ABSTRACT
Vancomycin-resistant Enterococcus faecium bloodstream infections (VREF-BSI) cause significant mortality, highlighting the need to optimize their treatment. We compared the effectiveness and safety of daptomycin (DAP) and linezolid (LZD) as continuous or sequential therapy for VREF-BSI in a national, retrospective, propensity score (PS)-matched cohort study of hospitalized Veterans Affairs patients (2004 to 2014). We compared clinical outcomes and adverse events among patients treated with continuous LZD, continuous DAP, or sequential LZD followed by DAP (LZD-to-DAP). Secondarily, we analyzed the impact of infectious diseases (ID) consultation and source of VREF-BSI. A total of 2,630 patients were included in the effectiveness analysis (LZD [n = 1,348], DAP [n = 1,055], LZD-to-DAP [n = 227]). LZD was associated with increased 30-day mortality versus DAP (risk ratio [RR], 1.11; 95% confidence interval [CI], 1.01 to 1.22; P = 0.042). After PS matching, this relationship persisted (RR, 1.13; 95% CI, 1.02 to 1.26; P = 0.015). LZD-to-DAP switchers had lower mortality than those remaining on LZD (RR, 1.29; 95% CI, 1.03 to 1.63; P = 0.021), suggesting a benefit may still be derived with sequential therapy. LZD-treated patients experienced more adverse events, including a ≥50% reduction in platelets (RR, 1.07; 95% CI, 1.03 to 1.11; P = 0.001). DAP was associated with lower mortality than was LZD in patients with endocarditis (RR, 1.20; 95% CI, 1.02 to 1.41; P = 0.024); however, there was no statistically significant association between treatment group and mortality with regard to other sources of infection. Therefore, source of infection appears to be important in selection of patients most likely to benefit from DAP over LZD.
KEYWORDS: Enterococcus, antimicrobial resistance, bloodstream infection, daptomycin, linezolid, transplant infectious diseases, vancomycin resistance
INTRODUCTION
Vancomycin-resistant Enterococcus faecium bloodstream infections (VREF-BSI) represent a significant health care-associated complication (1–4). The clinical impact of VREF-BSI is increasing, and most E. faecium strains are now vancomycin resistant (1, 2). As mortality associated with VREF-BSI is high, optimal treatment balancing effectiveness and safety is critical (1, 5).
Many small retrospective studies have compared daptomycin (DAP) and linezolid (LZD) for treatment of VREF-BSI (6–10). Two recent meta-analyses of these investigations found an apparent survival benefit associated with LZD treatment, despite the fact that none of the individual studies included found a statistically significant difference between the two agents in adjusted analyses (11, 12). The comparative effectiveness of DAP and LZD for VREF-BSI was recently evaluated in a large national cohort study of hospitalized patients in the Veterans Affairs (VA) health care system (13). In this study, DAP treatment was associated with improved clinical and microbiologic outcomes relative to LZD (13). While this study addressed several limitations of previous evaluations, many clinically important questions remain unanswered (13, 14). Specifically, patients who were treated sequentially with DAP and then LZD were excluded from previous analyses, and this may have produced biased results if a significant portion of patients switched therapy due to clinical failure (13, 14). The impacts of infectious diseases (ID) consultation and source of infection on outcomes in VREF-BSI have also not been extensively evaluated (13, 14). The present study sought to expand on findings of previous studies of VREF-BSI and address these gaps in the literature.
RESULTS
A total of 2,779 patients met study criteria (LZD, n = 1,348 [48.5% of total study patients]; DAP, n = 1,055 [38.0%]; LZD-to-DAP, n = 227 [8.2%]; DAP-to-LZD, n = 149 [5.4%]). These individuals were treated at 99 VA medical centers across 48 states, the District of Columbia, and Puerto Rico. Of these patients, 539/2,779 (19.4%) were included in a previous study of hospitalized VA patients with VRE-BSI (13). The median DAP dose was 6.15 mg/kg (total body weight; interquartile range [IQR], 5.57 to 7.07 mg/kg). All patients treated with LZD received 600 mg twice daily. The median duration of treatment was 13 days (IQR, 7 to 18 days) for DAP, 9 days (IQR, 5 to 15 days) for LZD, and 16 days (IQR, 11 to 27 days) for LZD-to-DAP. The median time to switching from LZD to DAP was 6 days (IQR, 2 to 10 days).
Baseline characteristics were compared between DAP, LZD, and LZD-to-DAP treatment groups, and many significant differences were noted (see Table S1 in the supplemental material). After propensity score (PS) derivation, 853 DAP patients were matched with 853 LZD patients and 217 LZD patients were matched to 217 LZD-to-DAP patients (Table 1). PS matching successfully balanced these treatment groups on baseline characteristics.
TABLE 1.
