Abstract
Patient: Female, 3-month-old
Final Diagnosis: Acute megakaryoblastic leukemia
Symptoms: Abdominal distension • fever
Medication: —
Clinical Procedure: —
Specialty: Pediatrics and Neonatology
Objective:
Challenging differential diagnosis
Background:
The reciprocal translocation t(1;22)(p13;q13) involving the RBM15 and MKL1 genes is an uncommon abnormality that occurs in a subset of acute myeloid leukemia with megakaryocytic differentiation (AMKL). Variant translocations have been infrequently described in this subtype of leukemia.
Case Report:
We describe the case of a 3-month-old girl who presented with progressive abdominal distension, vomiting, and fever. Although there was no morphologic evidence of leukemia in the bone marrow, cytogenetic and metaphase fluorescence in situ hybridization analysis identified an insertion of p13p31 bands of chromosome 1 onto the long arm of chromosome 22, resulting in the karyotype: 46,XX,ins(22;1)(q13;p13p31). Subsequent liver biopsy demonstrated extensive involvement by AMKL.
Conclusions:
AMKL can present with fewer than 20% blasts in the peripheral blood or bone marrow, necessitating careful evaluation for extramedullary disease. In other situations, bone marrow fibrosis can result in difficult marrow aspirations and a falsely decreased blast count. This case report highlights the critical role of careful cytogenetic and FISH testing in the diagnosis of AMKL.
MeSH Keywords: Cytogenetics; Leukemia, Megakaryoblastic, Acute; Pediatrics; Translocation, Genetic
Background
Acute myeloid leukemia (AML) with recurrent genetic abnormalities is a category of AML characterized by specific, recurrent cytogenetic changes that hold prognostic significance and impart characteristic morphological and immunophenotypic features [1,2]. These chromosomal abnormalities are generally balanced structural rearrangements that generate fusion genes and oncogenic proteins [3]. The reciprocal translocation t(1;22) (p13;q13) involving the RBM15 and MKL1 genes is an uncommon abnormality that occurs in a subset of AMLs with maturation in the megakaryocyte lineage (AML-megakaryoblastic or AMKL). This chromosomal aberration occurs most commonly in female infants and children younger than 3 years of age without Down syndrome, and comprises about 1% of all childhood de novo AML [1,4]. Clinically, the patients present with hepatosplenomegaly, anemia, and thrombocytopenia. About 60 cases with t(1;22) translocation have been described to date, of which only 3 had a variant t(1;22) translocation. Of these 3 cases, 2 showed a 3-way translocation and 1 case had a 4-way translocation [5–7]. We describe a patient with AMKL with a novel variant t(1;22) translocation in which the critical region of 1p was inserted onto the critical region of 22q.
Case Report
A 3-month-old female presented with a 2-week history of progressive abdominal distension, intermittent vomiting, and low-grade fever. Of note, the patient had no history of Down syndrome or dysmorphology. Laboratory testing showed an elevated white blood cell count at 35.9×103/μL with 64% lymphocytes and 2% blasts. The patient was anemic and thrombocytopenic with a hemoglobin of 5.7 g/dL and a platelet count of 86×109/μL. An abdominal ultrasound and CT scan revealed an enlarged spleen and liver measuring 11.5 cm and 11 cm, respectively, and ascites.
