LETTER
Patient-reported antibiotic allergies (so-called antibiotic allergy labels [AALs]) are associated with suboptimal prescribing and inferior clinical outcomes, especially in the immunocompromised (1, 2). The prevalence, type, and impact of AALs in liver transplant recipients (LTRs) remain ill defined. We report on AALs and their impact on a cohort of Australian LTRs.
A retrospective matched-cohort study was conducted over a 5-year period (2010 to 2015) at an Australian liver transplant center (Austin Health). Using a departmental liver transplant database, LTRs with an AAL (AAL group) were identified. The same number of matched controls (LTRs without an antibiotic allergy label [non-AAL group]) were randomly selected for comparison. AALs were evaluated and classified as type A adverse drug reactions (ADRs; nonimmune mediated), type B ADRs (immune mediated), or of unknown type (3). Baseline demographics, transplant history, and infection-related admission data were collected. From the time of transplant until 12 months afterward, antibiotics administered during infection-related admissions and their duration of administration were recorded. Readmission, intensive-care unit (ICU) admission, Clostridium difficile infection (CDI), multidrug-resistant (MDR) organism isolation, and mortality rate were captured. An MDR organism was defined as a bacterium resistant to at least one agent in three or more antibiotic classes (4).
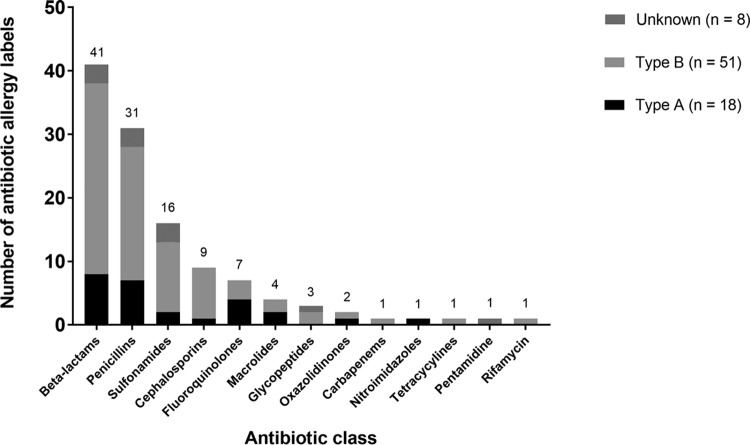
Of 313 LTRs, 51 (16%) had ≥1 AAL. Females predominated in the AAL group (75% female versus 25% male; P = 0.003), but there was no statistically significant differences in the rates of ICU admission and mortality between males and females (see Table S1 in the supplemental material). Seventy-seven AALs were recorded; of these, 23% (18/77) were type A ADRs, 66% were type B ADRs (51/77), and 10% (8/77) were of unknown type. Of the type A ADRs, 72% (13/18) were gastrointestinal upset, and of the type B ADRs, 6% (3/51) were severe cutaneous ADRs, 55% (28/51) were maculopapular exanthema, 35% (18/51) were anaphylaxis, urticaria, or angioedema, and 4% (2/51) were other reactions. The antibiotics implicated in AALs are demonstrated in Fig. 1.
FIG 1.
Antibiotic allergy labels per antibiotic class (n = 77).
AAL patients were associated with higher numbers of courses of cephalosporin (107/354 AAL patients [30%] versus 75/328 non-AAL patients [23%]; P = 0.03) and nitroimidazole (34/354 AAL patients [10%] versus 15/328 non-AAL patients [5%]; P = 0.02). When the AAL and non-AAL groups were compared, there was a nonsignificant reduction in the number of courses of penicillin V or G or aminopenicillin (26/354 AAL [7%] versus 36/328 non-AAL [11%]; P = 0.14) and beta-lactam or beta-lactamase inhibitors (43/354 AAL [12%] versus 49/328 non-AAL [15%]; P = 0.31). There was also an increasing trend in the number of MDR Gram-negative organisms isolated (4/51 AAL patients [8%] versus 1/52 non-AAL patients [2%]; P = 0.20) and CDI group (9 AAL patients [18%] versus 3 non-AAL patients [6%]; P = 0.07).
