Abstract
Patient: Female, 30
Final Diagnosis: Minimal change disease
Symptoms: Oliguria • systemic edema
Medication: —
Clinical Procedure: Steroid pulse therapy
Specialty: Obstetrics and Gynecology
Objective:
Rare disease
Background:
Nephrotic syndrome occurs very rarely, in only about 0.01–0.02% of all pregnancies, and de novo minimal change disease during pregnancy is especially rare. Nephrotic syndrome and, especially, minimal change disease are highly responsive to steroids, and preterm labor may be avoidable if the maternal condition is improved with steroid therapy. Therefore, prompt diagnosis and proper management are critical to maternal and fetal outcome when severe proteinuria occurs during pregnancy.
Case Report:
A 30-year-old pregnant Japanese woman presented with systemic edema, oliguria, and severe proteinuria and hypoalbuminemia at 25 weeks of gestation, although she was normotensive. The patient had high urinary protein selectivity. Her illness was diagnosed as de novo nephrotic syndrome with high steroid responsiveness rather than pre-eclampsia. She began steroid pulse therapy the day after admission. Complete remission was confirmed after 3 weeks. The patient did not relapse during pregnancy and delivered a healthy male baby at 37 weeks of gestation. A renal biopsy at a relapse after delivery confirmed minimal change disease.
Conclusions:
In pregnant women with de novo minimal change disease, serious maternal and/or fetal complications may occur if severe proteinuria and hypoalbuminemia are unabated for an extended time. Evaluation of urinary protein selectivity is noninvasive and useful for prediction of steroid responsiveness. Results of urinary protein selectivity can be obtained earlier than results of renal biopsy. Renal biopsy during pregnancy is not always necessary for initiation of steroid therapy. Rapid initiation of steroid pulse therapy may enable quicker achievement of remission and prevent serious perinatal complications.
MeSH Keywords: Nephrotic Syndrome, Oliguria, Pre-Eclampsia, Proteinuria
Background
Nephrotic syndrome is very rare, occurring in only about 0.01–0.02% of all pregnancies [1], and de novo minimal change disease (MCD) during pregnancy is especially rare [2]. It is difficult but important to distinguish de novo nephrotic syndrome from pre-eclampsia, which is cured only by termination of the pregnancy. Renal biopsy during pregnancy has the risk of complications. When a fetus is viable outside the uterus, a delivery may precede an invasive procedure and/or therapy. Nephrotic syndrome and, especially, MCD are highly responsive to steroids, and preterm labor may be avoidable if the maternal condition is improved with steroid therapy. Serious maternal and/or fetal complications may occur if severe proteinuria and hypoalbuminemia are unabated for an extended period. Therefore, prompt diagnosis and proper management are critical to maternal and fetal outcome when severe proteinuria occurs during pregnancy. Here, we report a case of successful management of pregnancy with de novo MCD that remitted completely with steroid pulse therapy.
Case Report
A 30-year-old pregnant Japanese woman, gravida 2 para 1, was admitted to our hospital at 25 weeks of gestation for further investigation of severe systemic edema, severe proteinuria, and oliguria. Her past medical history was unremarkable. During a previous pregnancy 7 years earlier, there had been no evidence of urinary abnormality or hypertension and she vaginally delivered a healthy baby weighing 2856 g at 38 weeks of gestation. In this pregnancy, she was normotensive and urinary protein dipstick was negative at all prior pregnancy checkups. The patient noticed rapidly worsening systemic edema and decreasing urinary amount several days before her referral to our hospital and visited the obstetrical clinic following her pregnancy. At this time, she had severe systemic edema, especially affecting her face and legs. Urinary protein, by dipstick, was strongly positive. The patient was referred to our hospital for same-day investigation and treatment.