Baseline characteristics among patients treated with continuous DAP, continuous LZD, or sequential DAP-to-LZD treatment for VREF-BSI, after propensity score matchinga
| Characteristic | No. (%) of patients with characteristic and P valueb for comparison |
|||||
|---|---|---|---|---|---|---|
| Continuous treatment (total n) |
Sequential treatment (total n) |
|||||
| DAP (853) | LZD (853) | P value | LZD (217) | LZD-to-DAP (217) | P value | |
| Age ≥ 65 yrs | 412 (48.3) | 420 (49.2) | 0.698 | 101 (50.7) | 102 (47.0) | 0.923 |
| Male gender | 830 (97.3) | 831 (97.4) | 0.880 | 209 (96.3) | 213 (98.2) | 0.242 |
| Concomitant pneumoniac | 33 (3.9) | 30 (3.5) | 0.700 | 21 (9.7) | 21 (9.7) | >0.999 |
| Facility complexity level | ||||||
| 1a (most complex) | 606 (71.0) | 596 (69.9) | 0.596 | 139 (64.1) | 129 (59.4) | 0.323 |
| 1b | 162 (19.0) | 148 (17.4) | 0.379 | 53 (24.4) | 47 (21.7) | 0.494 |
| 1c | 70 (8.2) | 81 (9.5) | 0.348 | 22 (10.1) | 31 (14.3) | 0.187 |
| 2 | 11 (1.3) | 19 (2.2) | 0.141 | 2 (0.9) | 8 (3.7) | 0.105 |
| 3 (least complex) | 4 (0.5) | 9 (1.1) | 0.164 | 1 (0.5) | 2 (0.9) | >0.999 |
| Yr of infection | ||||||
| 2004 | 60 (7.0) | 77 (9.0) | 0.130 | 17 (7.8) | 22 (10.1) | 0.401 |
| 2005 | 83 (9.7) | 85 (10.0) | 0.871 | 21 (9.7) | 26 (12.0) | 0.440 |
| 2006 | 73 (8.6) | 84 (9.8) | 0.357 | 26 (12.0) | 33 (15.2) | 0.327 |
| 2007 | 95 (11.1) | 107 (12.5) | 0.369 | 29 (13.4) | 25 (11.5) | 0.561 |
| 2008 | 102 (12.0) | 110 (12.9) | 0.557 | 26 (12.0) | 24 (11.1) | 0.764 |
| 2009 | 104 (12.2) | 104 (12.2) | >0.999 | 23 (10.6) | 23 (10.6) | >0.999 |
| 2010 | 85 (10.0) | 77 (9.0) | 0.509 | 26 (12.0) | 15 (6.9) | 0.071 |
| 2011 | 89 (10.4) | 69 (8.1) | 0.095 | 16 (7.3) | 14 (6.5) | 0.658 |
| 2012 | 71 (8.3) | 55 (6.4) | 0.139 | 19 (8.8) | 22 (10.1) | 0.622 |
| 2013 | 62 (7.3) | 52 (6.1) | 0.332 | 5 (2.3) | 4 (1.8) | 0.522 |
| 2014 | 29 (3.4) | 33 (3.9) | 0.605 | 9 (4.1) | 9 (4.1) | >0.999 |
| Infectious diseases consultd | 424 (49.7) | 421 (49.4) | 0.884 | 114 (52.5) | 124 (57.1) | 0.335 |
| Source of VREF-BSI | ||||||
| Genitourinary only | 99 (11.6) | 96 (11.3) | 0.819 | 27 (12.4) | 29 (13.4) | 0.775 |
| Abdominal/gastrointestinal only | 36 (4.2) | 42 (4.9) | 0.487 | 11 (5.1) | 7 (3.2) | 0.064 |
| Line-associated only | 89 (10.4) | 107 (12.5) | 0.172 | 12 (5.5) | 12 (5.5) | >0.999 |
| Endocarditis/cardiac device only | 57 (6.7) | 67 (7.9) | 0.351 | 30 (13.8) | 29 (13.4) | 0.889 |
| Wound/bone only | 25 (2.9) | 31 (3.6) | 0.415 | 12 (5.5) | 8 (3.7) | 0.684 |
| Multiple sources | 127 (14.9) | 113 (13.2) | 0.330 | 39 (18.0) | 46 (21.2) | 0.397 |
| Unknown source | 420 (49.2) | 397 (46.5) | 0.119 | 86 (39.6) | 86 (39.6) | >0.999 |
| Previous VRE stool colonizatione | 95 (11.1) | 85 (10.0) | 0.431 | 19 (8.8) | 30 (13.8) | 0.095 |
| Time to treatment (days)f [median (IQR)] | 3 (2–4) | 3 (2–4) | 0.657 | 3 (2–5) | 3 (2–4) | 0.489 |
| Concomitant β-lactam treatmentg | 662 (77.6) | 653 (76.6) | 0.604 | 193 (88.9) | 185 (85.3) | 0.252 |
| Concomitant aminoglycoside treatmenth | 90 (10.6) | 94 (11.0) | 0.755 | 38 (17.5) | 33 (15.2) | 0.516 |
| Intensive care unit admission | 271 (31.8) | 272 (31.9) | 0.959 | 72 (33.2) | 73 (33.6) | 0.919 |
| Malignancy | ||||||
| Any malignancy | 301 (35.3) | 292 (34.3) | 0.684 | 124 (57.1) | 124 (57.1) | >0.999 |
| Solid tumor | 194 (22.7) | 183 (21.5) | 0.521 | 91 (41.9) | 91 (41.9) | >0.999 |
| Hematologic | 186 (21.8) | 164 (19.2) | 0.187 | 40 (18.4) | 51 (23.5) | 0.195 |
