ABSTRACT
Stenotrophomonas maltophilia is an opportunistic pathogen with increasing prevalence, which is able to cause infections in immunocompromised patients or in those with a previous pathology. The treatment of the infections caused by this bacterium is often complicated due to the several intrinsic antibiotic resistance mechanisms that it presents. Multidrug efflux pumps are among the best-studied mechanisms of S. maltophilia antibiotic resistance. Some of these efflux pumps have a basal expression level but, in general, their expression is often low and only reaches high levels when the local regulator is mutated or bacteria are in the presence of an effector. In the current work, we have developed a yellow fluorescent protein (YFP)-based sensor with the aim to identify effectors able to trigger the expression of SmeVWX, an efflux pump that confers resistance to quinolones, chloramphenicol, and tetracycline when it is expressed at high levels. With this purpose in mind, we tested a variety of different compounds and analyzed the fluorescence signal given by the expression of YFP under the control of the smeVWX promoter. Among the tested compounds, vitamin K3, which is a compound belonging to the 2-methyl-1,4-naphthoquinone family, is produced by plants in defense against infection, and has increasing importance in human therapy, was able to induce the expression of the SmeVWX efflux pump. In addition, a decrease in the susceptibility of S. maltophilia to ofloxacin and chloramphenicol was observed in the presence of vitamin K3, in both wild-type and smeW-deficient strains.
KEYWORDS: inducible resistance, SmeVWX, Stenotrophomonas maltophilia, biosensors, efflux pumps
INTRODUCTION
Stenotrophomonas maltophilia is an emerging multidrug-resistant opportunistic pathogen involved in an increased number of infections (1). Among these infections, we can highlight septicemia, urinary infections, endocarditis, and respiratory infections in immunocompromised patients and in those with cystic fibrosis (2). In general, clinical isolates of S. maltophilia present low susceptibilities to a wide range of antibiotics, including macrolides, β-lactams, cephalosporins, trimethoprim-sulfamethoxazole, tetracyclines, polymyxins, aminoglycosides, chloramphenicol, carbapenems, and fluoroquinolones, making the infections caused by this bacterium difficult to treat (1). This low susceptibility to antibiotics is associated with several intrinsic resistance elements, such as antibiotic-modifying enzymes, low membrane permeability, the quinolone resistance protein SmQnr, and multidrug resistance (MDR) efflux pumps (3, 4).
Eight MDR efflux pumps (SmeABC, SmeDEF, SmeGH, SmeIJK, SmeMN, SmeOP, SmeVWX, and SmeYZ) belonging to the resistance-nodulation-cell division (RND) family have been identified in S. maltophilia K279a (4). The roles of six of them (SmeABC, SmeDEF, SmeIJK, SmeOP, SmeVWX, and SmeYZ) in intrinsic and acquired resistance to antibiotics have been analyzed (5–14).
The expression of MDR efflux pumps is generally tightly downregulated by specific transcriptional regulators, likely because their overexpression might compromise bacterial physiology (15). However, transient higher expression levels can be reached due to some particular physiological situations, as a response to stress or in the presence of effectors, including host-produced anti-infective molecules, such as bile, cationic peptides, or fatty acids (16–18), as well as agents used for therapeutic purposes, some of which might be relevant in the course of an infection (19). Knowing these effectors can be useful for understanding the role of these MDR efflux pumps besides resistance to antibiotics, as well as for predicting situations of transient antibiotic resistance that may occur in vivo (20).
RND efflux pump substrates are very diverse and include antibiotics, biocides, bile salts, detergents, aromatic hydrocarbons, homoserine lactones, and dyes (21); however, the number of known inducers that can trigger their expression is lower in comparison (20). In the present study, we have carried out a screening in order to identify potential effectors of the S. maltophilia RND efflux pump SmeVWX, using a yellow fluorescent protein (YFP)-based sensor. The operon encoding this MDR efflux system is composed of a membrane fusion protein gene (smeV), an inner membrane transporter gene (smeW), and an outer membrane protein gene (smeX). smeVWX genes are coexpressed with two short-chain-dehydrogenase/reductase (SDR) genes, which are located upstream smeV (smeU1) and between smeW and smeX (smeU2). The operon is regulated by the LysR-type transcriptional regulator SmeRv (11). SmeVWX does not participate in S. maltophilia intrinsic resistance, likely because its expression levels are too low (11); nevertheless, it is known that this efflux pump contributes to the acquisition of resistance mediated by mutations in the transcriptional regulator SmeRv, leading to the overexpression of the efflux system (22). Further, quinolone-resistant clinical isolates overexpressing this efflux pump have been found, indicating that SmeVWX overexpression may have clinical relevance (12, 22).
Among the compounds tested in this study, we have found that vitamin K3 (vitK3), also known as menadione (2-methylnaphtalene-1,4-dione), acts as an inducer and is likely a substrate of the SmeVWX efflux system. In addition to developing a biosensor for tracking inducers of the expression of SmeVWX and since vitK3 is present in some hemostatic drugs and is also becoming an important candidate for anticancer therapy, our study provides information about the potential implications that using vitK3 during the course of an infection caused by S. maltophilia might have.
RESULTS AND DISCUSSION
Reporter construction.
Fluorescent protein-based sensors have been extensively used for different purposes due to their high reliability, sensitivity, and simplicity in operation (23). In the current work, a YFP-based reporter was constructed in order to identify potential inducers of the expression of the S. maltophilia SmeVWX efflux pump, which are unknown so far. To carry this out, a DNA fragment containing the promoter sequence of smeVWX was amplified and cloned into the pSEVA237Y plasmid, which harbors the yellow fluorescent protein (YFP) (24), giving rise to the pPBT04 plasmid, as described in Materials and Methods. This allows the quantification of the expression of smeVWX through the fluorescence given by YFP. Using tripartite conjugation, the plasmid pPBT04 was introduced in S. maltophilia D457, with the resultant strain dubbed PBT02, and in S. maltophilia MBS287, the resultant strain was dubbed PBT06. The MBS287 strain, a mutant derived from the wild-type S. maltophilia D457 strain, constitutively expresses high levels of smeVWX due to a mutation (Gly266Asp) in the gene encoding its local regulator SmeRv. This strain can be used as a control for measuring the expression levels of smeVWX when the efflux pump is overexpressed (22). To test the smeVWX sensor, the fluorescence levels given by the PBT02 and PBT06 strains and the growth of both strains were measured for 18 h. As mentioned above, the expression levels of SmeVWX are very low; consequently, it is expected that the fluorescence given by the expression of YFP under the control of the smeVWX promoter in the PBT02 strain is lower than the one obtained in the overexpressing derivative strain PBT06. As shown in Fig. 1, higher levels of fluorescence given by the YFP are obtained in the case of the PBT06 strain than with PBT02 during growth, validating the smeVWX sensor developed in this work.
