ABSTRACT
Emergence of drug-resistant Plasmodium falciparum strains has led to a situation of haste in the scientific and pharmaceutical communities. Hence, all their efforts are redirected toward finding alternative chemotherapeutic agents that are capable of combating multidrug-resistant parasite strains. In light of this situation, scientists have come up with the concept of hybridization of two or more active pharmacophores into a single chemical entity, resulting in “antimalarial hybrids.” The approach has been applied widely for generation of lead compounds against deadly diseases such as cancer and AIDS, with a proven potential for use as novel drugs, but is comparatively new in the sphere of antimalarial drug discovery. A sudden surge has been evidenced in the number of studies on the design and synthesis of hybrids for treating malaria and may be regarded as proof of their potential advantages over artemisinin-based combination therapy (ACT). However, it is evident from recent studies that most of the potential advantages of antimalarial hybrids, such as lower toxicity, better pharmacokinetics, and easier formulation, have yet to be realized. A number of questions left unaddressed at present need to be answered before this approach can progress to the late stages of clinical development and prove their worth in the clinic. To the best of our knowledge, this compilation is the first attempt to shed light on the shortcomings that are surfacing as more and more studies on molecular hybridization of the active pharmacophores of known antimalarials are being published.
KEYWORDS: antimalarials, combination therapy, hybrids, covalent bitherapy
INTRODUCTION
Malaria continues to be a prominent killer of populations in the tropics. In the recent past, we had already been hit with a major setback in the effective control of malaria, when chloroquine (CQ) lost its position as a first-line antimalarial drug (1, 2). Consequently, most 4-aminoquinoline (AQ) drugs show cross-resistance due to their structural relationship to chloroquine. More recently, the endoperoxide sesquiterpene lactone-artemisinin (lactone-ART) (and its derivatives) became the mainstay for treating malaria. They were considered to represent the only class of potential drugs available to vitiate the impact of multidrug-resistant strains of Plasmodium. The World Health Organization (WHO), over the last decade, has been advocating the deployment of artemisinin-based combination therapy (ACT) as the “gold standard” for treatment of all malaria infections in areas afflicted by Plasmodium falciparum. It involves the simultaneous use of two or more blood schizonticidal drugs with independent modes of action and different biochemical targets in the parasite (3). However, recent reports on the emergence of artemisinin resistance have increased awareness of the risk of returning our efforts at reducing the worldwide malaria burden to ground zero (4, 5). As ACT is one of the last viable treatment options that we presently have, the current state of affairs is extremely worrisome.
Although true clinical resistance to artemisinins has not been confirmed in parasites collected from patients, there have been reports of clinical failures of artemisinin treatment (6). A small number of cases with poor responses to artesunate or artemether have also been reported in western Thailand, India, and Sierra Leone (7, 8). Some clinical parasite isolates from Nigeria and Madagascar appear to exhibit reduced sensitivity (9, 10).
Factors such as failure of mosquito control methods, development of parasite resistance to the existing antimalarials, lack of an effective antimalarial vaccine reaching clinical application, shortfall in drug supply versus demand, inadequate drug deployment, and poor patient compliance contribute to the severity of the malaria menace (11).
The present scenario has directed the attention of researchers toward exploring new, multifaceted avenues of drug discovery, the outcome of which has been the design and synthesis of antimalarial “hybrids.”
Hybrid compounds can be defined as chemical entities with two or more structural moieties with different biological functions, thus combining two or more pharmacophores in a single molecule (12, 13). In simple terms, it is a rational chemistry-based approach which involves the covalent linking of two molecules, each with its own antimalarial activity, to produce a single hybrid molecule with dual activity (14). Therefore, the concept, also known as “covalent biotherapy” or “double drugs,” can be regarded as an extension of the concept of a fixed-dose combination of two or more drugs in a single tablet. Hybridization of molecules is a powerful tool that has been utilized by several research groups to develop compounds with the potential to treat a number of diseases such as cancer, AIDS, and tuberculosis and is now gaining momentum in the field of antimalarial drug discovery.
Hybrid molecules can be classified on three different bases:
Mode of interaction of the individual pharmacophores with target
Nature/form of presentation
Nature of the linker unit employed
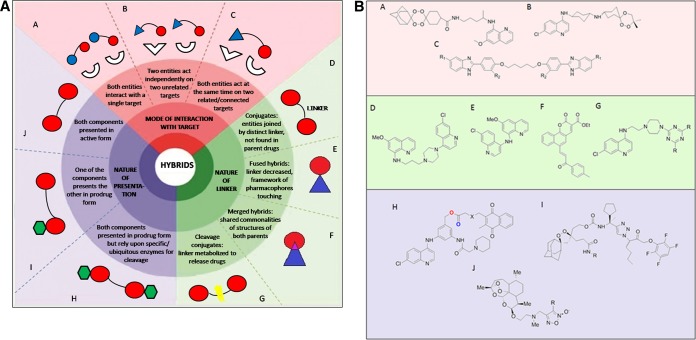
Figure 1 illustrates a detailed classification of hybrids based on the categories listed above, along with an example of a hybrid compound in each category (12–24).
FIG 1.
(A) Hybrid compounds classified according to (a) mode of interaction of the individual pharmacophores with target, (b) nature/form of presentation, and (c) nature of the linker unit employed. (B) Chemical structures of compounds representing antimalarial hybrids in each category. The chemical structure representing a merged hybrid (F) is that of a potential anticancer agent.
ADVANTAGES OF HYBRIDS
The suddenly accelerated pace of studies on the design and synthesis of hybrid antimalarial drugs stands strongly in support of the potential advantages of hybrids over ACTs, which are listed below.
Drug resistance.
The use of double-drug or dual molecules can be regarded as an approach that would reduce the risk of drug resistance development by the mutual protection of each pharmacophoric moiety. This is especially useful in designing drugs such as the aminoquinolines (for example, chloroquine), where resistance does not surface because of an altered target but because of an inability to latch onto the target. It will be interesting to evaluate whether the concept of “covalent bitherapy” can be exploited to develop hybrid molecules with the ability to restore the activity of members of other drug classes, such as antifolates (e.g., sulfadoxine/pyrimethamine), which have been rendered ineffective by emergence of resistance (25).
Solubility.
In case of fixed-dose combinations, different levels of bloodstream uptake occur due to differences in the solubility of the partner drugs. However, with a hybrid, one can surpass the fine-tuning required to ensure similar blood levels of drugs administered in the same tablet. If one moiety of the hybrid molecule is more soluble than the other, its uptake capacity can be used to contribute to the bioavailability of the other (26). Additionally, the nature of the linker employed can also contribute to the solubility of the entire unit. For example, the linker employed in chloroquine-pyrimethamine hybrids has two ethylene oxide units, which take part in hydrogen bonding with the water molecules and most likely contribute to its good solubility in both acidic and neutral media (27).
Synergism.