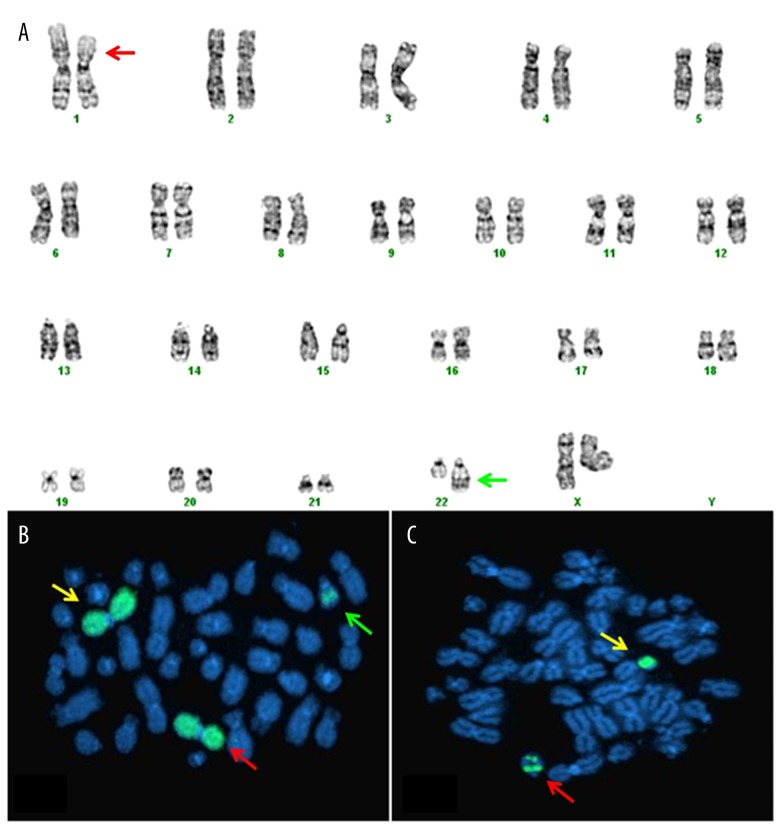
A bone marrow biopsy was performed, which showed normocellular marrow with active maturing trilineage hematopoiesis. Manual differential cell count performed on the bone marrow aspirate smear showed 6% blasts but flow cytometry using a broad array of myeloid and megakaryocytic markers did not identify an abnormal blast population. Cytogenetic analysis was performed according to standard protocol on G-banded chromosomes and demonstrated ins(22;1)(q13;p13p31) in 6 of 20 metaphases (Figure 1A). The remaining 14 cells showed a normal female karyotype. Metaphase FISH analysis, using spectrum green (SG)-labeled whole-chromosome painting probes for chromosomes 1 and 22, confirmed that 1p chromosomal material was inserted onto 22q, establishing the presence of a variant t(1;22) translocation as a result of an insertion (Figure 1B, 1C).
Figure 1.
(A) Karyotype showing 46,XX,ins(22;1)(q13;p13p31). The red arrow shows the region of deletion of p13q31 bands on chromosome 1. The green arrow shows the insertion of bands p13q31 of chromosome 1 on der(22) chromosome. (B) FISH using SG-labeled WCP 1 probe. Yellow arrow shows normal chromosome 1, red arrow indicates del(1), and green arrow shows der(22). (C) FISH using the SG-labeled WCP probe for chromosome 22. The red arrow shows the insertion of chromosome 1 bands. The yellow arrow indicates the normal chromosome 22.
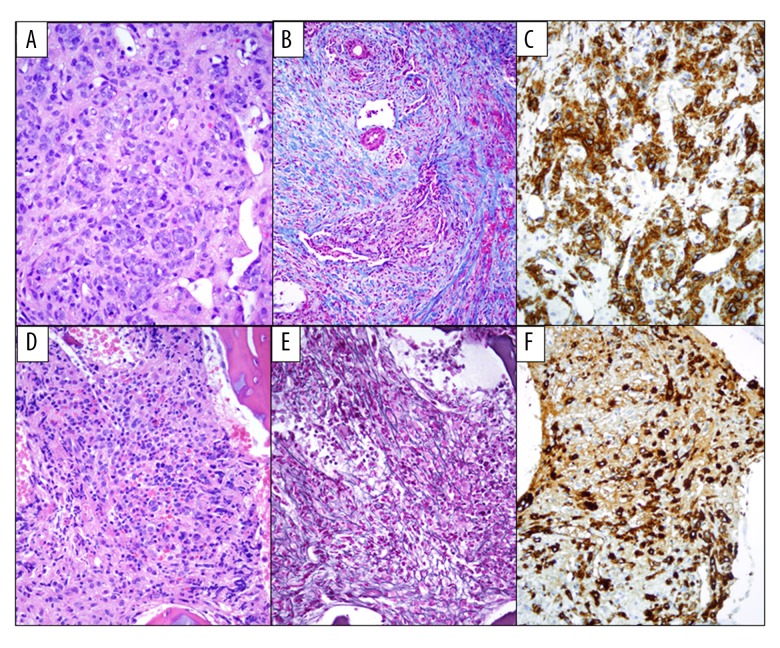
Subsequently, a liver biopsy was performed, revealing extensive disruption of the normal hepatic architecture by a diffuse proliferation of large atypical neoplastic cells with ample cytoplasm, fine chromatin, and prominent nucleoli. These neoplastic cells were associated with dissecting bands of dense collagen fibrosis, confirmed by Masson’s trichrome stain. By immunohistochemistry, these cells were positive for CD45, CD42b, CD41, CD117, and CD31. They were negative for CD34, MPO, CD68, B-cell and T-cell markers (Figure 2A–2C). In situ hybridization for EBV-RNA (EBER) was negative. A diagnosis of acute megakaryoblastic leukemia involving the liver was made. Intravenous chemotherapy with cytarabine, daunorubicin, and etoposide (modified ADE) was begun. The clinical course was complicated by E. coli septic shock, respiratory failure, and severe hepatic dysfunction, including veno-occlusive disease, which was treated with a course of defibrotide. The diagnosis of veno-occlusive disease was made following severe persistent direct hyperbilirubinemia and reversal of portal venous flow on ultrasound. On day 14, a bone marrow biopsy showed hypocellular marrow with extensive fibrosis and persistence of CD42b+, CD61+ atypical immature megakaryocytes with hypolobation, consistent with persistent AMKL (Figure 2D–2F). On day 28, a liver biopsy showed extensive obliteration of the sinusoids by dense fibrous tissue but no evidence of residual acute leukemia. Cytogenetic studies on the bone marrow aspirate showed a normal female karyotype. Maintenance therapy with low-dose cytarabine was begun post-induction. The patient remains in complete remission more than 2 years after the initial diagnosis.