FIG 1.
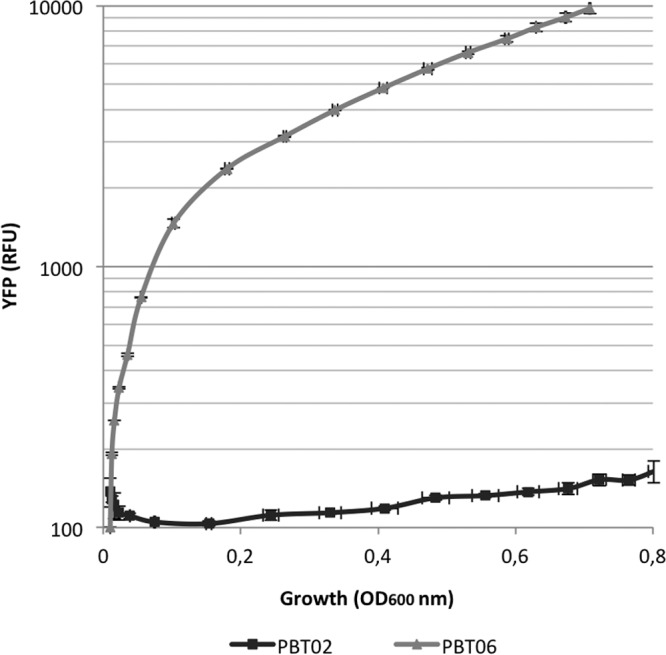
Fluorescence levels and growth of the sensor strains PBT02 and PBT06. The fluorescence of both strains was measured using a plate reader for 18 h in LB medium. The YFP levels given by the expression of smeVWX in PBT06 are higher than in the D457-derivative strain PBT02, since it overexpresses the efflux pump due to a mutation in the regulator SmeRv. There are not significant differences in the growth of both strains. Error bars indicate standard deviations of the results from three independent replicates. RFU, relative fluorescence units.
Vitamin K3 induces smeVWX expression.
Multidrug efflux pumps are relevant elements in the development of antibiotic resistance in bacterial populations. Expression of these determinants is usually repressed by specific regulators encoded by genes located upstream of the operons that contain the structural genes for these efflux pumps (19). In the opportunistic pathogen S. maltophilia, some of these RND multidrug efflux pumps, such as SmeDEF, SmeIJK, SmeOP, and SmeYZ, contribute to bacterial intrinsic resistance (4, 6, 9, 14), while others, such as SmeVWX, have very low levels of expression and its overexpression alone leads to acquired resistance (11, 22). In addition to genetic alterations, resistance can also be achieved by the presence of effectors or conditions that trigger the expression of multidrug efflux pumps, leading to the acquisition of transient phenotypic antibiotic resistance (25–27). A wide range of MDR efflux pump substrates are known. However, the number of known effectors that regulate their expression is lower in comparison (28). For instance, the AcrAB-TolC system in Escherichia coli can be induced by bile salts (29), and in Pseudomonas aeruginosa, the MexXY-OprM system is induced in response to antibiotics that target the ribosome and under oxidative stress conditions (30, 31). In the case of S. maltophilia, the SmeDEF efflux pump is induced by triclosan and some plant-produced compounds, which bind its local repressor SmeT, so that smeDEF transcription becomes activated (26, 32). To date, it is not known if there is any compound able to induce the expression of the SmeVWX efflux pump; consequently, we carried out a screening of such potential effectors in S. maltophilia PBT02 using compounds belonging to different categories, including antibiotics, compounds that produce oxidative stress, chelating agents, biocides, etc. The concentrations were chosen taking into consideration the MIC values of each compound.
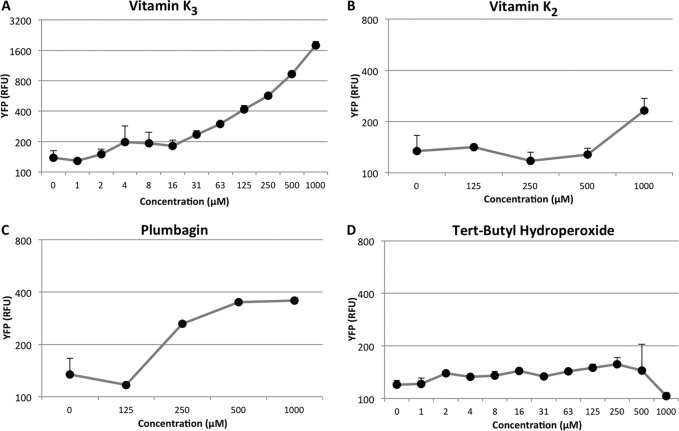
None of the tested compounds gave a clear increase in fluorescence levels, except vitK3, which caused an increment in the fluorescence obtained by YFP expression under control of the smeVWX promoter. The fluorescence levels were measured in both PBT02 and PBT06. As shown in Fig. 2, the fluorescence levels given by PBT02, when cells reached exponential phase (optical density at 600 nm [OD600], 0.6), are higher in the presence of 1 mM vitK3 than those obtained in the absence of any compound. To analyze whether or not the effect of vitK3 was specific and determine the minimal concentrations that may trigger smeVWX induction, a more detailed analysis was performed using as potential inducers vitK3, its structural analogues vitamin K2 (vitK2) and plumbagin, and the generator of oxidative stress, tert-butyl hydroperoxide. As shown in Fig. 3, vitK3 remains the best smeVWX inducer, even at concentrations as low as 4 μM, whereas a modest induction in the presence of vitK2 and plumbagin can be seen, and tert-butyl hydroperoxide does not induce the expression of smeVWX; this indicates that oxidative stress is not the cause (at least the unique cause) of the induction of smeVWX expression in the presence of vitK3.
FIG 2.