If the active moieties of the two partner drugs are linked and if the spacing is appropriate, they may interact synergistically and display higher activity than as free agents (13). Walsh et al. gave a proof of concept for the hybridization of artemisinin and quinine in a single molecule, which showed an enhanced antimalarial effect in comparison to that of each of the parent compounds as well as in comparison to that of a 1:1 mixture of artemisinin and quinine (28). Another example illustrating the synergistic effect of covalent linkage was furnished by Benoit-Vical and coworkers (29). They combined trioxane and chloroquine into a single moiety, forming a trioxaquine, which displayed better antimalarial activity than the two separate precursors. Also, in addition to all the properties of trioxane-containing molecules, the hybrid was also able to inhibit the polymerization of β-hematin, a property of chloroquine. Similar results have been obtained in the case of artemisinin-primaquine phosphate (artemisinin-Primaquine [PQ]) and stilbene-chalcone (30, 31) hybrids.
Pharmacokinetics.
The pharmacokinetic properties of a hybrid are easier to predict and hence to manipulate than those of the two individual drugs. Therefore, problems pertaining to pharmacokinetics, metabolic stability, or side effects of individual molecules are rectified in the form of a hybrid, as the entire drug molecule might be toxic in a few cases, but its pharmacophore might not necessarily be as toxic. If a toxicophore fragment of a given drug molecule does not overlap the pharmacophore, then it may be possible to redesign the molecule (32). For example, thalidomide derivatives devoid of teratogenic effects have been developed for treating chronic inflammation employing hybridization techniques, based on studies demonstrating that the teratogenicity is due to its distinct toxic subunit, glutarimide (33). PQ, an 8-aminoquinoline, has serious side effects and is known to induce hemolysis, especially in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals. However, Vangapandu and coworkers (34) demonstrated that 8-quinoline amine conjugates as well as the corresponding “double prodrugs” had promising in vivo activity in mice. If these compounds, in which the basic pharmacophore is primaquine phosphate, were modified to improve their blood schizonticide activity, they would have the capability to be employed as broad-spectrum (tissue and blood schizonticides) antimalarial agents.
Stability.
Joubert et al. (35) employed the differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) techniques to ascertain whether the addition of 9-aminoacridine moiety would impart stability to the otherwise unstable artemisinin pharmacophore. Affirming the stability of potential antimalarial compounds during the initial stages of development is of utmost importance, so as to ensure that the compound will be able to tolerate the extreme conditions present in regions of malaria endemicity. The hybrids that emerged in their study were extremely stable, with minimum weight losses. It is clearly evident from that study that the bulkiness and rigidity of the acridine ring imparted stability to the complete hybrid structure.
Incorporation of adducts.
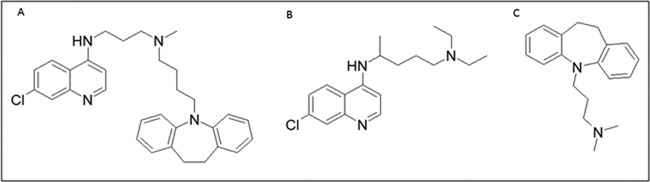
Forming hybrids allows grafting of suitable inhibitors of parasite resistance or water-soluble molecules to overcome poor solubility. For example, the first attempt to counteract chloroquine resistance in P. falciparum, by blocking its export from the parasite digestive vacuole (DV), involved linking a CQ-like moiety to a reversal agent (RA) via an alkyl linker (36). A number of calcium channel blockers, such as nifedipine and verapamil, and their derivatives, such as imipramine, dibenzylmethylamines, primaquine phosphate, and dihydroanthracenes, are known to restore sensitivity to chloroquine in resistant strains (37–40). Certain RAs are known to inhibit P. falciparum digestive vacuole membrane protein PfCRT (P. falciparum chloroquine resistance transporter)-associated export of chloroquine from its site of action in the DV. This occurs as a consequence of mutations in PfCRT. It was hypothesized that such a hybrid molecule would deliver the quinoline moiety and the RA in a 1:1 ratio, lowering the dose of reversal agent required in comparison to the dose that would be needed in cases in which the two components would be administered individually, making the effective dose much lower. The hybrid was termed a “reversed chloroquine” (Fig. 2), and although it was effective against chloroquine-resistant parasites both in vitro and in vivo, it did not move into further development on account of being highly lipophilic.
FIG 2.
Chemical structures of (A) reversed chloroquine (RCQ), as proposed by Burgess et al. (36), and (B) chloroquine and (C) imipramine (RA), employed for its synthesis.
Multistage antimalarial strategy.
Most antimalarial drugs in the current treatment strategies primarily target the erythrocytic stages of the malaria parasite in the human blood system. But to ensure malaria eradication, new drugs are urgently needed that restrict transmission of the parasite between the human host and the mosquito vector and that eliminate the parasite in its various stages during its cycle in the human body. The first study based on the covalent combination of molecules acting on different stages of the parasite life cycle was conducted by Capela et al. (30). The work describes the synthesis of hybrid molecules containing PQ and artemisinin (ART) pharmacophoric units, and their efficacies against Plasmodium hepatic and erythrocytic stages, both in vitro and in animal models of malaria. PQ is the only drug approved against liver stages of Plasmodium, including parasites acutely infecting the liver and hypnozoites. None of the other approved drugs reliably clear hypnozoites. PQ is also effective against sexual stages, i.e., the gametocytes, thereby disrupting the transmission of infection to mosquitoes. Therefore, PQ is administered in combination with a therapeutic agent that acts against blood-stage parasites. This strategy is aimed at reliably curbing infections with P. vivax or P. ovale and thereby preventing relapses due to the development of subsequent blood-stage infections from hypnozoites.
Miranda et al. (18) reported a series of hybrid compounds combining either a 1,2,4-trioxane or 1,2,4,5-tetraoxane and 8-aminoquinoline moieties. The hybrids were synthesized and screened for their antimalarial activity.
Both those studies indicated that peroxide-8-aminoquinoline hybrids can serve as promising lead compounds to develop potent agents that possess all the desired antimalarial multistage activities in a single chemical entity that may emerge as drugs of choice in malaria elimination campaigns.
Commercial aspects.
Goals such as cost-effective production (leading to greater chances of reaching the masses), better patient compliance, and freedom from patent restrictions, if achieved, would contribute to the path of hybrid compounds in emerging as the next-generation antimalarials.
WHICH ARE MORE EFFICACIOUS: HYBRIDS OR COMBINATIONS?
Although interesting from a chemical biology perspective, the same antimalarial potency of hybrid molecules might be achieved with combination therapy. The prime force driving the concept of hybrids is their potentially higher efficacy than that of either of the parent compounds administered as monotherapy or as a fixed-dose combination. This would account for all the efforts put into designing of the hybrid molecule rather than simply formulating a mixture of the drugs. However, it is a matter of concern that the majority of the published studies have neglected the fundamental control experiment, i.e., comparison of the activity of the hybrid with that of a 1:1 ratio combination of the individual drugs. Most of the published studies on design and synthesis of antimalarial hybrids involving different pharmacophore components, starting from 2001 and continuing until the present, have been listed in Table 1. As seen from the table, among the various classes of hybrids synthesized so far, viz., endoperoxide-quinoline-based hybrids, endoperoxide-chalcone-based hybrids, etc., only a few studies have reported such a comparison to date. The advantage of the covalent linkage of individual functional moieties for antimalarial activity over their combination has been proven experimentally and documented in the form of published literature in only a minute number of studies for cultured parasites and in no cases for animal models. The hybrid may actually be less efficacious than the combination of the individual constituents. Here, one should consider the fact that the strategy employed in forming a hybrid would add bulk to the molecule, which might hinder its passage into the parasite cell, which in turn would lead to reduced penetration and suppressed activity. Pretorius et al. (27) investigated this issue to ascertain the advantages of hybrids over equimolar combinations, in terms of antimalarial activity. They concluded that there were none. The most potent hybrid in their study was as effective as its two components, chloroquine and pyrimethamine, against strain D10 and only slightly superior to chloroquine alone against strain Dd2. Those results prove that all the possible partners should be thoroughly investigated and chosen for any hybrid antimalarial project and that the advantage(s) of the hybrid over the combination needs to be proven expeditiously. Taking into consideration the current preference for combination therapy in the treatment of malaria, the hybrid drug would have to compare favorably not only to the known single agents but also to the combinations in use.
