endothelial cell activity and induces angiogenesis. Lab Invest. 1997;76:267–276. [PubMed] [Google Scholar]
- 60.Zhu J, Li Y, Lu A, et al. Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis. Am J Pathol. 2011;179:915–930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krneta J, Kroll J, Alves F, et al. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 2006;66:5686–5695.. [DOI] [PubMed] [Google Scholar]
- 62.Chelenski A, Liu S, Baker LJ, et al. Neuroblastoma angiogenesis is inhibited with a folded synthetic molecule corresponding to the epidermal growth factor-like module of the follistatin domain of SPARC. Cancer Res. 2004;64:7420–7425. [DOI] [PubMed] [Google Scholar]
- 63.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katzel EB, Koltz PF, Tierney R, et al. A novel animal model for studying silicone gel-related capsular contracture. Plast Reconstr Surg. 2010;126:1483–1491.. [DOI] [PubMed] [Google Scholar]
- 65.Prantl L, Angele P, Schreml S, et al. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast Reconstr Surg. 2006;118:224–229.. [DOI] [PubMed] [Google Scholar]
- 66.Prantl L, Poppl N, Horvat N, et al. Serologic and histologic findings in patients with capsular contracture after breast augmentation with smooth silicone gel implants: is serum hyaluronan a potential predictor? Aesthet Plast Surg. 2005;29:510–518.. [DOI] [PubMed] [Google Scholar]
- 67.Ulrich D, Lichtenegger F, Eblenkamp M, et al. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implants. Plast Reconstr Surg. 2004;114:229–236.. [DOI] [PubMed] [Google Scholar]
- 68.Zimman OA, Toblli J, Stella I, et al. The effects of angiotensin-converting-enzyme inhibitors on the fibrous envelope around mammary implants. Plast Reconstr Surg. 2007;120:2025–2033.. [DOI] [PubMed] [Google Scholar]
- 69.Tan KT, Baildam AD, Juma A, et al. Hyaluronan, TSG-6, and inter-α-inhibitor in periprosthetic breast capsules: reduced levels of free hyaluronan and TSG-6 expression in contracted capsules. Aesthet Surg J. 2011;31:47–55.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang CK, Handel N. Effects of Singulair (montelukast) treatment for capsular contracture. Aesthet Surg J. 2010;30:404–408.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