| Solid organ transplant recipient | 31 (3.6) | 26 (3.0) | 0.501 | 13 (6.0) | 4 (1.8) | 0.065 |
| Kidney | 7 (0.8) | 8 (0.9) | 0.795 | 5 (2.3) | 1 (0.5) | 0.215 |
| Liver | 22 (2.6) | 17 (2.0) | 0.418 | 8 (3.7) | 3 (1.4) | 0.221 |
| Lung | 2 (0.2) | 0 (0.0) | 0.500 | 1 (0.5) | 0 (0.0) | >0.999 |
| Heart | 2 (0.2) | 1 (0.1) | >0.999 | 0 (0.0) | 0 (0.0) | |
| Comorbid conditions | ||||||
| Charlson comorbidity index [median (IQR)] | 7 (4–9) | 7 (4–9) | 0.672 | 6 (4–8) | 7 (4–9) | 0.121 |
| Moderate to severe renal disease | 444 (52.1) | 440 (51.6) | 0.846 | 117 (53.9) | 112 (51.6) | 0.631 |
| Severe liver disease | 79 (9.3) | 83 (9.7) | 0.741 | 26 (12.0) | 20 (9.2) | 0.349 |
| Metastatic solid tumor | 104 (12.2) | 110 (12.9) | 0.661 | 39 (18.0) | 39 (18.0) | >0.999 |
| HIV infection | 25 (2.9) | 29 (3.4) | 0.580 | 7 (3.2) | 7 (3.2) | >0.999 |
| Neutropenia | 90 (10.6) | 82 (9.6) | 0.520 | 23 (10.6) | 25 (11.5) | 0.760 |
| Thrombocytopenia | 231 (27.1) | 199 (23.3) | 0.074 | 60 (27.6) | 46 (21.1) | 0.118 |
| APACHE II scorei [median (IQR)] | 14 (10–17) | 13 (10–18) | 0.790 | 13 (9–17) | 14 (11–18) | 0.320 |
Propensity scores were derived from the following covariates: age ≥ 65 years, concomitant pneumonia, facility complexity level, year of infection, infectious diseases consultation, source of VREF-BSI, previous VRE colonization, concomitant β-lactam treatment, intensive care unit admission, malignancy, solid organ transplant, Charlson comorbidity index, moderate or severe renal disease, severe liver disease, neutropenia, and thrombocytopenia.
Categorical variables compared with the chi-square or Fisher's exact test; continuous variables were compared with the Mann-Whitney U test.
Within 72 h of index culture.
Within 48 h of treatment initiation.
Positive VREF stool screening within preceding 90 days; negative culture data not available.
Time from index VREF blood culture to first dose of daptomycin or linezolid.
At least one dose of the following: ampicillin, ampicillin-sulbactam, ticarcillin-clavulanate, aztreonam, cefazolin, cefotaxime, ceftazidime, ceftriaxone, cefepime, ertapenem, meropenem, doripenem, imipenem-cilastatin, or piperacillin-tazobactam.
At least one dose of the following: amikacin, gentamicin, or tobramycin.
APACHE II, Acute Physiology and Chronic Health Evaluation II study.
Continuous LZD versus DAP treatment.
Prior to PS matching, 30-day mortality was significantly higher among patients treated with LZD in comparison to DAP (34.6% versus 30.7%; risk ratio [RR], 1.11; 95% confidence interval [CI], 1.01 to 1.22; P = 0.042). Continuous LZD treatment also resulted in significantly higher infection-related mortality (5.3% versus 1.4%; RR, 1.04; 95% CI, 1.02 to 1.06; P < 0.001), persistent VREF-BSI (10.1% versus 6.3%; RR, 1.32; 95% CI, 1.02 to 1.71; P = 0.020), hospital mortality (36.6% versus 29.6%; RR, 1.20; 95% CI, 1.09 to 1.33; P < 0.001), longer median duration of VREF-BSI (3 days versus 2 days; P < 0.001), and longer median hospital length of stay among survivors (LOS; 26 days versus 20 days; P < 0.001).
After PS matching, the relationship between LZD treatment and increased 30-day mortality persisted (Table 2). LZD treatment was also significantly associated with increased infection-related mortality, persistent VREF-BSI, hospital mortality, and longer median duration of VREF-BSI and hospital LOS in the PS-matched cohort (Table 2).
TABLE 2.