Figure 2.
(A) H&E, 400×. Liver biopsy showing extensive involvement by acute megakaryoblastic leukemia. The liver architecture is distorted by the presence of numerous aggregates of large, markedly atypical neoplastic cells. (B) Trichrome special stain, 200×. Extensive collagen deposition. (C) CD42b, 400×. The atypical megakaryocytes are highlighted with immunohistochemical stains for CD42b. (D) H&E 400×. Cellular bone marrow with small hypolobated or immature megakaryoblasts. (E) Reticulin special stain, 400×. Marked bone marrow fibrosis. (F) CD42b, 400×. The atypical megakaryocytes are highlighted with immunohistochemical stains for CD42b.
Discussion
AMKL with t(1;22) is a rare form of AML that predominantly affects very young children; the presentation may be atypical for acute leukemia. While most forms of acute leukemia present with extensive bone marrow involvement with circulating blasts, AMKL, as in this case, may present as extramedullary disease with few peripheral blood blasts. As a result, initial diagnosis of leukemia may be delayed. Extensive bone marrow fibrosis in AMKL may impede bone marrow biopsy and aspiration, resulting in a dry tap. Thus, a thorough cytogenetic evaluation can be critical in the diagnosis of AMKL with t(1;22).
To the best of our knowledge, this is the first case of a variant t(1;22) translocation with insertion of 1p13p31 onto 22q13 seen by conventional cytogenetic analysis and confirmed by whole-chromosome painting. A search of the Mitelman database failed to identify any cases of AMKL with t(1;22) presenting as an insertion [8]. As in typical cases of RBM15-MKL1 rearranged leukemias, we hypothesize that insertion of 1p on 22q resulted in formation of the pathogenic fusion protein.
Several entities, both hematopoietic and non-hematopoietic, may be considered in the differential diagnosis of AMKL in neonates. The clinical presentation with abdominal organomegaly can suggest dissemination of a “small round blue cell tumor”, such as neuroblastoma [9]. Morphologically, the blast population in AMKL cannot be reliably distinguished from “small round blue cell tumors” or other AML subtypes. Immunohistochemistry may help with this differential, but cytogenetic studies revealing the presence of t(1;22)(p13;q13) help establish the final diagnosis [4,10]. Recent studies of cytogenetic subgroups and outcomes in de novo AMKL have shown that patients with t(1;22) translocation have an intermediate risk [11]. However, prior studies have shown favorable responses to intensive chemotherapy regimens, especially in patients without Down syndrome [12,13].