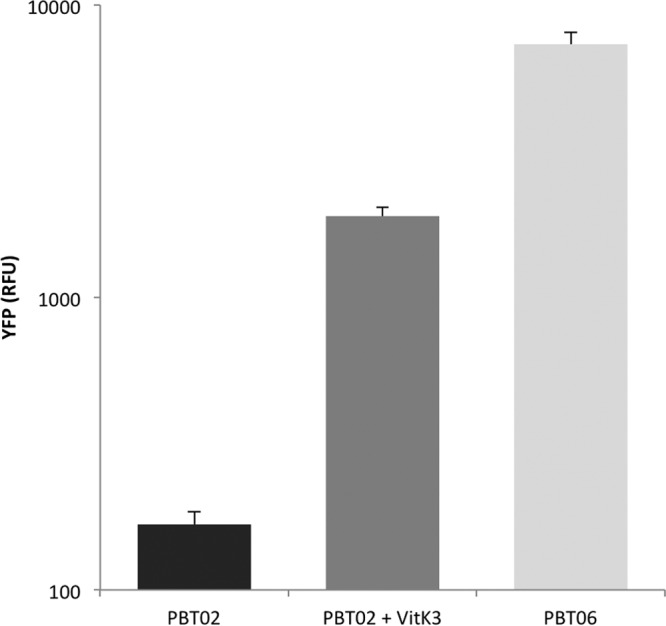
Effect of vitK3 on smeVWX expression. The fluorescence levels obtained by smeVWX expression when OD600 of 0.6 is reached in PBT02 strain, PBT02 strain after incubation with 1 mM vitK3, and PBT06 strain are shown. vitK3 increases YFP expression in comparison with the untreated strain, although the level reached is lower than in PBT06. Error bars indicate standard deviations of the results from three independent replicates.
FIG 3.
Effects of different compounds on smeVWX expression. Fluorescence levels for: vitamin K3 (A), vitamin K2 (B), plumbagin (C), and tert-butyl hydroperoxide (D) given by the expression of smeVWX in PBT02 strain when OD600 of 0.6 is reached at several concentrations. Plumbagin and vitamin K2 slightly increase the YFP levels in comparison with vitamin K3 (A to C); tert-butyl hydroperoxide does not cause any changes (D). Error bars indicate standard deviations of the results from three independent replicates.
In order to further address whether or not vitK3 induces the expression of smeVWX, smeV mRNA levels were measured by real-time reverse transcription-PCR (RT-PCR) in the presence and absence of vitK3. As shown in Fig. 4, the expression levels of smeV are increased by 105-fold in the presence of vitK3 in the wild-type strain D457 in comparison with those levels obtained without this compound. In S. maltophilia MBS287, where smeVWX is overexpressed, the smeV levels are increased by 286-fold in comparison with those of the wild-type strain D457. These data show that vitK3 increases the expression of smeVWX mRNA, a feature in agreement with the results obtained using the above-described fluorescent reporter, indicating that the sensor is valid for the detection of inducer compounds.
FIG 4.
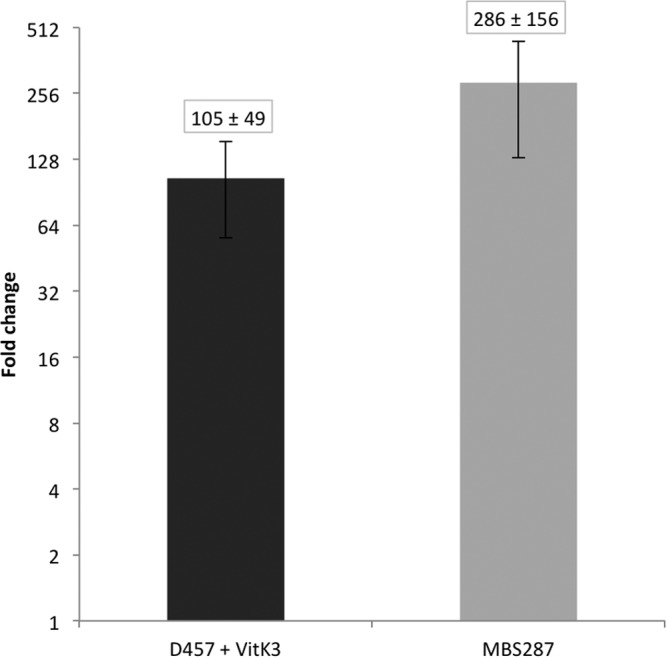
vitK3 increases smeV mRNA levels. smeV mRNA levels were measured by real-time RT-PCR in the presence of vitK3 in the wild-type D457 strain and in the MBS287 strain, which overexpresses smeVWX. The data show that expression of smeV is induced by vitK3. Fold changes were estimated with respect to the value given by strain D457 in the absence of vitK3. Error bars indicate standard deviations of the results from three independent experiments.
Vitamin K3 might be extruded by SmeVWX efflux pump.
As described above, vitK3 is able to induce smeVWX expression, and it is possible that this compound is a substrate of the efflux pump. To test this possibility, a D457-derivative mutant with a partial deletion in smeW (MBS704) was constructed by homologous recombination, as described in Materials and Methods. Both strains, the wild type (D457) and the smeW mutant (MBS704), were grown in the presence and absence of vitK3 (1 mM) for 20 h at 37°C, and the optical density at 600 nm was measured every 10 min using a Tecan Infinite 200 plate reader (Tecan). As shown in Fig. 5, in the absence of vitK3, the two strains exhibit similar growth; however, when vitK3 is added to the medium, the MBS704 strain growth is impaired in comparison with D457. This result suggests that SmeVWX extrudes vitK3, and in the absence of this efflux pump, this compound inhibits S. maltophilia growth.
FIG 5.
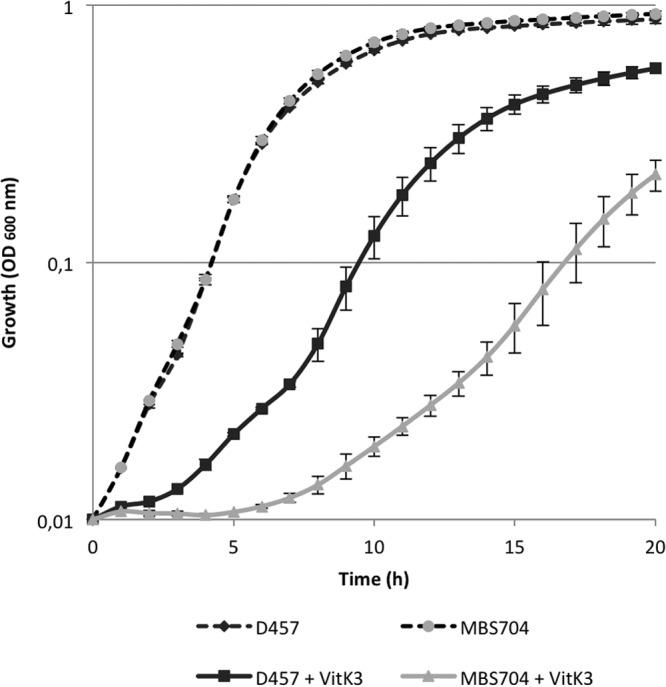
vitK3 might be extruded by the SmeVWX multidrug efflux pump. The growth in the presence and absence of 1 mM vitK3 in D457 strain and MBS704 (ΔsmeW) was measured. In the presence of vitK3, the smeW mutant presents a diminished growth in comparison with the wild-type strain D457, suggesting that vitK3 might be extruded by the SmeVWX MDR efflux pump. Error bars indicate standard deviations of the results from three independent replicates.