Comparison of clinical outcomes among patients treated continuously with DAP versus LZD and LZD versus LZD-to-DAP for VREF-BSI, after propensity score matchinga
| Outcome | Continuous treatment |
Sequential treatment |
||||||
|---|---|---|---|---|---|---|---|---|
| DAP (n = 853) | LZD (n = 853) | Risk ratio (95% CI) | P value | LZD (n = 217) | LZD-to-DAP (n = 217) | Risk ratio (95% CI) | P value | |
| 30-day all-cause mortality [n (%)] | 251 (29.4) | 298 (34.9) | 1.13 (1.02–1.26) | 0.015 | 75 (34.6) | 53 (24.4) | 1.29 (1.03–1.63) | 0.021 |
| Infection-related mortalityb [n (%)] | 12 (1.4) | 46 (5.4) | 1.47 (1.19–2.09) | <0.001 | 16 (7.4) | 15 (6.9) | 1.04 (0.71–1.51) | 0.852 |
| Hospital mortality [n (%)] | 250 (29.3) | 305 (35.8) | 1.16 (1.05–1.39) | 0.004 | 88 (40.6) | 71 (32.7) | 1.19 (0.97–1.46) | 0.090 |
| Persistent VREF-BSIc,d [n (%)] | 37 (6.4) | 54 (10.0) | 1.30 (1.02–1.67) | 0.027 | 19 (10.3) | 8 (5.4) | 1.54 (0.85–2.80) | 0.107 |
| Duration of VREF-BSI (days)d,e [median (IQR)] | 2 (1–3) | 3 (2–4) | <0.001 | 2 (1–5) | 2 (1–4) | 0.248 | ||
| Hospital length of stay (days) [median (IQR)] | 21 (12–43) | 25 (14–47) | 0.001 | 24 (14–51) | 22 (10–45) | 0.165 | ||
Categorical variables were compared by using a chi-square or Fisher's exact test. Continuous variables were compared by using the Mann-Whitney U test. Propensity scores were derived from the following covariates: age ≥ 65 years, concomitant pneumonia, facility complexity level, infectious diseases consultation, source of VREF-BSI, previous VRE colonization, concomitant β-lactam treatment, intensive care unit admission, malignancy, solid organ transplant, Charlson comorbidity index, moderate to severe renal disease, severe liver disease, neutropenia, or thrombocytopenia.
Death during treatment with DAP or LZD without microbiological clearance from bloodstream.
Lack of microbiological clearance after ≥7 days.
Comparison among those with at least 1 follow-up blood culture while on treatment (LZD [n = 539], DAP [n = 578]).
Comparison among those with at least 1 follow-up blood culture while on treatment (LZD [n = 184], LZD-to-DAP [n = 147]).
Continuous LZD versus sequential LZD-to-DAP treatment.
Among the 227 patients treated with sequential LZD-to-DAP, 138 (60.8%) were switched due to clinical failure, 69 (30.4%) due to an adverse event, 9 (4.0%) due to physician preference, and 11 (4.8%) due to an unspecified reason. After PS matching, LZD treatment was associated with increased 30-day mortality (34.6% versus 24.4%; RR, 1.29; 95% CI, 1.03 to 1.63; P = 0.021) (Table 2) and lower overall survival (log-rank test, P < 0.001). No significant differences in other measured clinical outcomes were observed in this cohort. Longer median time to switching was associated with increased 30-day mortality (3 days versus 6 days; P < 0.001). In a Cox regression controlling for treatment as a time-dependent covariate, switching to DAP was associated with improved survival compared with remaining on LZD treatment (hazard ratio, 1.21; 95% CI, 1.06 to 1.44; P < 0.001).
Infection source.
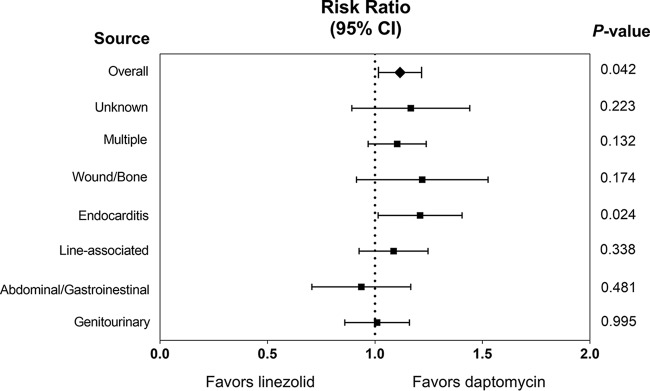
A comparison of 30-day mortality by treatment with LZD or DAP stratified by source of infection is displayed in Fig. 1. Among patients meeting criteria for definite infective endocarditis who were treated with continuous therapy, LZD was significantly associated with increased 30-day mortality compared to DAP (30.9% [n = 30/97] versus 17.2% [n = 17/99]; RR, 1.20; 95% CI, 1.02 to 1.41; P = 0.024). This mortality benefit was sustained at 60 days (47.4% [n = 46/97] versus 31.3% [n = 31/99]; RR, 1.31; 95% CI, 1.04 to 1.65; P = 0.021) among patients with VREF endocarditis. There was no statistically significant association between treatment and 30-day mortality with regard to other sources of VREF-BSI. While not statistically significant, DAP appeared to be associated with proportionally lower mortality than LZD for patients with multiple sources or a wound/bone source of VREF-BSI. There was no difference in 30-day mortality between LZD and DAP among those with a nonendocarditis source of infection (34.9% versus 32.1%; RR, 1.04; 95% CI, 0.98 to 1.11; P = 0.165).
FIG 1.
Comparison of 30-day mortality by treatment with LZD or DAP, stratified by source of infection.
Infectious diseases consultation.
In the full cohort of patients treated with continuous or sequential LZD or DAP (n = 2,779), ID consultation was associated with decreased 30-day mortality (27.7% [n = 370/1,335] versus 38.0% [n = 549/1,444]; RR, 0.86; 95% CI, 0.82 to 0.90; P < 0.001). The effect of ID consultation was further analyzed within stratum for each treatment group. ID consultation was associated with decreased 30-day mortality among DAP patients (26.1% [n = 149/571] versus 36.2% [n = 175/484]; RR, 0.86; 95% CI, 0.80 to 0.94; P < 0.001) and LZD patients (28.8% [n = 158/548] versus 38.6% [n = 309/800]; RR, 0.86; 95% CI, 0.80 to 0.93; P < 0.001). Among LZD-to-DAP patients, a similar reduction in 30-day mortality was observed in cases with ID consultation (29.8% [n = 36/121] versus 39.6% [n = 42/106]; RR, 0.86; 95% CI, 0.71 to 1.04; P = 0.118).