TABLE 1.
Comparison of the efficacies of antimalarial hybrids, their component drugs, and 1:1 mixturesa
| Component 1 | Component 2 | Activity in culture (IC50) | Activity in animal model | Activity with respect to components 1 and 2 in culture (component no.) | Activity with respect to mixture (1:1) in culture | Reference |
|---|---|---|---|---|---|---|
| Aminoquinoline | Trioxane | ∼40 to 48 nmol/liter | ND | ND | ND | 41 |
| Aminoquinoline | Trioxane or trioxolane | 22 to 536 nM | ED50, CD50, 0.7 to >25 mg/kg/day | ND | ND | 42 |
| Aminoquinoline | Aminopeptidase inhibitor | 150 nM to 1.4 μM | ND | ND | ND | 43 |
| Aminoquioline | Hexanoic acid derivative (GR inhibitor) | 107 to 259 nM | ND | ND | ND | 44 |
| Primaquine | Statine | 0.4 to 6.2 μM | ND | ND | ND | 45 |
| Aminoquinoline | Isatin derivative | 33 nM to 1.5 μM | ND | ND | ND | 46 |
| Trifluoromethyl-artemisinin | Mefloquine | 2.4 to 17.2 nM | Effective at 35 μmol/kg/day | Higher (2) | ND | 47 |
| Primaquine | Statine (PS777621, plasmepsin inhibitor) | 0.1 to 4.5 μM | ND | Higher (2) | ND | 48 |
| Chloroquine | Imipramine | 2.9 to 5.3 nM | Effective at 64 mg/kg/day | Higher (1) | ND | 36 |
| Ferroquine | Thiosemi-carbazone | 0.1 to 96 μM | ND | Lower (1) | ND | 49 |
| Artemisinin | Quinine | 9.0 to 10 nM | ND | Higher (1 & 2) | Higher (3-fold) | 28 |
| Aminoquinoline | Trioxolane | 4.0 to 32.0 nM | ED50, CD50, 0.7 to >25 mg/kg/day | Higher | Higher | 29 |
| Aminoquinoline | Tetraoxane | 2.0 to 33 nM | Most effective at 320 mg/kg/day, partly effective at 80 mg/kg/day | Higher (1) | ND | 50 |
| Aminoquinoline | Triazine | 4.4 to 256 ng/ml | Partly effective at 50 mg/kg/day | Lower (1) | ND | 51 |
| Atovaquone and derivatives | Statine | 0.61 to 5.3 nM | ND | ND | ND | 52 |
| Aminoquinoline/aminoacridine | Clotrimazole derivatives | 3.0 nM to 1.4 μM | Some compounds effective at 50 mg/kg/day; ED50 of one compound, 6.3 mg/kg/day | Variable with strains (1), higher (2) | ND | 53 |
| Chloroquine | Astemizole | 0.02 to 0.61 μM | Effective at 80 to 200 mg/kg | Higher (1), higher (2) | ND | 54 |
| Artemisinin | Dipeptidyl vinyl sulfone | 2.08 to 4.81 nM | ND | Higher (1), higher (2) | ND | 55 |
| Chalcone | Thiolactone/Isatin | 0.68 to 14.9 μM | ND | Higher (2) | ND | 56 |
| Artemisinin (1,2,4-trioxane pharmacophore) | Ferroquine (ferrocenel-quinoline) | 16 to 43 nM | 10 mg/kg/day | Comparable to artemisinin, ferroquine, & trioxaquine; higher than chloroquine | ND | 57 |
| Artemisinin | Primaquine | 9.1 to 12.5 nM | 9 mg/kg/day | Lower (1), higher (2) | ND | 30 |
| Chloroquine | Thiazolidinone | 0.02 to >22 μM | Two compounds effective at 10 mg/kg/day | Variable with strains (1) | ND | 58 |
| Artemisinin/naphthoquinone | Aminoquinoline | 0.02 to 0.64 μM | ND | Comparable (1), comparable (2) | ND | 59 |
| Stilbene | Chalcone | 1.4 to 6.4 μM | ND | Higher (1), higher (1) | Higher | 31 |
| Chloroquine | Chalcone | 29 to 314 nM | ND | Variable with strains (1) | ND | 60 |
| Coumarin | Trioxane | 39 to >500 ng/ml | One compound partly effective at 96 mg/kg/day | ND | ND | 61 |
| Chloroquine | Chalcone | 3.6 to 380 ng/ml | Two compounds effective at 100 mg/kg/day | Lower (1) | ND | 62 |
| Amodiaquine | Phenyl furoxan | 0.630 μM | ND | Higher | ND | 63 |
| Chloroquine | Primaquine | 0.08 to 0.64 μM | Effective at 60 mg/kg/day | Variable with strains (1 & 2) | Higher (strain K1), lower (strains 3D7 & Dd2) | 64 |
| Artemisinin | Quinoline | 5.0 to 21.5 nM | ED50, <0.8 to 1.4 mg/kg/day | Lower (1), higher (2) | ND | 65 |
| Aminoquinoline | Triazine | 0.17 to 1.7 nM | ND | Variable with strains (1) | ND | 66 |
| Tetraoxane | Dipeptidyl vinyl sulfone | 10.7 to 175 nM | ND | Variable with strains (1) | ND | 67 |
| Chloroquine | Hydroxy-pyridone | 0.004 to 71.6 μM | ND | Higher (1) | ND | 68 |
| Quinoline | Triazole | 1.4 to 109 μM | ND | Lower (1) | ND | 69 |
| Artemisinin | Triazine | 5.5 to 85 nM | ND | Comparable (1), higher (2) | Lower (strain NF54), comparable (strain Dd2) | 70 |
| Artemisinin | Acridine | 2.6 to 430 nM | ND | Lower (1) | ND | 35 |
| Quinoline | Diarylether | 20.3 to >1,200 nM | ND | Lower (1) | ND | 71 |
| Tetraoxane | Pyrimidine | 9.8 to 81.2 nM | ND | ND | ND | 72 |
| Trioxane | Egonol and/or ferrocene | 0.3 to 88 nM | ND | Higher (1), lower (2) | ND | 73 |
| Quinoline | Pyrimidine | 22 to 4,310 nM | ND | Lower (1), lower (2) | ND | 74 |
| Artesunate | Indoloquinoline | 0.4 to 3.2 nM | One compound effective at 40 mg/kg/day | Higher (1), higher (1) | ND | 75 |
| Chloroquine | Hydroxi-pyridone | 0.04 to 0.46 μM | Partly effective at 8 mg/kg/day | Variable with strains (1) | ND | 76 |
| Aminoquinoline | Pyrazolopyrimidine | 0.005 to 1.6 μM | Variable with strains (1) | ND | 77 | |
| Primaquine | Pyrimidine | 1.93 to 6.46 μM | ND | Lower (1) | ND | 78 |
| Quinoline | Sulfonamide | 0.05 to 1.63 μM | Partly effective at 10 mg/kg/day | Higher (1), higher (1) | ND | 79 |
| Trioxane | Ferrocene | 7.2 to 30.2 nM | ND | Lower (1), higher (2) | ND | 80 |
| Aminoquinoline | s-Triazine | 0.22 to 8.3 μM | ND | Higher (1) | ND | 81 |
| Aminoquinoline | Atorvastatin | 0.4 to 6.39 μM | ND | Higher (1), higher (2) | ND | 82 |
CD50, 50% cytotoxic dose; ED50, 50% effective dose; GR, glucocorticoid receptor; kg, kilograms of body weight; ND, not determined.