Conclusions
In summary, we present a case of AMKL with a novel variant t(1;22) translocation resulting in formation of the RBM15-MKL1 fusion gene due to an insertion of 1p to 22q. AMKL is associated with abdominal organomegaly, with the liver frequently representing the primary site of disease [9,10]. In some children, as in our case, AMKL can present with fewer than 20% blasts in the peripheral blood or bone marrow, necessitating careful evaluation for extramedullary disease. In other situations, bone marrow fibrosis can result in difficult marrow aspirations and a falsely decreased blast count [1]. Our case highlights the critical role of careful cytogenetic and FISH testing in the diagnosis of AMKL, as morphologic and flow cytometric examination of the bone marrow did not identify a significant blast population.
Acknowledgments
We give thanks to Ilham Atir, Svetlana Kleyman, May Wong, and Ana Revelo-Sanchez for their technical support.
Footnotes
Conflict of interest and disclosure
The authors have no conflicts of interest to disclose.
References:
- 1.Arber DA, Brunning R, Beau ML, et al. Acute myeloid leukemia with recurrent genetic abnormalities. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed edn. International Agency for Research on Cancer; Lyon, France: 2008. p. 110. [Google Scholar]
- 2.Arber DA, Carter NH, Ikle D, Slovak ML. Value of combined morphologic, cytochemical, and immunophenotypic features in predicting recurrent cytogenetic abnormalities in acute myeloid leukemia. Hum Pathol. 2003;34:479–83. doi: 10.1016/s0046-8177(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 3.Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2002;2:502–13. doi: 10.1038/nrc840. [DOI] [PubMed] [Google Scholar]
- 4.Carroll A, Civin C, Schneider N, et al. The t(1;22) (p13;q13) is nonrandom and restricted to infants with acute megakaryoblastic leukemia: A Pediatric Oncology Group Study. Blood. 1991;78:748–52. [PubMed] [Google Scholar]
- 5.Mercher T, Coniat MB, Monni R, et al. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc Natl Acad Sci USA. 2001;98:5776–79. doi: 10.1073/pnas.101001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres L, Lisboa S, Vieira J, et al. Acute megakaryoblastic leukemia with a four-way variant translocation originating the RBM15-MKL1 fusion gene. Pediatr Blood Cancer. 2011;56:846–49. doi: 10.1002/pbc.22765. [DOI] [PubMed] [Google Scholar]
- 7.Dastugue N, Lafage-Pochitaloff M, Pages MP, et al. Cytogenetic profile of childhood and adult megakaryoblastic leukemia (M7): A study of the Groupe Francais de Cytogenetique Hematologique (GFCH) Blood. 2002;100:618–26. doi: 10.1182/blood-2001-12-0241. [DOI] [PubMed] [Google Scholar]
- 8.Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer, 2015
- 9.Kawasaki Y, Makimoto M, Nomura K, et al. Neonatal acute megakaryoblastic leukemia mimicking congenital neuroblastoma. Clin Case Rep. 2015;3:145–49. doi: 10.1002/ccr3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein J, Dastugue N, Haas OA, et al. Nineteen cases of the t(1;22) (p13;q13) acute megakaryblastic leukaemia of infants/children and a review of 39 cases: Report from a t(1;22) study group. Leukemia. 2000;14:216–18. doi: 10.1038/sj.leu.2401639. [DOI] [PubMed] [Google Scholar]
- 11.Inaba H, Zhou Y, Abla O, et al. Heterogeneous cytogenetic subgroups and outcomes in childhood acute megakaryoblastic leukemia: A retrospective international study. Blood. 2015;126:1575–84. doi: 10.1182/blood-2015-02-629204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchayne E, Fenneteau O, Pages MP, et al. Acute megakaryoblastic leukaemia: A national clinical and biological study of 53 adult and childhood cases by the Groupe Francais d’Hematologie Cellulaire (GFHC) Leuk Lymphoma. 2003;44:49–58. doi: 10.1080/1042819021000040279. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien MM, Cao X, Pounds S, et al. Prognostic features in acute megakaryoblastic leukemia in children without Down syndrome: A report from the AML02 multicenter trial and the Children’s Oncology Group Study POG 9421. Leukemia. 2013;27:731–34. doi: 10.1038/leu.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]