Adverse events.
Overall, elevated creatine phosphokinase (CPK) was rare and occurred in only 3.0% of evaluable patients (n = 37/1,249). There were, however, statistically significant differences in CPK elevation between treatment groups (P < 0.001) (Table 3). No significant difference in CPK elevation was observed between patients treated with DAP versus those treated with LZD (P = 0.161). Patients who received DAP at any time were no more likely to experience elevated CPK than those with only LZD exposure (2.9% [n = 29/1,000] versus 3.2% [n = 8/249]; P = 0.687). However, patients who switched from DAP to LZD had significantly more frequent CPK elevation than all other patients (P < 0.001).
TABLE 3.
Frequency of adverse events, by antimicrobial treatment group for VREF-BSI
| Adverse event | Continuous treatment |
Sequential treatment |
Overall P valuea | ||||
|---|---|---|---|---|---|---|---|
| DAP only | LZD only | P value | LZD to DAP | DAP to LZD | P value | ||
| Thrombocytopeniab | 101/1,023 (9.9) | 145/1,301 (11.1) | 0.478 | 60/219 (27.4) | 24/144 (16.7) | 0.130 | <0.001 |
| Platelets decreased 50% from baselinec | 166/978 (17.0) | 217/1,225 (17.7) | 0.648 | 87/203 (42.9) | 36/141 (25.5) | 0.011 | <0.001 |
| CPK elevationd | 13/747 (1.7) | 8/249 (3.2) | 0.161 | 4/150 (2.7) | 12/103 (11.7) | 0.006 | <0.001 |
Adverse events were compared for all groups by using the chi-square test.
Thrombocytopenia was defined as a platelet count of <50,000/μl.
Our analysis included only those with ≥1 follow-up platelet count.
CPK elevation was defined based on a patient having one of two conditions: (i) among patients with normal baseline CPK, an elevated CPK value ≥3 times the upper limit of normal based on two sequential measurements during the period from day 4 of treatment to 3 days after therapy, with one of these measures being ≥5 times the upper limit of normal; or (ii) among patients with elevated baseline CPK, an elevated CPK value ≥5 times the upper limit of normal based on two sequential measurements during the period from day 4 of treatment to 3 days after therapy.
Thrombocytopenia occurred in 12.3% (n = 330/2,687), and there was a ≥50% drop in platelet count in 19.9% (n = 506/2,547) of evaluable patients. These frequencies varied significantly between treatment groups (P < 0.001) (Table 3). As shown, thrombocytopenia was relatively rare among patients treated continuously with LZD and was most frequent among patients who were switched from LZD to DAP. Patients with LZD exposure at any time were no more likely to develop thrombocytopenia than those who only received DAP (13.8% [n = 229/1,664] versus 9.9% [n = 101/1,023]; P = 0.411). However, patients with any LZD exposure were significantly more likely to experience a ≥50% drop in platelet count than were those with only DAP exposure (21.7% [n = 340/1,569] versus 17.0% [n = 166/978]; P = 0.001).
DISCUSSION
In a national PS-matched cohort study, DAP was associated with lower 30-day mortality, infection-related mortality, hospital mortality, persistent VREF-BSI, median duration of VREF-BSI, and hospital LOS compared to LZD. Additionally, switching from LZD to DAP was associated with improved survival compared to continuing LZD; which is a novel and clinically important finding.
The mortality rates we observed were similar to those of previous studies comparing DAP and LZD for VREF-BSI (7–13, 15). Infection-related mortality was low relative to the overall mortality observed in the present study. This may reflect the limited virulence of E. faecium in the context of a patient population with a high comorbidity burden and baseline mortality risk (16, 17). As no consensus definition for infection-related mortality or persistent infection exists, the definitions used were arbitrary and our data may represent conservative estimates.
The finding of improved 30-day mortality associated with DAP versus LZD was previously described in a study of VA patients (13). The mechanism for the more favorable clinical outcomes we observed with DAP treatment remains to be elucidated, but it may be related to the bactericidal activity of DAP against VREF in comparison to the bacteriostatic agent LZD (18). One other explanation is the potential synergy with concomitant β-lactam or aminoglycoside treatment (19–21). However, daptomycin combination therapy failed to improve outcomes in a previous study of VA patients with VREF-BSI (13). Cases of VREF-BSI refractory to monotherapy or with an associated elevated DAP MIC may represent unique populations that could benefit from concomitant β-lactam or aminoglycoside therapy; we were unable to characterize this (22). Patients receiving combination therapy likely have a higher baseline risk of mortality and complex treatment courses; thus, the comparative effectiveness of daptomycin monotherapy versus combination therapy in VREF-BSI warrants further investigation.