vitK3 is an analogue of vitamins K1 and K2, with all of them belonging to the 2-methyl-1,4-naphthoquinone family (33, 34). vitK3 is also known as menadione, a compound that has been isolated from fungi and phanerogams (35), where it was first studied as a plant regulator (36). The function of menadione in the defense against plant pathogens has been shown in several studies (37–39). This contribution can be carried out in two ways: first, vitK3 is able to increase the activity of the H+-ATPase in plant cells due to its redox properties, contributing to the immune response against phytopathogens (40, 41); second, vitK3 itself is toxic for bacteria as a result of its capability of generating reactive oxygen species (ROS), elevating the intracellular production of O2− and H2O2 (42).
S. maltophilia is an ubiquitous bacterium which has been isolated from several sources, including those associated with the plant rhizosphere (1, 43). One of the roles of MDR efflux pumps is the prevention of the accumulation of toxic compounds inside the cell by extruding them (44). Since S. maltophilia is a rhizosphere-related bacterium, the SmeVWX efflux pump is likely involved in nature in the detoxification of vitK3 and its analogues, which might be produced by plants during S. maltophilia root colonization.
Vitamin K3 decreases S. maltophilia antibiotic susceptibility even in the absence of smeW.
It is known that the SmeVWX efflux pump is able to extrude quinolones and chloramphenicol, and its overexpression causes resistance to these antibiotics (11, 12). Since vitK3 is able to induce smeVWX expression, it is possible that the susceptibility to such antibiotics decreases in the presence of this agent. To analyze this hypothesis, a susceptibility assay using either an ofloxacin disc (5 μg) or a chloramphenicol disc (30 μg) placed next to a vitK3-containing disc (2 μmol) was carried out. The inhibition halos around the antibiotic discs present deformations in the region nearby the vitK3 disc (not shown), indicating that this compound decreases S. maltophilia susceptibility to antibiotics. To further confirm that vitK3 decreases susceptibility to antibiotics, a checkerboard assay was performed. A 96-well microtiter plate containing serial concentrations of either ofloxacin or chloramphenicol (also a substrate of SmeVWX) in combination with vitK3 was inoculated with cultures of S. maltophilia D457 or the smeW-defective mutant MBS704 at an OD600 of 0.01. After 24 h of incubation at 37°C, the OD600 was measured in each well. The fractional inhibitory concentration (FIC) values are shown in Table 1. Noteworthy, the effect of vitK3 was antagonistic in all cases, independently of the type of the antibiotic used and of the presence or the absence of smeW. This indicates that the effect of vitK3 on S. maltophilia goes beyond the induction of smeVWX, a topic that is currently under study in our laboratory.
TABLE 1.
FIC values of vitK3 in combination with either chloramphenicol or ofloxacina
| Strain | Chloramphenicol + vitK3 |
Ofloxacin + vitK3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CvitK3 | MICvitK3 | Cchlor | MICchlor | ΣFIC | CvitK3 | MICvitK3 | Coflox | MICoflox | ΣFIC | |
| D457 | 2.56 | 2.56 | 16 | 16 | 2 | 5.12 | 5.12 | 6 | 4 | 2.5 |
| MBS704 | 5.12 | 2.56 | 16 | 12 | 3 | 2.56 | 2.56 | 6 | 3 | 3 |
See "FIC index analysis" in Materials and Methods for details on the terms used here. chlor, chloramphenicol; oflox, ofloxacin.
Concluding remarks.
Resistance to antibiotics has become an increasing problem in public health, with MDR efflux pumps being relevant elements in the development of this resistance. As mentioned above, MDR efflux pump expression is usually repressed by specific transcriptional regulators (15). Expression of MDR efflux pumps is likely induced under physiological conditions when their activity is required, and bacteria can determine this need by detecting growing conditions or compounds in the environment that act as effectors. However, antibiotics are not usually good inducers of efflux pumps, whereas nonantibiotic compounds can induce such expression (21).
In this study, we have developed a fluorescent biosensor useful for detecting inducers of the S. maltophilia SmeVWX MDR efflux pump. Using this biosensor, we have determined that vitK3 (a potential substrate of this efflux pump involved in the defense of plants against pathogens) is able to induce the expression of the S. maltophilia SmeVWX efflux pump. Vitamin K is an essential nutrient which acts as a cofactor in the production of some factors of blood coagulation in mammals, such as factors II (prothrombin), VII (proconvertin), IX, and X (33). vitK3 has improved antihemorrhagic activity compared with the natural vitamin K, so it is used as an agent of choice for the treatment of vitamin K deficiency, hemorrhagic diathesis, and hypoprothrombinemia, prophylactically before and after surgery to prevent bleeding, and it is administered to newborns with low levels of prothrombin to prevent hemorrhagic diseases (45). Besides, vitK3 is gaining importance as an anticancer agent because of its cytotoxic effect against cancer cells (34, 46). Recent work has shown that the plasma concentration of menadione upon the administration of 10 mg of menadiol sodium diphosphate to healthy subjects can reach a peak of 3 μM (47), within the concentration range at which we begin to observe induction of smeVWX expression.
S. maltophilia mainly causes nosocomial infections (48), although community-acquired infections can also occur (49). The population at risk is mostly composed of immunocompromised hosts, including intensive care unit (ICU) patients, patients with dialysis catheters, hematological diseases, and cystic fibrosis, and those treated with a wide spectrum of antibiotics (50–53). Although our results indicate that vitK3 might induce the expression of the smeVWX efflux pump even at low concentrations and antagonizes the effects of ofloxacin and chloramphenicol against S. maltophilia, both in the presence and in the absence of this efflux pump, the clinical significance of these findings remains to be clearly established.
In addition, the fact that vitK3 is produced by plants and that S. maltophilia is a common plant inhabitant (54) strongly suggests that SmeVWX might be involved in bacterium-plant interactions in nature, a functional role that has been already demonstrated for the S. maltophilia SmeDEF efflux pump (32).
MATERIALS AND METHODS
Bacterial strains and growing conditions.