Sixteen LTRs (31%) in the AAL group had a trimethoprim-sulfamethoxazole (TMP-SMX) ADR history noted, with the majority (11 ADRs [68%]) classified as type B (7 ADRs [43%] were delayed, and 4 [25%] were immediate), 2 (13%) classified as type A, and 3 (19%) classified as unknown. With regard to Pneumocystis jirovecii pneumonia prophylaxis, aerosolized pentamidine was employed in 56% (9/16) of these patients with a TMP-SMX ADR history immediately posttransplantation. Only one patient received dapsone, without an ADR. Sixty-three percent (10/16) of the TMP-SMX ADR patients were toxoplasmosis IgG positive, of which 80% (8/10) received no directed toxoplasmosis prophylaxis. In relation to treatment doses, there was a reduction in TMP-SMX usage as a proportion of the total number of antibiotic courses in the AAL group (0/354 AAL versus 9/328 non-AAL [3%]; P = 0.001).
Although the conclusions are limited by the small cohort size, we identified in LTRs a high prevalence of AALs, predominately AALs to sulfonamides and beta-lactams. Significantly, a large proportion of AALs were type A (23%), amendable to immediate “delabeling.” In LTRs with a sulfonamide allergy, TMP-SMX or dapsone may also have been unnecessarily avoided if one considers that antibiotic sulfonamide cross-reactivity is lower than previously thought (5) and TMP-SMX desensitization or rechallenge is often effective (6, 7).
A trend comparable to that shown by nontransplant patients, in whom AALs were found to be associated with increased microbiological resistance and CDI (8), was noted in our cohort. In light of recent increasing cephalosporin resistance (9) and CDI in LTRs (10) and our data demonstrating both, aggressive antibiotic allergy testing and delabeling should be evaluated (11). Use of cephalosporin and other broad-spectrum antibiotics is often a common target for antimicrobial stewardship programs; the addition of antibiotic allergy testing to high-risk antibiotic usage populations, such as transplant recipients, is likely to support antimicrobial stewardship programs by enhancing the use of first-line, narrow-spectrum agents (12). The largest benefit of antibiotic allergy testing is likely to occur pretransplantation, to effectively reduce AAL prevalence and improve antibiotic prescribing in this vulnerable cohort.
Supplementary Material
ACKNOWLEDGMENTS
J.A.T. is supported by an NHMRC postgraduate research scholarship.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00078-17.
REFERENCES
- 1.Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA, National Antimicrobial Prescribing Survey. 2016. Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother 71:1715–1722. doi: 10.1093/jac/dkw008. [DOI] [PubMed] [Google Scholar]
- 2.Trubiano JA, Leung VK, Chu MY, Worth LJ, Slavin MA, Thursky KA. 2015. The impact of antimicrobial allergy labels on antimicrobial usage in cancer patients. Antimicrob Resist Infect Control 4:23. doi: 10.1186/s13756-015-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichler W. 2007. Drug hypersensitivity. S Karger Publishing, Basel, Switzerland. [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Strom BL, Schinnar R, Apter AJ, Margolis DJ, Lautenbach E, Hennessy S, Bilker WB, Pettitt D. 2003. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med 349:1628–1635. doi: 10.1056/NEJMoa022963. [DOI] [PubMed] [Google Scholar]
- 6.Pyle RC, Butterfield JH, Volcheck GW, Podjasek JC, Rank MA, Li JT, Harish A, Poe KL, Park MA. 2014. Successful outpatient graded administration of trimethoprim-sulfamethoxazole in patients without HIV and with a history of sulfonamide adverse drug reaction. J Allergy Clin Immunol Pract 2:52–58. doi: 10.1016/j.jaip.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Leoung GS, Stanford JF, Giordano MF, Stein A, Torres RA, Giffen CA, Wesley M, Sarracco T, Cooper EC, Dratter V, Smith JJ, Frost KR, American Foundation for ARC-BCTN. 2001. Trimethoprim-sulfamethoxazole (TMP-SMZ) dose escalation versus direct rechallenge for Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with previous adverse reaction to TMP-SMZ. J Infect Dis 184:992–997. doi: 10.1086/323353. [DOI] [PubMed] [Google Scholar]
- 8.Macy E, Contreras R. 2014. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 133:790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Chaulk J, Carbonneau M, Qamar H, Keough A, Chang HJ, Ma M, Kumar D, Tandon P. 2014. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: a single-centre experience and summary of existing studies. Can J Gastroenterol Hepatol 28:83–88. doi: 10.1155/2014/429536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paudel S, Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E. 2015. Prevalence of Clostridium difficile infection among solid organ transplant recipients: a meta-analysis of published studies. PLoS One 10:e0124483. doi: 10.1371/journal.pone.0124483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trubiano J, Phillips E. 2013. Antimicrobial stewardship's new weapon? A review of antibiotic allergy and pathways to ‘de-labeling.’ Curr Opin Infect Dis 26:526–537. doi: 10.1097/QCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.