The benefits of sequential therapy have been well-described in the literature for other Gram-positive BSIs, but they had not been explored in VREF-BSI prior to the present study. Notably, the majority of LZD-to-DAP patients were switched relatively early during the course of infection, and a shorter time to switching was associated with improved mortality. Additionally, approximately 40% of these therapeutic switches were due to a reason other than clinical failure, and many of these patients may have had a favorable outcome if LZD had been continued. Inherent to any study of sequential treatment is the possibility of survivor bias, in which patients who live longer have a prolonged opportunity to switch therapy. However, when treatment was modeled as a time-dependent covariate in a Cox regression to account for this effect, the relationship between switching from LZD to DAP and improved survival persisted. These results suggest that switching treatment from LZD to DAP, particularly early in the course of infection, may lead to more favorable outcomes than would continuation of LZD.
Regarding infection source, DAP was associated with improved 30-day mortality among patients with VREF endocarditis. Recently published clinical practice guidelines recommend either high-dose DAP or LZD first-line therapy for these infections (23). The results of the present study suggest that DAP treatment may be associated with improved mortality in VREF endocarditis, a relationship which should be further evaluated in a study properly adjusting for DAP dose and other potential confounders, such as surgical intervention. Importantly, DAP was not associated with improved outcomes in any other subgroup of infection source. This suggests that source of VREF-BSI may play an important role in outcomes and could be useful in guiding treatment selection. It should be noted, however, that daptomycin had a numerically lower risk of mortality for all distinct sources of infections, except for abdominal/gastrointestinal infections. These subgroups also featured a limited number of patients and were likely underpowered. Further evaluation of the relationship between infection source and outcomes in VREF-BSI is warranted.
To our knowledge, the present study is also the first to demonstrate the impact of ID consultation on clinical outcomes among patients with VREF-BSI, regardless of treatment. This finding was in an unadjusted secondary analysis and should be hypothesis generating, requiring confirmation in other data sets with consideration of potential confounding factors. However, ID specialist consultation has previously been associated with improved clinical outcomes in other Gram-positive BSIs, perhaps due to more diligent and aggressive source identification and control (24, 25). In a recent analysis of hospitalized VA patients with VRE-BSI treated with daptomycin, ID specialist consultation was also independently associated with improved survival (26). We believe future analyses of VREF-BSI should consider the potential influence of ID consultation on clinical outcomes.
This investigation included a safety analysis of DAP and LZD used to treat VREF-BSI. Despite a large sample size, DAP-exposed patients were no more likely to experience elevated CPK than LZD-exposed patients. In contrast, LZD-exposed patients were significantly more likely to experience a ≥50% decrease in platelet count from baseline, a threshold frequently used to indicate drug-induced thrombocytopenia (27). Additionally, an adverse event to LZD was the primary reason for switching in nearly one-third of cases with sequential therapy.
The present study is not without limitations. While the use of PS matching balanced the treatment groups with regard to baseline characteristics and enhanced internal validity, we cannot exclude the potential influence of unmeasured or residual confounders (28, 29). The large-scale investigation of national clinical databases improved the external validity of this study; however, we were unable to collect potentially important data which were not available from these data sources at the time of the study, including time-to-culture positivity, source control, and perceived source of infection as documented by a treating physician. We attempted to overcome the latter by identification of microbiologically confirmed VREF infection from other sites; however, no such data existed for nearly half of the included patients. The screening methodology used to identify cases of VREF endocarditis would also have been unable to capture those without an ICD-9 code. Additionally, VRE colonization status was not known for all patients and the timing and frequency of follow-up blood cultures was left to physician discretion. The present study featured a primarily male, elderly cohort with a relatively small number of solid organ and hematopoietic stem cell transplant recipients; therefore, these results should be interpreted within the context of the population studied.
In conclusion, DAP was associated with improved clinical outcomes and fewer adverse events than LZD in the treatment of VREF-BSI. Improved outcomes associated with DAP use were most apparent in patients with VREF endocarditis, and there was no statistically significant advantage of DAP therapy in patients with other sources of infection. Therefore, consideration of infection source appears to be important in the selection of VREF-BSI patients most likely to benefit from DAP therapy over LZD. We recommend consideration of early switching to DAP among those treated initially with LZD for VREF-BSI, as delays in switching were associated with poorer survival. Finally, ID specialist consultation was associated with decreased mortality irrespective of treatment modality and should be considered where possible for patients with VREF-BSI.
MATERIALS AND METHODS
Study population and data sources.
We conducted a national retrospective cohort study of hospitalized patients admitted to any VA medical center (VAMC) from 2004 to 2014. All adult patients with ≥1 blood culture positive for vancomycin-resistant E. faecium were included. Exclusion criteria included the following: (i) total duration of treatment with DAP, LZD, or sequential therapy of <48 h; (ii) treatment with another active anti-VRE agent; (iii) VREF-BSI caused by a microbiologically confirmed DAP-nonsusceptible or LZD-resistant isolate. For the comparative effectiveness analyses, patients were classified into three treatment groups: (i) continuous DAP; (ii) continuous LZD; (iii) sequential LZD to DAP (LZD-to-DAP). Two comparisons were made: (i) continuous DAP versus continuous LZD; (ii) continuous LZD versus LZD-to-DAP (to assess the effects of sequential therapy). Patients treated with sequential DAP to LZD (DAP-to-LZD) were included in the adverse events analysis only, as we hypothesized DAP would be associated with more favorable outcomes based on previous research (13). This was a follow-up analysis to a previously published study, featuring an expanded cohort due to upgrades to the data sources used and less-stringent exclusion criteria, to include sequential therapy and additional patients (13). This study was approved by the Kansas City VAMC institutional review board.