All bacterial strains and plasmids used in this study are listed in Table 2. Cells were grown in LB medium at 37°C, unless otherwise stated. When required, the following antibiotics were added: ampicillin (Ap; 100 μg/ml) for Escherichia coli harboring the pGEM-T Easy vector and the pPBT02 and pBS51 plasmids, and kanamycin (Km; 50 μg/ml and 500 μg/ml) for E. coli and S. maltophilia D457, respectively, for pSEVA237Y and pPBT04 plasmid selection. Km at 50 μg/ml was added to LB liquid medium to maintain pSEVA237Y and pPBT04 plasmids in both E. coli and S. maltophilia; tetracycline (Tc; 10 μg/ml) was added in the case of E. coli harboring pEx18Tc and pBS52 plasmids. Tc at 10 μg/ml and imipenem (Imp; 20 μg/ml) were added for the selection of S. maltophilia D457 exconjugants. Medium was supplemented with 80 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for the detection of β-galactosidase production.
TABLE 2.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| OmniMAX | Strain used in transformation; F′ {proAB+ lacIq lacZΔM15 Tn10(Tetr)} mcrA Δ(mrr-hsdRMS-mcrBC) Φ80(lacZ)ΔM15 Δ(lacZYA-argF) U169 endA1 supE44 thi-1 gyrA96 relA1 deoR tonA panD | Invitrogen, Life Technologies |
| TGI | Strain used in transformation; supE thi-1 Δ(lac-proAB) Δ(mcrB-hsdSM)5 (rK− mK−) [F′ traD36 proAB+ lacIq ZΔM15] | 61 |
| CC118λpir | Donor cell in conjugation; strain CC118 lysogenized with λ pir phage (Tc′) Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE (Am) recA1 | 56 |
| 1047 (pRK2013) | Helper cell in conjugation harboring pRK2013 (Kanr) plasmid | 62 |
| S. maltophilia | ||
| D457 | Clinical strain | 63 |
| MBS287 | D457-derived mutant (SmeRv G266N) overexpressing SmeVWX efflux pump | 22 |
| MBS704 | D457 ΔsmeW | This work |
| PBT02 | D457 with pPBT04 plasmid | This work |
| PBT06 | MBS287 with pPBT04 plasmid | This work |
| Plasmids | ||
| pGEM-T Easy vector | Cloning vector, Ampr | Promega |
| pSEVA237Y | Plasmid containing YFP; replication origin pBBR1; Kanr | 24 |
| pPBT02 | pGEMT-derived plasmid containing smeVWX promoter region | This work |
| pPBT04 | pSEVA237Y-derived plasmid containing smeVWX promoter region | This work |
| pEX18Tc | Gene replacement vector; sacB, Tcr | 23 |
| pBS51 | pGEMT-derived plasmid containing the 5′ and 3′ regions of smeW gene | This work |
| pBS52 | pEX18Tc-derived plasmid containing the 5′ and 3′ regions of smeW gene | This work |
Tetr, tetracycline resistance; Kanr, kanamycin resistance; Ampr, ampicillin resistance.
Reporter construction.
S. maltophilia D457 genomic DNA was extracted according to the Gnome DNA kit protocol (MP Biomedicals). The 384-bp region between the smeRv gene (SMD_1762) and the smeU1 gene (SMD_1763), which contains the smeVWX promoter region, was amplified using the FailSafe PCR system (Epicentre) with primers SmeVWX_F (5′-GAATTCGATCCTGGACGTCG-3′, EcoRI site underlined) and SmeVWX_R (5′-AAGCTTGACATTTCCTCCCAAATC-3′, HindIII site underlined). The thermocycler was programmed for 25 cycles of 94°C for 30 s of denaturation, 56°C for 30 s of annealing, and 72°C for a 36-s extension, with an initial denaturation at 94°C for 5 min and a final extension at 72°C for 7 min. The PCR product was ligated into pGEM-T Easy vector (Promega), according to the manufacturer's instructions, obtaining the pPBT02 plasmid, which was introduced by transformation into E. coli OmniMAX (Invitrogen). The construction was verified by DNA sequencing. The pPBT02 plasmid was extracted with the QIAprep Spin miniprep kit 250 (Qiagen), according to the manufacturer's instructions, and digested with EcoRI and HindIII restriction enzymes (New England BioLabs). The product corresponding to the smeVWX promoter region was purified with the purification kit (GE Healthcare) from a 1% agarose gel and cloned into pSEVA237Y using the same restriction enzymes and the T4 DNA ligase (New England BioLabs). The obtained plasmid, pPBT04, was introduced by transformation in E. coli OmniMAX (Invitrogen) competent cells. The presence of the plasmid was confirmed by PCR, as described above, with primers pSEVA227Y_F (5′-GCGGATAACAATTTCACACA-3′) and pSEVA227Y_R (5′-TTGCTCACCATATGTTTTTCC-3′).
The pPBT04 plasmid was introduced by transformation in E. coli CC118λpir. Afterwards, the plasmid was introduced into the S. maltophilia D457 and MBS287 strains by tripartite matting using the strains E. coli CC118λpir (donor cell), E. coli 1047/pRK2013 (helper cell), and S. maltophilia (receptor cell), in a 4:2:1 proportion (receptor:donor:helper). Kanamycin (500 μg/ml) was added for selecting the S. maltophilia clones containing the pPBT04 plasmid, and imipenem (20 μg/ml) was added for eliminating the E. coli strains. For plasmid confirmation, PCR was performed as described above with primers pSEVA227Y_F and pSEVA227Y_R.
Deletion of the smeW gene.
An S. maltophilia D457 mutant with a partial deletion of smeW gene was generated by homologous recombination. A fragment homologous to both the 5′ end (498 bp) and the 3′ end (513 bp) of the smeW gene was obtained by overlapping PCR, using the PCR master mix (Promega). In the first PCR, the primers SmeWA (5′-CGGGATCCTTAGCTGCCGGCGCCAG-3′, BamHI site underlined) and SmeWB (5′-CAGGATCTTCTGCGTAGTCA-3′) for the 5′ end and SmeWC (5′-CAGGATCTTCTGCGTAGTCA-3′) and SmeWD (5′-CCCAAGCTTGATGCATGCCTTGTGG3′, HindIII site underlined) for the 3′ end were used. The PCR products were purified with the QIAquick PCR purification kit (Qiagen), according to the manufacturer's instructions, and used as the template for a second PCR with SmeWA and SmeWD primers. The PCR product, purified from an agarose gel with the QIAquick gel extraction kit (Qiagen), was cloned in pGEM-T Easy vector (Promega), generating pBS51 plasmid, which was introduced in E. coli TG1. The right sequence was confirmed by sequencing, and the fragment containing the 5′ and 3′ ends of the smeW gene was cloned into the suicide vector pEX18Tc (55) using the BamHI-HindIII sites, generating the pBS52 plasmid. This plasmid was introduced into E. coli CC118λpir and mobilized afterwards into S. maltophilia D457 by tripartite conjugation (56). The exconjugants containing pBS52 were selected on LB agar containing 10 mg/ml tetracycline and 20 mg/ml imipenem. Tetracycline-resistant colonies were streaked onto 10% sucrose-LB agar to select double recombinants with the partial deletion of the smeW gene. S. maltophilia D457 ΔsmeW (MBS704) was confirmed by PCR, with external (SmeWA/SmeWD) and internal (SmeW5 [5′-GAACCGTTGCCGAACAGC-3′]/SmeW6 [5′-GACAGGCCTTCCTCGATG-3′]) primers to the smeW gene.