NEW METABOLIC LIABILITIES THAT ARISE BY MOLECULAR HYBRIDIZATION
Hybridization can lead to loss or gain of favorable absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME/PK) properties of the individual pharmacophoric moieties. Thelingwani et al. (83) addressed the various metabolic challenges that arise in covalently linking two active pharmacophores by characterizing artemisinin-chloroquinoline hybrids (47) and concluded that though the technique combines the desirable properties, certain unfavorable properties are also carried along in the process and need to be accounted for. The experiment investigating metabolic stability and metabolite identification showed that the hybrids were not extensively metabolized, with the major amount of the parent compound remaining unchanged after 1 h of incubation in hepatocytes. As a result, the proportions of the detected metabolites were very low compared to those of the parent hybrid molecule. Contrastingly, in another study by the same group, metabolism of 4-aminoquinoline-3-hydroxypyridin-4-one hybrids was predicted to be extensive in cryopreserved human hepatocytes (mainly via the linker chain), leading to formation of various primary and secondary metabolites (84).
The long half-life property of chloroquine was lost in both artemisinin-chloroquinoline and 4-aminoquinoline-3-hydroxypyridin-4-one hybrids, which in turn behaved more like the other pharmacophore present (artemisinin in the first case) by displaying a short half-life. Intermediate to fast clearance in hepatocytes signifies that their effective regimen should be dosing more than once daily in the treatment or prophylaxis of malaria. The most potent compounds were the fastest cleared. Therefore, the strategy for molecular hybridization should focus on designing more metabolically stable hybrids, thereby achieving the desired regimen of dosing once a day.
Additionally, the artemisinin-chloroquinoline hybrid compounds were observed to inhibit the enzymes involved in their own metabolism, the cytochrome P450 (CYP) enzymes. Hence, they inhibited their own metabolism and that of other compounds as well that share a common clearance mechanism. This gives rise to a liability because of the risk of drug-drug interactions. Although the parent compounds have been reported to inhibit a few CYP enzymes, a striking difference here is the inhibition of all the five isoforms by the hybrids (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4). The 50% inhibitory concentration (IC50) for CYP3A4 inhibition was noted to be less than 1 μM, further substantiating the risk for drug-drug interactions. Intermediate risk of interactions with the substrates of CYP3A4 and CYP2D6 was observed for 4-aminoquinoline-3-hydroxypyridin-4-one hybrids, with IC50s ranging from 1 to 10 μM. Hence, hybridization, instead of masking an undesirable property of the parent compounds, led to broadening it.
Joubert et al. (35) observed unfavorable drug-likeness properties in artemisinin-acridine hybrids. The hybrids showed extremely low solubility and absorption levels under physiological conditions and undefined blood-brain barrier (BBB) penetration levels, which they proposed to result from the blocking of the polar primary/secondary amine groups in the intermediates, thereby leading to a reduction in the formation of hydrogen bonds. Another possible reason could be the presence the lipophilic artemisinin moiety. Also, the hybrids were unable to serve efficaciously against the chloroquine-resistant P. falciparum Dd2 strain, although dihydroartemisinin was active against the same strain. Hence, one can infer that hybridization of the artemisinin and acridine moieties resulted in an antagonistic interaction and that the presence of acridine rendered artemisinin ineffective against the Dd2 strain.
Ferrocene-pyrimidine conjugates were also reported by Chopra et al. (85) to exhibit moderate to poor aqueous solubility, and the lack of hydrophilicity was distinctly reflected in their average levels of in vitro antiplasmodial activity against CQS NF54 strain. The hybrid with the lowest IC50 was the one with the highest hydrophilicity. That study proved that water solubility should be regarded as an essential property of newly synthesized anyimalarial hybids to ensure adequate absorption and plasma concentrations.
SOME METABOLIC LIABILITIES PERSIST AFTER MOLECULAR HYBRIDIZATION
It has been proposed that the side effects manifested by individual drugs may be masked when they are linked covalently in a hybrid. Contrastingly, some hybrids display the same metabolic liabilities as are exhibited by their constituents. For example, the 4-aminoquinolines, which have been drugs of choice for various antimalarial hybrid development programs, have been implicated in the occurrence of clinically significant cardiovascular effects (86). They cause significant prolongation of the electrocardiograph QT interval, raising the risk for fatal ventricular arrhythmias such as “torsades de pointes” and sudden cardiac death (87). When injected rapidly, chloroquine is potentially hypotensive (88).
Very low solubility and absorption levels of artemisinin-acridine hybrids have been attributed to inherent pharmacokinetic limitations of artemisinin, viz., poor water solubility, absorption, and plasma bioavailability (35). Hepatotoxicity of acridine and neurotoxicity of dihydroartemisinin are also well reported (89, 90). The process could neither reduce the toxicities associated with the acridine or artemisinin moieties nor overcome the resistance of parasites to chloroquine.
LINKER SELECTION
In the design of hybrid drugs for malaria, very little attention has been given to the relative proximity of the reputed cellular targets and the distance between the two components of the hybrid. Although the data may be difficult to obtain in practice, they nevertheless should be considered in the design of hybrid drugs, especially in situations where a metabolically resistant linker unit is employed.
Lombard et al. (65) have devised a number of strategies to covalently link quinolines and artemisinins, making use of different linkers. Their hybrids displayed activity either similar to or higher than that of chloroquine against CQ-sensitive P. falciparum strains and activity greater than that of chloroquine against CQ-resistant P. falciparum strains. The results of all their studies indicated that cyclic linkers should be avoided as they contribute to decreased antiplasmodial properties and that the length of the linker should be curtailed to two or three carbon atoms. Hybrids with a linker chain length of greater than three were found to be less potent than chloroquine. The members of the O'Neill group, which worked on synthetic 1,2,4-trioxolaquines (91), and several others (62, 92), have reported similar results. Therefore, the length and nature of the linker exert a strong influence on the antimalarial efficacy of the conjugates.