Data collection and definitions.
Clinical and administrative data were abstracted from national databases comprised of records from all VAMCs to identify patients. Data collected included patient demographics, laboratory and microbiological data (positive and negative cultures), echocardiography reports, vital signs, antimicrobial treatment data, comorbidities, admissions records (bed type, length of stay), ID specialist consultation within 48 h, stool VRE colonization, and date of death. Potential cases of VREF endocarditis were initially identified by ICD-9 codes. These cases were further assessed by retrospective review of the electronic medical record, and definite infective endocarditis cases were identified by the modified Duke criteria (23, 30). Cases of line-associated VREF-BSI were identified based on the presence of additional positive catheter tip cultures. All other sources of VREF-BSI were determined by microbiologic culture confirmation from another site growing VREF, drawn within 72 h of index blood culture, when available. For patients who were treated with sequential therapy, retrospective review of the electronic medical record was also conducted to determine the reason for switching. Susceptibilities to antimicrobial agents were determined during routine clinical care per institution-specific procedures, and quantitative MIC data were not available.
Clinical outcomes.
The primary clinical outcome was 30-day all-cause mortality, defined from the time of index VREF-positive blood culture. Secondary outcomes included infection-related mortality (death during treatment with DAP or LZD for VREF-BSI without microbiologic clearance), hospital mortality (mortality while hospitalized for VREF-BSI), duration of VREF-BSI (time from index VRE-positive blood culture until the first negative blood culture), persistent VREF-BSI (lack of microbiologic clearance after ≥7 days treatment with DAP or LZD), and hospital LOS (time from index VREF-positive blood culture until discharge, excluding patients who died during hospitalization).
Adverse events.
We evaluated the following adverse events: (i) thrombocytopenia (platelets < 50,000/μl); (ii) decrease in platelet count of ≥50% during treatment; (iii) CPK elevation, according to previously defined criteria (27, 31, 32). Patients with baseline thrombocytopenia were excluded from analyses of the effect of treatment on platelet count.
Statistical analysis.
Baseline patient characteristics were compared by χ2 test or Fisher's exact test for categorical data and Student's t test or Mann-Whitney U test for continuous data. To address potential confounding by indication, a series of PS-matched analyses were conducted. The first analysis included patients who were treated with DAP versus those treated with LZD, and the second included patients treated with LZD versus those treated with LZD and then switched to DAP. For these analyses, PS were derived from unconditional logistic regression, controlling for variables which were associated with the primary outcome or treatment group (P < 0.2) at baseline. PS matches were performed 1:1 with replacement using a greedy nearest-neighbor algorithm (caliper width, 0.2). The effect of sequential therapy on survival was assessed in the PS-matched cohort using the Kaplan-Meier method and log-rank test and in a Cox regression with treatment group status modeled as a time-dependent covariate to account for potential survival bias. Reason for switching therapy was categorized into the following groups: (i) clinical failure (persistent leukocytosis [white blood cell count of ≥12,000/μl], persistent fever [body temperature of ≥38°C], persistent BSI [lack of microbiologic clearance prior to switching treatment], or perceived clinical failure as documented by a treating physician); (ii) adverse event (development of thrombocytopenia, elevated CPK, or as documented by a treating physician); (iii) physician preference; (iv) unspecified (no reason for switching could be identified). The associations between treatment group and adverse events were compared by partitioned χ2 analysis. Statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC) with a two-sided P value of <0.05 considered statistically significant for all tests.
Secondary analyses.
The comparative effectiveness of DAP and LZD was further analyzed after stratification by source of VREF-BSI and ID specialist consultation. An a priori assumption was made that ID specialist consultation could serve as a confounding or modifying variable. To address this, stratified analyses were performed to determine if the relationship between treatment group and 30-day mortality was modified by ID specialist consultation, prior to PS matching (33). Because this variable was considered a potential confounder, it was also included in the PS-matching algorithm.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Mary Oehlert (Dwight D. Eisenhower VAMC) for institutional support and study coordination and Allen Faler for assistance in data queries.
This work was supported by the National Institutes of Health (grant TL1TR000120-03 to Nicholas S. Britt). This material is the result of work supported with resources and the use of facilities at the Dwight D. Eisenhower VAMC, Leavenworth, KS, USA.