Screening of potential inducers of the expression of smeVWX.
Different compounds from different categories were used in this assay at serial concentrations. Among them were the antibiotics erythromycin (512, 256, and 128 μg/ml), gentamicin (32, 16, and 8 μg/ml), co-trimoxazole (trimethoprim-sulfamethoxazole, 1:5) (1, 0.5, and 0.25 μg/ml), chloramphenicol (16, 8, and 4 μg/ml), tobramycin (512, 256, and 128 μg/ml), ofloxacin (4, 2, and 1 μg/ml), kanamycin (512, 256, and 128 μg/ml), tetracycline (4, 2, and 1 μg/ml), polymyxin B (2, 1, and 0.5 μg/ml), and colistin (24, 12, and 6 μg/ml); heavy metals ZnSO4 (5, 2.5, and 1.25 mM), CuSO4 (5, 2.5, and 1.25 mM), CdSO4 (5, 2.5, and 1.25 mM), CoSO4 (5, 2.5, and 1.25 mM), and FeCl3 (5, 2.5, and 1.25 mM); oxidative stress compounds paraquat (5, 2.5, and 1.25 mM), vitamin K3 (2, 1, and 0.5 mM), and tert-butyl hydroperoxide (1, 0.5, and 0.25 mM); biocides triclosan (10, 5, and 2.5 μg/ml) and hexachlorophene (100, 50, and 25 μg/ml); plant-produced flavonoids phloretin (10, 5, and 2.5 μg/ml), quercetin (10, 5, and 2.5 μg/ml), and genistein (10, 5, and 2.5 μg/ml); detergents SDS (200, 100, and 50 mM) and Tween 20 (200, 100, and 50 mM); chelating agents EDTA (2, 1, and 0.5 mM) and EGTA (2, 1, and 0.5 mM); the analgesic metamizol (10, 5, and 2.5 mg/ml); and the inhibitor of oxidative phosphorylation carbonyl cyanide m-chlorophenyl hydrazone (CCCP; 20, 10, and 5 μM). The compounds vitamin K3, vitamin K2, plumbagin, and tert-butyl hydroperoxide were studied in more detail using a wider range of concentrations.
The stock solutions of the different compounds were diluted in LB medium to obtain the required concentrations. The assay was performed in Corning Costar 96-well black clear-bottom plates (Corning Incorporated). Ten microliters of cell culture was inoculated in 140 μl of medium in each well to a final OD600 of 0.01. Bacteria were grown at 37°C for 18 h, and growth (OD600) and fluorescence were measured every 10 min (although data are represented on an hourly basis) using a Tecan Infinite 200 plate reader (Tecan) set with an excitation wavelength at 508 nm and emission wavelength at 540 nm for YFP detection.
Assays of induction of antibiotic resistance.
Two hundred microliters of S. maltophilia D457 culture at an OD600 of 0.01 was seeded in an agar-LB medium plate. A 9-mm sterile disc (Macherey-Nagel) was placed in the plate with vitK3 (2 μmol). An ofloxacin (5 μg) or a chloramphenicol disc (30 μg) (Oxoid) was placed 18 mm from the vitK3 disc. The plate was incubated for 24 h at 37°C.
The effect of vitK3 on the susceptibility of S. maltophilia to ofloxacin or chloramphenicol was analyzed using the checkerboard technique in a 96-well microtiter plate, where 10 μl of bacterial culture of D457 or MBS704 strain was added to each well to a final OD600 of 0.01. Eleven vitK3 concentrations were combined with 7 ofloxacin or chloramphenicol concentrations to carry out this assay. The plates were incubated at 37°C, and the OD600 was recorded after 24 h using a Tecan Infinite 200 plate reader (Tecan).
FIC index analysis.
The fractional inhibitory concentration (FIC) index (57) was determined for each strain in order to define if there is some interaction between the antibiotics and vitK3. The sum of the FICs is defined as ΣFIC = FICA + FICB = (CA/MICA) + (CB/MICB), where A is vitK3 and B is ofloxacin or chloramphenicol. The MIC is defined as the minimum inhibitory concentration of the compounds alone, and C is defined as the MIC of the compounds in combination. Synergy is defined as an FIC of ≤0.5, an FIC value between 0.5 and 2 is considered indifferent, and antagonism is defined as an FIC value of ≤2.
RNA preparation and real-time RT-PCR.
Two flasks containing 20 ml of LB medium were inoculated with an overnight culture of S. maltophilia D457 to a final OD600 of 0.01 and were incubated until exponential phase was reached (OD600, 0.6). This step was repeated, inoculating two new flasks with culture from the previous ones. When the culture reached an OD600 of 0.6, vitK3 (1 mM) was added to one of the flasks and cells were incubated for a further 30 min. Ten milliliters of each culture was centrifuged at 8,000 rpm and 4°C for 20 min. Five hundred seventy microliters of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) and 30 μl of lysozyme (Sigma) from a 20 mg/ml stock, for a final concentration of 1 mg/ml, were added to each sample. Afterwards, the samples were mixed by vortexing for 10 s and were incubated at room temperature for at least 10 min with regular vortexing. A volume of 2,100 μl of buffer RLT (Qiagen) was added, and samples were sonicated twice for 5 to 10 s (constant frequency, 0.45 Hz). Then, 1,410 μl of ethanol was added and the protocol continued using the RNeasy minikit (Qiagen), according to the manufacturer's instructions. In order to eliminate any residual DNA, a DNase I (Qiagen) treatment was carried out, according to the manufacturer's instructions. A second treatment was performed using Turbo DNA-free (Ambion). RNA integrity was verified on a 1% agarose gel, and the absence of DNA was confirmed by PCR using primers sme27 and sme48, which amplify a 347-bp fragment belonging to the smeT gene from strain D457 (58). cDNA was obtained from 10 μg of RNA using the High-Capacity cDNA reverse transcription kit (Applied Biosystems). Real-time RT-PCR was performed using the Power SYBR green PCR master mix (Applied Biosystems) in the ABI Prism 7500 real-time system (Applied Biosystems). The first denaturation step, 95°C for 10 min, was followed by 40 temperature cycles (95°C for 15 s, 60°C for 1 min) for amplification and quantification. Fifty nanograms of cDNA was used in each reaction. Primers SmeV-RT.fw and SmeV-RT.rv, which amplify the smeV gene (22), were used at 400 nM. Primers FtsZ1 and FtsZ2 were used to amplify the housekeeping gene ftsZ (22). Differences in the relative amounts of mRNA were determined according to the 2−ΔΔCT method (59, 60). In all cases, the mean values for relative mRNA expression were obtained from three independent experiments.