It is noteworthy that, in contrast with the studies described above, another study to do with linking astemizole derivatives with an aminoquinoline via a piperazine/aminopiperidine linker concluded that hybrids with conformationally constrained cyclic linkers also exhibited potent activity against a CQ-resistant K1 strain (54). Similarly, the two most potent hybrids from a series of quinoline-pyrimidine hybrids evaluated by Pretorius et al. (27) contained rigid aromatic and piperazine linkers. This indicates that the flexibility of the linker between the two pharmacophores does not govern the activity of these compounds.
CONCLUDING REMARKS
The potential advantages of antimalarial hybrids, such as lower toxicity, better pharmacokinetics, and easier formulation, have yet to be realized. The paucity of information about pharmacokinetics, pharmacodynamics, and rational dosing of drugs represents a critical knowledge gap that needs to be addressed in order to use current drugs in conjunction with other tools to reduce malaria transmission, as well as to provide rationally designed treatment strategies. The hybrid approach is interesting in itself but likely no more so than the others. However, it can take a substantial position in the strategy for searching for new antimalarials. It is important to indicate, that instead of exhibiting potential promising pharmacological interest, no antimalarial hybrid drug is currently either in development and or at a preclinical step in this portfolio. From over 100 trioxaquines tested so far, the trioxaquine PA1103/SAR116242 was selected by Palumed in collaboration with Sanofi-Aventis as a drug development candidate for treatment of uncomplicated malaria as the first “fusion” antimalarial (93). However, it no longer appears in the global malaria portfolio as it was abandoned in preclinical development. Our opinion is that for any hybrid antimalarial project, the choice of partners needs careful justification, and the advantage(s) of the hybrid over the combination needs to be proven at the earliest opportunity. Before going ahead with designing a hybrid, both the benefits and demerits of the product, and the strategy employed, should be thoroughly investigated and the hybrid candidate should then be taken a level up only when the advantages overshadow the drawbacks.
ACKNOWLEDGMENTS
D.A. thanks the Department of Science and Technology-UK India Education Research Initiative (DST-UKIERI), R.D.G. thanks Faculty of Life Sciences and Biotechnology, South Asian University, India, and S.K.A. thanks University of Delhi, Delhi, India, for financial assistance.
REFERENCES
- 1.Wellems TE, Plowe CV. 2001. Chloroquine-resistant malaria. J Infect Dis 184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 2.Chinappi M, Via A, Marcatili P, Tramontano A. 2010. On the mechanism of chloroquine resistance in Plasmodium falciparum. PLoS One 5:e14064. doi: 10.1371/journal.pone.0014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2004. Roll back malaria. The RBM partnership's global response; a programmatic strategy 2004–2008. http://www.rollbackmalaria.org/files/files/board/meetings/docs/strategy_rev.pdf.
- 4.Talundzic E, Okoth SA, Congpuong K, Plucinski MM, Morton L, Goldman IF, Kachur PS, Wongsrichanalai C, Satimai W, Barnwell JW, Udhayakumar V. 2015. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog 11:e1004789. doi: 10.1371/journal.ppat.1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal D, Sharma M, Dixit SK, Dutta RK, Singh AK, Gupta RD, Awasthi SK. 2015. In vitro synergistic effect of fluoroquinolone analogues in combination with artemisinin against Plasmodium falciparum; their antiplasmodial action in rodent malaria model. Malar J 14:48. doi: 10.1186/s12936-015-0561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White NJ. 2004. Antimalarial drug resistance. J Clin Invest 113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noedl H, Se Y, Sriwichai S, Schaecher K, Teja-Isavadharm P, Smith B, Rutvisuttinunt W, Bethell D, Surasri S, Fukuda MM, Socheat D, Chan Thap L. 2010. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin Infect Dis 51:e82–. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 8.Pillai DR, Lau R, Khairnar K, Lepore R, Via A, Staines HM, Krishna S. 2012. Artemether resistance in vitro is linked to mutations in PfATP6 that also interact with mutations in PfMDR1 in travellers returning with Plasmodium falciparum infections. Malar J 11:131. doi: 10.1186/1475-2875-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andriantsoanirina V, Ratsimbasoa A, Bouchier C, Jahevitra M, Rabearimanana S, Radrianjafy R, Andrianaranjaka V, Randriantsoa T, Rason MA, Tichit M, Rabarijaona LP, Mercereau-Puijalon O, Durand R, Ménard D. 2009. Drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrob Agents Chemother 53:4588–4597. doi: 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meremikwu MM, Odey F, Oringanje C, Oyo-Ita A, Effa E, Esu EB, Eyam E, Oduwole O, Asiegbu V, Alaribe A, Ezedinachi EN. 2012. Open-label trial of three dosage regimens of fixed-dose combination of artemisinin and naphthoquine for treating uncomplicated falciparum malaria in calabar, Nigeria. Malar J 11:413. doi: 10.1186/1475-2875-11-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.Meunier B. 2008. Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res 41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JJ, Bell A. 2009. Hybrid drugs for malaria. Curr Pharm Des 15:2970–2985. doi: 10.2174/138161209789058183. [DOI] [PubMed] [Google Scholar]
- 14.Yadav N, Sharma C, Awasthi SK. 2014. Diversification in the synthesis of trioxane and tetraoxane analogs. RSC Adv 4:5469–5498. doi: 10.1039/c3ra42513d. [DOI] [Google Scholar]
- 15.Morphy R, Kay C, Rankovic Z. 2004. From magic bullets to designed multiple ligands. Drug Discov Today 9:641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 16.Davioud-Charvet E, Delarue S, Biot C, Schwöbel B, Boehme CC, Müssigbrodt A, Maes L, Sergheraert C, Grellier P, Schirmer RH, Becker K. 2001. A prodrug form of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J Med Chem 44:4268–4276. doi: 10.1021/jm010268g. [DOI] [PubMed] [Google Scholar]
- 17.Morphy R, Rankovic Z. 2005. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 48:6523–6543. [DOI] [PubMed] [Google Scholar]