The contents of this report do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02216-16.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 58:163–170. doi: 10.1016/j.diagmicrobio.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Reik R, Tenover FC, Klein E, McDonald LC. 2008. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn Microbiol Infect Dis 62:81–85. doi: 10.1016/j.diagmicrobio.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey AM, Zilberberg MD. 2009. Secular trends of hospitalization with vancomycin-resistant enterococcus infection in the United States, 2000-2006. Infect Control Hosp Epidemiol 30:184–186. doi: 10.1086/593956. [DOI] [PubMed] [Google Scholar]
- 5.Prematunge C, MacDougall C, Johnstone J, Adomako K, Lam F, Robertson J, Garber G. 2015. VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 37:26–35. doi: 10.1017/ice.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. 2011. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect 139:1342–1350. doi: 10.1017/S0950268810002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mave V, Garcia-Diaz J, Islam T, Hasbun R. 2009. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J Antimicrob Chemother 64:175–180. doi: 10.1093/jac/dkp154. [DOI] [PubMed] [Google Scholar]
- 8.Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. 2012. Vancomycin-resistant Enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J Hosp Med 7:243–248. doi: 10.1002/jhm.994. [DOI] [PubMed] [Google Scholar]
- 9.Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, Segreti J. 2010. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin Ther 32:1713–1719. doi: 10.1016/j.clinthera.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher JC, Perez ME, Marino EA, LoCastro LG, Abrardo LA, MacDougall C. 2009. Daptomycin therapy for vancomycin-resistant enterococcal bacteremia: a retrospective case series of 30 patients. Pharmacotherapy 29:792–799. doi: 10.1592/phco.29.7.792. [DOI] [PubMed] [Google Scholar]
- 11.Balli EP, Venetis CA, Miyakis S. 2014. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother 58:734–739. doi: 10.1128/AAC.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang YC, Wang JT, Lin HY, Chang SC. 2014. Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: systematic review and meta-analysis. BMC Infect Dis 14:687. doi: 10.1186/s12879-014-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britt NS, Potter EM, Patel N, Steed ME. 2015. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of Veterans Affairs patients. Clin Infect Dis 61:871–878. doi: 10.1093/cid/civ444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinnell JA, Arias CA. 2015. Editorial commentary: linezolid vs daptomycin for vancomycin-resistant enterococci: the evidence gap between trials and clinical experience. Clin Infect Dis 61:879–882. doi: 10.1093/cid/civ449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, Liang L, Ji L, Chen D, Zhang Y, Zhu Y, Patel K. 2016. Similar efficacy and safety of daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bloodstream infections: a meta-analysis. Int J Antimicrob Agents 48:231–238. doi: 10.1016/j.ijantimicag.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Tavadze M, Rybicki L, Mossad S, Avery R, Yurch M, Pohlman B, Duong H, Dean R, Hill B, Andresen S, Hanna R, Majhail N, Copelan E, Bolwell B, Kalaycio M, Sobecks R. 2014. Risk factors for vancomycin-resistant enterococcus bacteremia and its influence on survival after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 49:1310–1316. doi: 10.1038/bmt.2014.150. [DOI] [PubMed] [Google Scholar]
- 17.Lautenbach E, Bilker WB, Brennan PJ. 1999. Enterococcal bacteremia: risk factors for vancomycin resistance and predictors of mortality. Infect Control Hosp Epidemiol 20:318–323. [DOI] [PubMed] [Google Scholar]
- 18.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. 2012. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 56:3174–3180. doi: 10.1128/AAC.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. 2014. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 58:1494–1500. doi: 10.1128/AAC.02274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JR, Barber KE, Raut A, Aboutaleb M, Sakoulas G, Rybak MJ. 2015. Beta-lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 70:1738–1743. doi: 10.1093/jac/dkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JR, Barber KE, Raut A, Rybak MJ. 2015. Beta-lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother 59:2842–2848. doi: 10.1128/AAC.00053-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla BS, Shelburne S, Reyes K, Kamboj M, Lewis JD, Rincon SL, Reyes J, Carvajal LP, Panesso D, Sifri CD, Zervos MJ, Pamer EG, Tran TT, Adachi J, Munita JM, Hasbun R, Arias CA. 2016. Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: is it time to change the breakpoint? Clin Infect Dis 62:1514–1520. doi: 10.1093/cid/ciw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. A scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. 2008. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 46:1000–1008. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 25.Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, Chiu A, Raybardhan S, Science M, Fernando E, Tomlinson G, Bell CM, Morris AM. 2015. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 60:1451–1461. doi: 10.1093/cid/civ120. [DOI] [PubMed] [Google Scholar]
- 26.Britt NS, Potter EM, Patel N, Steed ME. 22 December 2016. Comparative effectiveness and safety of standard-, medium-, and high-dose daptomycin strategies for the treatment of vancomycin-resistant enterococcal bacteremia among Veterans Affairs patients. Clin Infect Dis doi: 10.1093/cid/civ815. [DOI] [PubMed] [Google Scholar]
- 27.Aster RH, Bougie DW. 2007. Drug-induced immune thrombocytopenia. N Engl J Med 357:580–587. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 28.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. 2005. Weaknesses of goodness-of-fit tests for evaluating propensity score models: the case of the omitted confounder. Pharmacoepidemiol Drug Saf 14:227–238. doi: 10.1002/pds.986. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum PR, Rubin DB. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55. [Google Scholar]
- 30.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 31.Wazny LD, Ariano RE. 2000. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy 20:292–307. doi: 10.1592/phco.20.4.292.34883. [DOI] [PubMed] [Google Scholar]
- 32.Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 50:1568–1574. doi: 10.1086/652767. [DOI] [PubMed] [Google Scholar]
- 33.Liem YS, Wong JB, Hunink MM, de Charro FT, Winkelmayer WC. 2010. Propensity scores in the presence of effect modification: a case study using the comparison of mortality on hemodialysis versus peritoneal dialysis. Emerg Themes Epidemiol 7:1. doi: 10.1186/1742-7622-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.