On examination, her blood pressure was 137/77 mmHg. Her body weight was 70.5 kg, reflecting an 11.5 kg increase over the previous 4 weeks. Chest auscultation and X-ray showed no evidence of heart failure. An abdominal ultrasound showed normal fetal growth, amniotic fluid volume, and placenta. The urinary protein/creatinine ratio was 10.99. The 24-h urinary volume was 273 mL/day and urinary protein was 12.2 g/day. Serum creatinine was 0.54 mg/dL, and serum total protein and albumin were 3.6 g/dL and 0.7 g/dL, respectively. Disease screening for secondary nephrotic syndrome, including for autoimmune disease, viral infection, and diabetes, was negative. The proteinuria selectivity index (SI) demonstrates the selectivity of urinary protein and is calculated according to the formula:
where Ig is immunoglobulin and Tf is transferrin. For this patient, the SI was 0.13 (i.e., high urinary protein selectivity); renal biopsy was avoided because of the pregnancy. We reasoned that the most likely cause of her proteinuria was primary nephrotic syndrome rather than pre-eclampsia.
The patient was treated with steroid pulse therapy (methyl prednisolone 1 g/day) from day 2 to day 4 of admission to bring about rapid disease remission. On day 5, oral prednisolone (50 mg/day) was started. By day 14 (at 27 weeks of gestation), urinary protein had decreased to 0.76 g/day. Complete remission of the disease was confirmed on day 21 (at 28 weeks of gestation). As the urinary protein decreased, the oliguria improved. The patient’s body weight decreased gradually. Steroids were tapered accordingly. The patient did not relapse during pregnancy and vaginally delivered a healthy male baby weighing 2342 g at 37 weeks 3 days of gestation.
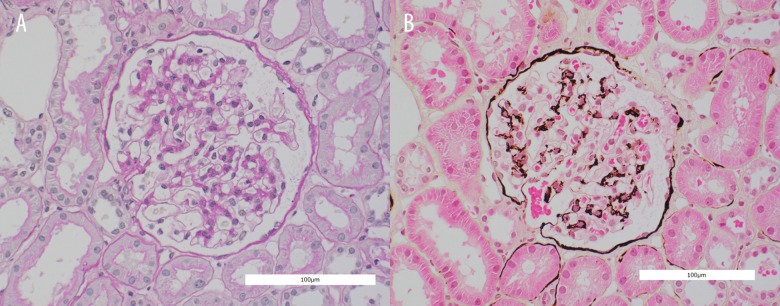
The patient relapsed about 40 days after delivery. Steroid pulse therapy was again administered, and complete remission was achieved. At this time, renal biopsy was performed. The renal biopsy was consistent with MCD. On light microscopy, there were 25 glomeruli, none of which showed segmental sclerosis or thickening of glomerular basement membrane. All glomeruli appeared structurally normal (Figure 1A, 1B). Immunofluorescence studies were negative for glomerular deposits of immunoglobulin A and G, immunoglobulin light chains, and Clq.
Figure 1.
Microscopic findings of renal biopsy (A – Periodic acid-Schiff stain, B – Periodic acid-methenamine-silver stain). Neither segmental sclerosis nor thickening of glomerular basement membrane was shown. All glomeruli appeared structurally normal.
Discussion
When proteinuria was initially detected during the pregnancy, pre-eclampsia or gestational proteinuria was suspected to be the leading cause. A previous observational study concluded that gestational proteinuria is a risk factor in pre-eclampsia and precedes hypertension in 20% of patients with pre-eclampsia; [3] however, Murakami et al. reported that women who had proteinuria prior to 30 weeks of gestation were more likely to have had underlying renal disease [4]. Nephrotic syndrome is specifically defined by the presence of severe proteinuria (>3.5 g/24 h), hypoalbuminemia (<3 g/dL), and peripheral edema. MCD is the predominant cause of nephrotic syndrome in children but was found to be present in only 10%–15% of adult cases [5]. In our patient, termination of the pregnancy and preterm labor would have been indicated as treatment in the event of pre-eclampsia; however, pre-term labor at 25 weeks of gestation may lead to serious neurological sequelae in the newborn. Thus, it was important to distinguish de novo nephrotic syndrome from pre-eclampsia or gestational proteinuria.
Pre-eclampsia can be diagnosed without proteinuria if there is end-organ damage, although hypertension is an essential diagnostic criterion for pre-eclampsia [6]. However, patients with proteinuria who are not hypertensive do not always have underling kidney disease. Renal biopsy is usually performed for histopathological confirmation of nephrotic syndrome in nonpregnant patients. Renal biopsy is not absolutely contraindicated for all pregnant women. A systematic review of renal biopsy during pregnancy concluded that the risks of complications were higher in pregnancy (relative to postpartum biopsy), with a possible peak at around 25 weeks of gestation [7]. During pregnancy, noninvasive kidney evaluation is very important. Wang et al. reported that Virtual Touch Quantification technology is a useful method for noninvasive assessment of tissue elasticity to examine the kidney injury in gestational hypertension [8]. Renal biopsy was avoided during pregnancy in our case and we based our diagnosis and treatment on noninvasive investigations.