- 18.Miranda D, Capela R, Albuquerque IS, Meireles P, Paiva I, Nogueira F, Amewu R, Gut J, Rosenthal PJ, Oliveira R, Mota MM, Moreira R, Marti F, Prudêncio M, O'Neill PM, Lopes F. 2014. Novel endoperoxide-based transmission-blocking antimalarials with liver- and blood-schizontocidal activities. ACS Med Chem Lett 5:108–112. doi: 10.1021/ml4002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coslédan F, Fraisse L, Pellet A, Guillou F, Mordmüller B, Kremsner PG, Moreno A, Mazier D, Maffrand JP, Meunier B. 2008. Selection of a trioxaquine as an antimalarial drug candidate. Proc Natl Acad Sci U S A 105:17579–17584. doi: 10.1073/pnas.0804338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Gómez H, Hernández-Núñez E, León-Rivera I, Guerrero-Alvarez J, Cedillo-Rivera R, Moo-Puc R, Argotte-Ramos R, Rodríguez-Gutiérrez Mdel C, Chan-Bacab MJ, Navarrete-Vázquez G. 2008. Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids. Bioorg Med Chem Lett 18:3147–3151. doi: 10.1016/j.bmcl.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Lödige M, Hiersch L. 2015. Design and synthesis of novel hybrid molecules against malaria. Int J Med Chem 2015:458319. doi: 10.1155/2015/458319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S. 2010. Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett 20:7205–7211. doi: 10.1016/j.bmcl.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan SS, Due E, Lauterwasser EMW, Leyva MJ, Ellman JA, Bogyo M, Renslo AR. 2011. A fragmenting hybrid approach for targeted delivery of multiple therapeutic agents to the malaria parasite. ChemMedChem 6:415–419. doi: 10.1002/cmdc.201100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertinaria M, Orjuela-Sanchez P, Marini E, Guglielmo S, Hofer A, Martins YC, Zanini GM, Frangos JA, Gasco A, Fruttero R, Carvalho LJ. 2015. NO-donor dihydroartemisinin derivatives as multitarget agents for the treatment of cerebral malaria. J Med Chem 58:7895–7899. doi: 10.1021/acs.jmedchem.5b01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muregi FW, Ishih A. 2010. Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug Dev Res 71:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frantz S. 2006. The trouble with making combination drugs. Nat Rev Drug Discov 5:881–882. doi: 10.1038/nrd2188. [DOI] [PubMed] [Google Scholar]
- 27.Pretorius SI, Breytenbach WJ, de Kock C, Smith PJ, N′Da DD. 2013. Synthesis, characterization and antimalarial activity of quinoline-pyrimidine hybrids. Bioorg Med Chem 21:269–277. doi: 10.1016/j.bmc.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JJ, Coughlan D, Heneghan N, Gaynor C, Bell A. 2007. A novel artemisinin–quinine hybrid with potent antimalarial activity. Bioorg Med Chem Lett 17:3599–3602. doi: 10.1016/j.bmcl.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Benoit-Vical F, Lelièvre J, Berry A, Deymier C, Dechy-Cabaret O, Cazelles J, Loup C, Robert A, Magnaval JF, Meunier B. 2007. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob Agents Chemother 51:1463–1472. doi: 10.1128/AAC.00967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capela R, Cabal GG, Rosenthal PJ, Gut J, Mota MM, Moreira R, Lopes F, Prudêncio M. 2011. Design and evaluation of primaquine-artemisinin hybrids as a multistage antimalarial strategy. Antimicrob Agents Chemother 55:4698–4706. doi: 10.1128/AAC.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma N, Mohanakrishnan D, Shard A, Sharma A, Saima Sinha AK, Sahal D. 2012. Stilbene-chalcone hybrids: design, synthesis, and evaluation as a new class of antimalarial scaffolds that trigger cell death through stage specific apoptosis. J Med Chem 55:297–311. doi: 10.1021/jm201216y. [DOI] [PubMed] [Google Scholar]
- 32.Nogrady T, Weaver DF. Medicinal chemistry: a molecular and biochemical approach, 3rd ed Oxford University Press, New York, NY, USA. [Google Scholar]
- 33.Bosquesi PL, Melo TRF, Vizioli EO, Santos JL, Chung MC. 2011. Anti-inflammatory drug design using a molecular hybridization approach. Pharmaceuticals (Basel) 4:1450–1474. doi: 10.3390/ph4111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Kaul CL, Jain R. 2003. 8-quinolinamines and their pro prodrug conjugates as potent blood-schizontocidal antimalarial agents. Bioorg Med Chem 11:4557–4568. doi: 10.1016/j.bmc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Joubert JP, Smit FJ, du Plessis L, Smith PJ, N′Da DD. 2014. Synthesis and in vitro biological evaluation of aminoacridines and artemisinin-acridine hybrids. Eur J Pharm Sci 56:16–27. doi: 10.1016/j.ejps.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. 2006. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J Med Chem 49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- 38.Martin SK, Oduola AM, Milhous WK. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 39.van Schalkwyk DA, Egan TJ. 2006. Quinoline-resistance reversing agents for the malaria parasite Plasmodium falciparum. Drug Resist Updat 9:211–226. doi: 10.1016/j.drup.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Guantai E, Chibale K. 2010. How can natural products serve as a viable source of lead compounds for the development of new/novel anti-malarials? Curr Drug Deliv 7:312. doi: 10.2174/156720110793360577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basco LK, Dechy-Cabaret O, Ndounga M, Meche FS, Robert A, Meunier B. 2001. In vitro activities of DU-1102, a new trioxaquine derivative, against Plasmodium falciparum isolates. Antimicrob Agents Chemother 45:1886–1888. doi: 10.1128/AAC.45.6.1886-1888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dechy-Cabaret O, Benoit-Vical F, Robert A, Magnaval JF, Séguéla JP, Meunier B. 2003. Synthesis and biological evaluation of a new trioxaquine containing a trioxane moiety obtained by halogenocyclisation of a hemiperoxyacetal. C R Chim 6:153–160. doi: 10.1016/S1631-0748(03)00007-9. [DOI] [Google Scholar]
- 43.Flipo M, Florent I, Grellier P, Sergheraert C, Deprez-Poulain R. 2003. Design, synthesis and antimalarial activity of novel, quinoline-based, zinc metallo-aminopeptidase inhibitors. Bioorg Med Chem Lett 13:2659–2662. doi: 10.1016/S0960-894X(03)00550-X. [DOI] [PubMed] [Google Scholar]
- 44.Biot C, Dessolin J, Grellier P, Davioud-Charvet E. 2003. Double-drug development against antioxidant enzymes from Plasmodium falciparum. Redox Rep 8:280–283. doi: 10.1179/135100003225002916. [DOI] [PubMed] [Google Scholar]
- 45.Romeo S, Dell'Agli M, Parapini S, Rizzi L, Galli G, Mondani M, Sparatore A, Taramelli D, Bosisio E. 2004. Plasmepsin II inhibition and antiplasmodial activity of primaquine-statine ‘double-drugs’. Bioorg Med Chem Lett 14:2931–2934. doi: 10.1016/j.bmcl.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Chiyanzu I, Clarkson C, Smith PJ, Lehman J, Gut J, Rosenthal PJ, Chibale K. 2005. Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorg Med Chem 13:3249–3261. doi: 10.1016/j.bmc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 47.Grellepois F, Grellier P, Bonnet-Delpon D, Bégué JP. 2005. Design, synthesis and antimalarial activity of trifluoromethylartemisinin-mefloquine dual molecules. ChemBioChem 6:648–652. doi: 10.1002/cbic.200400347. [DOI] [PubMed] [Google Scholar]
- 48.Dell'Agli M, Parapini S, Galli G, Vaiana N, Taramelli D, Sparatore A, Liu P, Dunn BM, Bosisio E, Romeo S. 2006. High antiplasmodial activity of novel plasmepsins I and II inhibitors. J Med Chem 49:7440–7449. doi: 10.1021/jm061033d. [DOI] [PubMed] [Google Scholar]
- 49.Biot C, Pradines B, Sergeant MH, Gut J, Rosenthal PJ, Chibale K. 2007. Design, synthesis, and antimalarial activity of structural chimeras of thiosemicarbazone and ferroquine analogues. Bioorg Med Chem Lett 17:6434–6438. doi: 10.1016/j.bmcl.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Opsenica I, Opsenica D, Lanteri CA, Anova L, Milhous WK, Smith KS, Solaja BA. 2008. New chimeric antimalarials with 4-aminoquinoline moiety linked to a tetraoxane skeleton. J Med Chem 51:6216–6219. doi: 10.1021/jm8006905. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A, Srivastava K, Raja Kumar S, Puri SK, Chauhan PM. 2008. Synthesis and bioevaluation of hybrid 4-aminoquinoline triazines as a new class of antimalarial agents. Bioorg Med Chem Lett 18:6530–6533. doi: 10.1016/j.bmcl.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 52.Romeo S, Parapini S, Dell'Agli M, Vaiana N, Magrone P, Galli G, Sparatore A, Taramelli D, Bosisio E. 2008. Atovaquone-statine “double-drugs” with high antiplasmodial activity. ChemMedChem 3:418–420. doi: 10.1002/cmdc.200700166. [DOI] [PubMed] [Google Scholar]
- 53.Gemma S, Campiani G, Butini S, Joshi BP, Kukreja G, Coccone SS, Bernetti M, Persico M, Nacci V, Fiorini I, Novellino E, Taramelli D, Basilico N, Parapini S, Yardley V, Croft S, Keller-Maerki S, Rottmann M, Brun R, Coletta M, Marini S, Guiso G, Caccia S, Fattorusso C. 2009. Combining 4-aminoquinoline- and clotrimazole-based pharmacophores toward innovative and potent hybrid antimalarials. J Med Chem 52:502–513. doi: 10.1021/jm801352s. [DOI] [PubMed] [Google Scholar]
- 54.Musonda CC, Whitlock GA, Witty MJ, Brun R, Kaiser M. 2009. Chloroquine–astemizole hybrids with potent in vitro and in vivo antiplasmodial activity. Bioorg Med Chem Lett 19:481–484. doi: 10.1016/j.bmcl.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 55.Capela R, Oliveira R, Gonçalves LM, Domingos A, Gut J, Rosenthal PJ, Lopes F, Moreira R. 2009. Artemisinin-dipeptidyl vinyl sulfone hybrid molecules: design, synthesis and preliminary SAR for antiplasmodial activity and falcipain-2 inhibition. Bioorg Med Chem Lett 19:3229–3232. doi: 10.1016/j.bmcl.2009.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hans RH, Gut J, Rosenthal PJ, Chibale K. 2010. Comparison of the antiplasmodial and falcipain-2 inhibitory activity of beta-amino alcohol thiolactone-chalcone and isatin-chalcone hybrids. Bioorg Med Chem Lett 20:2234–2237. doi: 10.1016/j.bmcl.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Bellot F, Coslédan F, Vendier L, Brocard J, Meunier B, Robert A. 2010. Trioxaferroquines as new hybrid antimalarial drugs. J Med Chem 53:4103–4109. doi: 10.1021/jm100117e. [DOI] [PubMed] [Google Scholar]
- 58.Rojas Ruiz FA, García-Sánchez RN, Estupiñan SV, Gómez-Barrio A, Torres Amado DF, Pérez-Solórzano BM, Nogal-Ruiz JJ, Martínez-Fernández AR, Kouznetsov VV. 2011. Synthesis and antimalarial activity of new heterocyclic hybrids based on chloroquine and thiazolidinone scaffolds. Bioorg Med Chem 19:4562–4573. doi: 10.1016/j.bmc.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Feng TS, Guantai EM, Nell M, van Rensburg CE, Ncokazi K, Egan TJ, Hoppe HC, Chibale K. 2011. Effects of highly active novel artemisinin-chloroquinoline hybrid compounds on β-hematin formation, parasite morphology and endocytosis in Plasmodium falciparum. Biochem Pharmacol 82:236–247. doi: 10.1016/j.bcp.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Sashidhara KV, Avula SR, Palnati GR, Singh SV, Srivastava K, Puri SK, Saxena JK. 2012. Synthesis and in vitro evaluation of new chloroquine-chalcone hybrids against chloroquine-resistant strain of Plasmodium falciparum. Bioorg Med Chem Lett 22:5455–5459. doi: 10.1016/j.bmcl.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 61.Sashidhara KV, Kumar A, Dodda RP, Krishna NN, Agarwal P, Srivastava K, Puri SK. 2012. Coumarin-trioxane hybrids: synthesis and evaluation as a new class of antimalarial scaffolds. Bioorg Med Chem Lett 22:3926–3930. doi: 10.1016/j.bmcl.2012.04.100. [DOI] [PubMed] [Google Scholar]
- 62.Sashidhara KV, Kumar M, Modukuri RK, Srivastava RK, Soni A, Srivastava K, Singh SV, Saxena JK, Gauniyal HM, Puri SK. 2012. Antiplasmodial activity of novel keto-enamine chalcone-chloroquine based hybrid pharmacophores. Bioorg Med Chem 20:2971–2981. doi: 10.1016/j.bmc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Mott BT, Cheng CC, Guha R, Kommer VP, Williams DL, Vermeire JJ, Cappello M, Maloney DJ, Rai G, Jadhav A, Simeonov A, Inglese J, Posner GH, Thomas CJ. 2012. A furoxan-amodiaquine hybrid as a potential therapeutic for three parasitic diseases. Medchemcomm 3:1505–1511. doi: 10.1039/c2md20238g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lödige M, Lewis MD, Paulsen ES, Esch HL, Pradel G, Lehmann L, Brun R, Bringmann G, Mueller AK. 2013. A primaquine-chloroquine hybrid with dual activity against Plasmodium liver and blood stages. Int J Med Microbiol 303:539–547. doi: 10.1016/j.ijmm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Lombard MC, N′Da DD, Tran Van Ba C, Wein S, Norman J, Wiesner L, Vial H. 2013. Potent in vivo anti-malarial activity and representative snapshot pharmacokinetic evaluation of artemisinin-quinoline hybrids. Malar J 12:71. doi: 10.1186/1475-2875-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manohar S, Khan SI, Rawat DS. 2013. 4-Aminoquinoline-triazine-based hybrids with improved in vitro antimalarial activity against CQ-sensitive and CQ-resistant strains of P. falciparum. Chem Biol Drug Des 81:625–630. doi: 10.1111/cbdd.12108. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira R, Newton AS, Guedes RC, Miranda D, Amewu RK, Srivastava A, Gut J, Rosenthal PJ, O'Neill PM, Ward SA, Lopes F, Moreira R. 2013. An endoperoxide-based hybrid approach to deliver falcipain inhibitors inside malaria parasites. ChemMedChem 8:1528–1536. doi: 10.1002/cmdc.201300202. [DOI] [PubMed] [Google Scholar]
- 68.Andayi WA, Egan TJ, Gut J, Rosenthal PJ, Chibale K. 2013. Synthesis, antiplasmodial activity, and β-hematin inhibition of hydroxypyridone-chloroquine hybrids. ACS Med Chem Lett 4:642–646. doi: 10.1021/ml4001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boechat N, Ferreira MDLG, Pinheiro LC, Jesus AM, Leite MM, Júnior CC, Aguiar AC, de Andrade IM, Krettli AU. 2014. New compounds hybrids 1H-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem Biol Drug Des 84:325–332. doi: 10.1111/cbdd.12321. [DOI] [PubMed] [Google Scholar]