SI is a useful and safe biomarker. SI results can be obtained earlier than results of renal biopsy. In our present case, the SI result indicated that urinary protein selectivity was high and that steroid responsiveness was likely to be good. While SI cannot accurately predict MCD, urinary protein selectivity was found to be low in pregnant women with pre-eclampsia and renal disease other than MCD [9]. We would have performed renal biopsy during pregnancy if urinary protein selectivity had been low or if steroid therapy had been ineffective.
A literature search revealed only 4 previous case reports of de novo MCD in pregnancy [2,10–12]. Two of the cases were histologically confirmed by renal biopsy during the pregnancy. In both cases, the nephrotic syndrome occurred at 19 weeks of gestation [11,12]. The other cases were not confirmed histologically during pregnancy [2,10]; in 1 case, nephrotic syndrome occurred at 33 weeks of gestation and the SI was examined [2] and this case had no other signs of pre-eclampsia. The authors concluded that correct specific treatment for nephrotic syndrome can confer safer prolongation of gestation. In our patient, prolongation of pregnancy was achieved with corticosteroid therapy.
In pregnant women with de novo nephrotic syndrome, fetal and/or maternal complications may occur if severe proteinuria continues for an extended period [12,13]. In a previous report, severe fetal growth restriction occurred, followed by intrauterine fetal death [13], and in another case, dialysis was required for maternal renal failure [12]. Waldman et al. reported that the risk factors for acute kidney injury in patients with MCD were male sex, old age, hypertension, low serum albumin, and elevated protein excretion [14]. Chen et al. reported that severe hypoalbuminemia (<1 g/dL) was a significant risk factor for acute kidney injury in nephrotic syndrome [15], while Silva et al. reported that oliguria was a poor prognostic factor for acute kidney injury during pregnancy [16]. Acute kidney injury occurred in 1 of the 4 reported cases of de novo MCD during pregnancy [12]. In that case, the severest proteinuria (15.4 g/day) was detected at admission to care, while in the other 3 cases proteinuria was never higher than 10 g/day. Among these 4 cases of de novo MCD during pregnancy and in our case, serum albumin before treatment was the lowest in our case. Although the serum creatinine was normal in our patient, severe hypoalbuminemia and oliguria indicated severe maternal intravascular volume depletion. Therefore, we decided that treatment for nephrotic syndrome would be effective and that it should be rapidly started to avoid threatened acute kidney injury.
To prevent worsening of the maternal and/or fetal condition, rapid achievement of complete remission was very important. MCD is generally a steroid-sensitive disease; however, the rate of response of MCD to corticosteroid therapy is lower in adults compared with children, and more prolonged therapy is required to achieve a remission [17]. A systematic review of the treatment of MCD concluded that patients treated with steroid pulse therapy achieved complete remission sooner and showed fewer adverse events than did patients receiving oral steroid monotherapy [18], but complete remission rates and relapse rates were similar in both groups. Steroid pulse therapy was not performed in the previously reported 4 cases of de novo MCD during pregnancy; however, steroid pulse therapy should be particularly considered for pregnant women with nephrotic syndrome, for whom acute kidney injury threatens.
Conclusions
De novo MCD during pregnancy is very rare; however, serious maternal and/or fetal complication may occur if severe proteinuria and hypoalbuminemia are unabated for an extended period. Prompt initiation of steroid pulse therapy after diagnosis may enable quicker achievement of remission. Renal biopsy during pregnancy has a higher risk of complications than in the postpartum period. Evaluation of urinary protein selectivity is noninvasive and useful for prediction of steroid responsiveness. Results of urinary protein selectivity can be obtained earlier than results of renal biopsy. Renal biopsy is not always necessary for initiation of steroid therapy. In pregnant women with de novo minimal change disease, serious maternal and/or fetal complication may occur if severe proteinuria and hypoalbuminemia are unabated for an extended period. Rapid initiation of steroid pulse therapy may enable quicker achievement of remission and prevent serious perinatal complications. Further research on the evaluation of the severity of nephrotic syndrome during pregnancy and the selection of treatments is required.
Acknowledgments
The authors report no conflicts of interest in this work.
References:
- 1.Pandya BK, Gibson SP, Robertson IG. Nephrotic syndrome in early pregnancy – is renal biopsy always necessary? Nephrol Dial Transplant. 2002;17:672–74. doi: 10.1093/ndt/17.4.672. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton P, Myers J, Gillham J, et al. Urinary protein selectivity in nephrotic syndrome and pregnancy: Resurrection of a biomarker when renal biopsy is contraindicated. Clin Kidney J. 2014;7:595–98. doi: 10.1093/ckj/sfu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada T, Obata-Yasuoka M, Hamada H, et al. Isolated gestational proteinuria preceding the diagnosis of preeclampsia – an observational study. Acta Obstet Gynecol Scand. 2016;95(9):1048–54. doi: 10.1111/aogs.12915. [DOI] [PubMed] [Google Scholar]
- 4.Murakami S, Saitoh M, Kubo T, et al. Renal disease in women with severe preeclampsia or gestational proteinuria. Obstet Gynecol. 2000;96:945–49. doi: 10.1016/s0029-7844(00)01055-3. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JS. Histology, protein clearances, and response to treatment in the nephrotic syndrome. Br Med J. 1968;4:352–56. doi: 10.1136/bmj.4.5627.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooq AS, Malik RI, Feiz H, Ledford R. Preeclampsia presenting in a post-partum woman without proteinuria: Is that possible? Med Sci Case Rep. 2015;2:25–28. [Google Scholar]
- 7.Piccoli GB, Daidola G, Attini R, et al. Kidney biopsy in pregnancy: Evidence for counselling? A systematic narrative review. BJOG. 2013;120:412–27. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Feng Y, Yang X, et al. Clinical values of studying kidney elasticity with virtual touch quantification in gestational hypertension patients. Med Sci Monit. 2016;22:403–7. doi: 10.12659/MSM.895567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood SM, Burnett D, Studd JWW. Selectivity of proteinuria during pregnancy assessed by different methods. In: Lindheimer MD, Katz AI, Zuspan FP, editors. Hypertension in Pregnancy. New York: JohnWiley and Sons; 1977. pp. 61–73. [PubMed] [Google Scholar]
- 10.McCleave TC., Jr Lipoid nephrosis during and after pregnancy. Calif Med. 1951;74:440–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson DB. Minimal change glomerulopathy in pregnancy. Nephrol Nurs J. 2003;30:45–50. [PubMed] [Google Scholar]
- 12.Lo JO, Kerns E, Rueda J, Marshall NE. Minimal change disease in pregnancy. J Matern Fetal Neonatal Med. 2014;27:1282–84. doi: 10.3109/14767058.2013.852178. [DOI] [PubMed] [Google Scholar]
- 13.Basgul A, Kavak ZN, Sezen D, et al. A rare case of early onset nephrotic syndrome in pregnancy. Clin Exp Obstet Gynecol. 2006;33:127–28. [PubMed] [Google Scholar]
- 14.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–53. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Lv Y, Lin F, Zhu J. Acute kidney injury in adult idiopathic nephrotic syndrome. Ren Fail. 2011;33:144–49. doi: 10.3109/0886022X.2011.553301. [DOI] [PubMed] [Google Scholar]
- 16.Silva GB, Jr, Monteiro FA, Mota RM, et al. Acute kidney injury requiring dialysis in obstetric patients: A series of 55 cases in Brazil. Arch Gynecol Obstet. 2009;279:131–37. doi: 10.1007/s00404-008-0682-8. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M, Katafuchi R, Yanase T, et al. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis. 2002;39:503–12. doi: 10.1053/ajkd.2002.31400. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Cheng J, Zhou J, et al. Enhanced steroid therapy in adult minimal change nephrotic syndrome: A systematic review and meta-analysis. Intern Med. 2015;54:2101–8. doi: 10.2169/internalmedicine.54.3927. [DOI] [PubMed] [Google Scholar]