- 70.Cloete TT, de Kock C, Smith PJ, N′Da DD. 2014. Synthesis, in vitro antiplasmodial activity and cytotoxicity of a series of artemisinin-triazine hybrids and hybrid-dimers. Eur J Med Chem 76:470–481. doi: 10.1016/j.ejmech.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 71.Mishra A, Batchu H, Srivastava K, Singh P, Shukla PK, Batra S. 2014. Synthesis and evaluation of new diaryl ether and quinoline hybrids as potential antiplasmodial and antimicrobial agents. Bioorg Med Chem Lett 24:1719–1723. doi: 10.1016/j.bmcl.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira R, Guedes RC, Meireles P, Albuquerque IS, Gonçalves LM, Pires E, Bronze MR, Gut J, Rosenthal PJ, Prudêncio M, Moreira R, O'Neill PM, Lopes F. 2014. Tetraoxane-pyrimidine nitrile hybrids as dual stage antimalarials. J Med Chem 57:4916–4923. doi: 10.1021/jm5004528. [DOI] [PubMed] [Google Scholar]
- 73.Reiter C, Capcı Karagöz A, Fröhlich T, Klein V, Zeino M, Viertel K, Held J, Mordmüller B, Emirdağ Öztürk S, Anıl H, Efferth T, Tsogoeva SB. 2014. Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells. Eur J Med Chem 75:403–412. doi: 10.1016/j.ejmech.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 74.Singh K, Kaur H, Smith P, de Kock C, Chibale K, Balzarini J. 2014. Quinoline-pyrimidine hybrids: synthesis, antiplasmodial activity, SAR, and mode of action studies. J Med Chem 57:435–448. doi: 10.1021/jm4014778. [DOI] [PubMed] [Google Scholar]
- 75.Wang N, Wicht KJ, Shaban E, Ngoc TA, Wang MQ, Hayashi I, Hossain MI, Takemasa Y, Kaiser M I, El Tantawy El Sayed, Egan TJ, Inokuchi T. 2014. Synthesis and evaluation of artesunate–indoloquinoline hybrids as antimalarial drug candidates. Medchemcomm 5:927–931. doi: 10.1039/c4md00091a. [DOI] [Google Scholar]
- 76.Dambuza NS, Smith P, Evans A, Norman J, Taylor D, Andayi A, Egan T, Chibale K, Wiesner L. 2015. Antiplasmodial activity, in vivo pharmacokinetics and anti-malarial efficacy evaluation of hydroxypyridinone hybrids in a mouse model. Malar J 14:505. doi: 10.1186/s12936-015-1032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kannan M, Raichurkar AV, Khan FR, Iyer PS. 2015. Synthesis and in vitro evaluation of novel 8-aminoquinoline-pyrazolopyrimidine hybrids as potent antimalarial agents. Bioorg Med Chem Lett 25:1100–1103. doi: 10.1016/j.bmcl.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Kaur H, Machado M, de Kock C, Smith P, Chibale K, Prudêncio M, Singh K. 2015. Primaquine-pyrimidine hybrids: synthesis and dual-stage antiplasmodial activity. Eur J Med Chem 101:266–273. doi: 10.1016/j.ejmech.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 79.Pinheiro LC, Boechat N, Ferreira MDLG, Júnior CC, Jesus AM, Leite MM, Souza NB, Krettli AU. 2015. Anti-Plasmodium falciparum activity of quinoline-sulfonamide hybrids. Bioorg Med Chem 23:5979–5984. doi: 10.1016/j.bmc.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 80.Reiter C, Fröhlich T, Zeino M, Marschall M, Bahsi H, Leidenberger M, Friedrich O, Kappes B, Hampel F, Efferth T, Tsogoeva SB. 2015. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids Eur J Med Chem 97:164–172. doi: 10.1016/j.ejmech.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 81.Rodrigues CA, Frade RF, Albuquerque IS, Perry MJ, Gut J, Machado M, Rosenthal PJ, Prudêncio M, Afonso CA, Moreira R. 2015. Targeting the erythrocytic and liver stages of malaria parasites with s-triazine-based hybrids. ChemMedChem 10:883–890. doi: 10.1002/cmdc.201500011. [DOI] [PubMed] [Google Scholar]
- 82.Carvalho RC, Martins WA, Silva TP, Kaiser CR, Bastos MM, Pinheiro LC, Krettli AU, Boechat N. 2016. New pentasubstituted pyrrole hybrid atorvastatin-quinoline derivatives with antiplasmodial activity. Bioorg Med Chem Lett 26:1881–1884. doi: 10.1016/j.bmcl.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 83.Thelingwani R, Leandersson C, Bonn B, Smith P, Chibale K, Masimirembwa C. 2016. Characterisation of artemisinin-chloroquinoline hybrids for potential metabolic liabilities. Xenobiotica 46:234–240. doi: 10.3109/00498254.2015.1070975. [DOI] [PubMed] [Google Scholar]
- 84.Thelingwani R, Bonn B, Chibale K, Masimirembwa C. 2014. Physicochemical and drug metabolism characterization of a series of 4-aminoquinoline-3-hydroxypyridin-4-one hybrid molecules with antimalarial activity. Expert Opin Drug Metab Toxicol 10:1313–1324. doi: 10.1517/17425255.2014.954547. [DOI] [PubMed] [Google Scholar]
- 85.Chopra R, de Kock C, Smith P, Chibale K, Singh K. 2015. Ferrocene-pyrimidine conjugates: synthesis, electrochemistry, physicochemical properties and antiplasmodial activities. Eur J Med Chem 100:1–9. doi: 10.1016/j.ejmech.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 86.White NJ. 2007. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 87.Fermini B, Fossa AA. 2003. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov 2:439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 88.Ajayi AA, Adigun AQ. 2002. Syncope following oral chloroquine administration in a hypertensive patient controlled on amlodipine. Br J Clin Pharmacol 53:404–405. doi: 10.1046/j.1365-2125.2002.01572_2.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grace JM, Aguilar AJ, Trotman KM, Peggins JO, Brewer TG. 1998. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab Dispos 26:313–317. [PubMed] [Google Scholar]
- 90.Plymale DR, de la Iglesia FA. 1999. Acridine-induced subcellular and functional changes in isolated human hepatocytes in vitro. J Appl Toxicol 19:31–38. doi:. [DOI] [PubMed] [Google Scholar]
- 91.Araújo NC, Barton V, Jones M, Stocks PA, Ward SA, Davies J, Bray PG, Shone AE, Cristiano ML, O'Neill PM. 2009. Semi-synthetic and synthetic 1,2,4-trioxaquines and 1,2,4-trioxolaquines: synthesis, preliminary SAR and comparison with acridine endoperoxide conjugates. Bioorg Med Chem Lett 19:2038–2043. doi: 10.1016/j.bmcl.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Raj R, Biot C, Carrere-Kremer S, Kremer L, Guerardel Y, Gut J, Rosenthal PJ, Kumar V. 2014. 7-Chloroquinoline-isatin conjugates: antimalarial, antitubercular, and cytotoxic evaluation. Chem Biol Drug Des 83:622–629. doi: 10.1111/cbdd.12273. [DOI] [PubMed] [Google Scholar]
- 93.Bousejra-El Garah F, Claparols C, Benoit-Vical F, Meunier B, Robert A. 2008. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob Agents Chemother 52:2966–2969. doi: 10.1128/AAC.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]