ACKNOWLEDGMENTS
Work in our laboratory is supported by grants from the Instituto de Salud Carlos III (Spanish Network for Research on Infectious Diseases [RD16/0016/0011]) and from the Spanish Ministry of Economy and Competitivity (grants BIO2014-54507-R and JPI Water StARE JPIW2013-089-C02-01). P.B. is the recipient of a FPI fellowship and F.C. is the recipient of a JAE fellowship funded by the European Social Fund.
REFERENCES
- 1.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Kastoris AC, Vouloumanou EK, Dimopoulos G. 2009. Community-acquired Stenotrophomonas maltophilia infections: a systematic review. Eur J Clin Microbiol Infect Dis 28:719–730. doi: 10.1007/s10096-009-0709-5. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez MB. 2015. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front Microbiol 6:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N, Adlem E, Kerhornou A, Lord A, Murphy L, Seeger K, Squares R, Rutter S, Quail MA, Rajandream MA, Harris D, Churcher C, Bentley SD, Parkhill J, Thomson NR, Avison MB. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li XZ, Zhang L, Poole K. 2002. SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 46:333–343. doi: 10.1128/AAC.46.2.333-343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso A, Martinez JL. 2000. Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 44:3079–3086. doi: 10.1128/AAC.44.11.3079-3086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Li XZ, Poole K. 2001. SmeDEF multidrug efflux pump contributes to intrinsic multidrug resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 45:3497–3503. doi: 10.1128/AAC.45.12.3497-3503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YW, Liou RS, Lin YT, Huang HH, Yang TC. 2014. A linkage between SmeIJK efflux pump, cell envelope integrity, and sigmaE-mediated envelope stress response in Stenotrophomonas maltophilia. PLoS One 9:e111784. doi: 10.1371/journal.pone.0111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CW, Huang YW, Hu RM, Yang TC. 2014. SmeOP-TolCSm efflux pump contributes to the multidrug resistance of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 58:2405–2408. doi: 10.1128/AAC.01974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YW, Hu RM, Yang TC. 2013. Role of the pcm-tolCsm operon in the multidrug resistance of Stenotrophomonas maltophilia. J Antimicrob Chemother 68:1987–1993. doi: 10.1093/jac/dkt148. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Huang CC, Chung TC, Hu RM, Huang YW, Yang TC. 2011. Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 55:5826–5833. doi: 10.1128/AAC.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-León G, Ruiz de Alegria Puig C, Garcia de la Fuente C, Martinez-Martinez L, Martinez JL, Sanchez MB. 2015. High-level quinolone resistance is associated with the overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin Microbiol Infect 21:464–467. doi: 10.1016/j.cmi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Gould VC, Okazaki A, Avison MB. 2013. Coordinate hyperproduction of SmeZ and SmeJK efflux pumps extends drug resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 57:655–657. doi: 10.1128/AAC.01020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YT, Huang YW, Chen SJ, Chang CW, Yang TC. 2015. The SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics, and virulence in mice. Antimicrob Agents Chemother 59:4067–4073. doi: 10.1128/AAC.00372-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso A, Morales G, Escalante R, Campanario E, Sastre L, Martinez JL. 2004. Overexpression of the multidrug efflux pump SmeDEF impairs Stenotrophomonas maltophilia physiology. J Antimicrob Chemother 53:432–434. doi: 10.1093/jac/dkh074. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Cagliero C, Guo B, Barton YW, Maurel MC, Payot S, Zhang Q. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Bacteriol 187:7417–7424. doi: 10.1128/JB.187.21.7417-7424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido E, Yamaguchi A, Nishino K. 2008. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J Biol Chem 283:24245–24253. doi: 10.1074/jbc.M804544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grkovic S, Brown MH, Skurray RA. 2001. Transcriptional regulation of multidrug efflux pumps in bacteria. Semin Cell Dev Biol 12:225–237. doi: 10.1006/scdb.2000.0248. [DOI] [PubMed] [Google Scholar]
- 20.Blanco P, Hernando-Amado S, Reales-Calderon J, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez MB, Martinez JL. 2016. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms 4:14. doi: 10.3390/microorganisms4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez JL, Sanchez MB, Martinez-Solano L, Hernandez A, Garmendia L, Fajardo A, Alvarez-Ortega C. 2009. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev 33:430–449. doi: 10.1111/j.1574-6976.2008.00157.x. [DOI] [PubMed] [Google Scholar]
- 22.García-León G, Salgado F, Oliveros JC, Sanchez MB, Martinez JL. 2014. Interplay between intrinsic and acquired resistance to quinolones in Stenotrophomonas maltophilia. Environ Microbiol 16:1282–1296. doi: 10.1111/1462-2920.12408. [DOI] [PubMed] [Google Scholar]
- 23.D'Souza SF. 2001. Microbial biosensors. Biosens Bioelectron 16:337–353. doi: 10.1016/S0956-5663(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 24.Silva-Rocha R, Martinez-Garcia E, Calles B, Chavarria M, Arce-Rodriguez A, de Las Heras A, Paez-Espino AD, Durante-Rodriguez G, Kim J, Nikel PI, Platero R, de Lorenzo V. 2013. The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res 41:D666–D675. doi: 10.1093/nar/gks1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Z, Pu XY, Zhang Q. 2011. Salicylate functions as an efflux pump inducer and promotes the emergence of fluoroquinolone-resistant Campylobacter jejuni mutants. Appl Environ Microbiol 77:7128–7133. doi: 10.1128/AEM.00763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández A, Ruiz FM, Romero A, Martinez JL. 2011. The binding of triclosan to SmeT, the repressor of the multidrug efflux pump SmeDEF, induces antibiotic resistance in Stenotrophomonas maltophilia. PLoS Pathog 7:e1002103. doi: 10.1371/journal.ppat.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corona F, Martinez JL. 2013. Phenotypic resistance to antibiotics. Antibiotics (Basel) 2:237–255. doi: 10.3390/antibiotics2020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Ortega C, Olivares J, Martínez JL. 2013. RND multidrug efflux pumps: what are they good for? Front Microbiol 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol 48:1609–1619. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 30.Jeannot K, Sobel ML, El Garch F, Poole K, Plesiat P. 2005. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J Bacteriol 187:5341–5346. doi: 10.1128/JB.187.15.5341-5346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraud S, Poole K. 2011. Oxidative stress induction of the MexXY multidrug efflux genes and promotion of aminoglycoside resistance development in Pseudomonas aeruginosa. Antimicrob Agents Chemother 55:1068–1074. doi: 10.1128/AAC.01495-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-León G, Hernández A, Hernando-Amado S, Alavi P, Berg G, Martínez JL. 2014. A function of SmeDEF, the major quinolone resistance determinant of Stenotrophomonas maltophilia, is the colonization of plant roots. Appl Environ Microbiol 80:4559–4565. doi: 10.1128/AEM.01058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klotz LO, Hou X, Jacob C. 2014. 1,4-Naphthoquinones: from oxidative damage to cellular and inter-cellular signaling. Molecules 19:14902–14918. doi: 10.3390/molecules190914902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verrax J, Taper H, Buc Calderon P. 2008. Targeting cancer cells by an oxidant-based therapy. Curr Mol Pharmacol 1:80–92. doi: 10.2174/1874467210801010080. [DOI] [PubMed] [Google Scholar]
- 35.Binder RG, Benson ME, Flath RA. 1989. Eight 1,4-naphthoquinones from Juglans. Phytochemistry 28:2799–2801. doi: 10.1016/S0031-9422(00)98092-0. [DOI] [Google Scholar]
- 36.Rama Rao AV, Ravichandran K, David SB, Ranade S. 1985. Menadione sodium bisulphite: a promising plant growth regulator. Plant Growth Regul 3:111–118. doi: 10.1007/BF01806050. [DOI] [Google Scholar]
- 37.Borges AA, Cools HJ, Lucas JA. 2003. Menadione sodium bisulphite: a novel plant defence activator which enhances local and systemic resistance to infection by Leptosphaeria maculans in oilseed rape. Plant Pathol 52:429–436. doi: 10.1046/j.1365-3059.2003.00877.x. [DOI] [Google Scholar]
- 38.Carrillo-Perdomo E, Jimenez-Arias D, Aller A, Borges AA. 2016. Menadione sodium bisulphite (MSB) enhances the resistance response of tomato, leading to repel mollusc pests. Pest Manag Sci 72:950–960. doi: 10.1002/ps.4074. [DOI] [PubMed] [Google Scholar]
- 39.Borges AA, Dobon A, Exposito-Rodriguez M, Jimenez-Arias D, Borges-Perez A, Casanas-Sanchez V, Perez JA, Luis JC, Tornero P. 2009. Molecular analysis of menadione-induced resistance against biotic stress in Arabidopsis. Plant Biotechnol J 7:744–762. doi: 10.1111/j.1467-7652.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 40.De Nisi P, Manzotti P, Zocchi G. 2006. Effect of vitamin K3 on plasma membrane-bound H+-ATPase and reductase activities in plants. Plant Sci 170:936–941. doi: 10.1016/j.plantsci.2005.12.019. [DOI] [Google Scholar]
- 41.Elmore JM, Coaker G. 2011. The role of the plasma membrane H+-ATPase in plant-microbe interactions. Mol Plant 4:416–427. doi: 10.1093/mp/ssq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan HM, Fridovich I. 1979. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys 196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 43.Berg G, Eberl L, Hartmann A. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 44.Fernández L, Hancock REW. 2012. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25:661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassan GS. 2013. Menadione. Profiles Drug Subst Excip Relat Methodol 38:227–313. doi: 10.1016/B978-0-12-407691-4.00006-X. [DOI] [PubMed] [Google Scholar]
- 46.Lamson DW, Plaza SM. 2003. The anticancer effects of vitamin K. Altern Med Rev 8:303–318. [PubMed] [Google Scholar]
- 47.Liu R, Wang M, Ding L. 2014. A novel liquid chromatography-tandem mass spectrometry method for determination of menadione in human plasma after derivatization with 3-mercaptopropionic acid. Talanta 128:51–57. doi: 10.1016/j.talanta.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Denton M, Kerr KG. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev 11:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heath T, Currie B. 1995. Nosocomial and community-acquired Xanthomonas maltophilia infection in tropical Australia. J Hosp Infect 30:309–313. doi: 10.1016/0195-6701(95)90266-X. [DOI] [PubMed] [Google Scholar]
- 50.Calza L, Manfredi R, Chiodo F. 2003. Stenotrophomonas (Xanthomonas) maltophilia as an emerging opportunistic pathogen in association with HIV infection: a 10-year surveillance study. Infection 31:155–161. [DOI] [PubMed] [Google Scholar]
- 51.Cho SY, Lee DG, Choi SM, Park C, Chun HS, Park YJ, Choi JK, Lee HJ, Park SH, Choi JH, Yoo JH. 2015. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 15:69. doi: 10.1186/s12879-015-0801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kataria A, Lata S, Khillan V. 2015. Hemodialysis catheter-related bacteremia caused by Stenotrophomonas maltophilia. Indian J Nephrol 25:318–319. doi: 10.4103/0971-4065.157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Silva Filho LV, Tateno AF, de F Velloso L, Levi JE, Fernandes S, Bento CN, Rodrigues JC, Ramos SR. 2004. Identification of Pseudomonas aeruginosa, Burkholderia cepacia complex, and Stenotrophomonas maltophilia in respiratory samples from cystic fibrosis patients using multiplex PCR. Pediatr Pulmonol 37:537–547. doi: 10.1002/ppul.20016. [DOI] [PubMed] [Google Scholar]
- 54.Berg G, Martinez JL. 2015. Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front Microbiol 6:241. doi: 10.3389/fmicb.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 56.de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol 235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 57.Van der Auwera P. 1985. Interaction of gentamicin, dibekacin, netilmicin and amikacin with various penicillins, cephalosporins, minocycline and new fluoro-quinolones against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 16:581–587. doi: 10.1093/jac/16.5.581. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez P, Alonso A, Martinez JL. 2002. Cloning and characterization of SmeT, a repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. Antimicrob Agents Chemother 46:3386–3393. doi: 10.1128/AAC.46.11.3386-3393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 62.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonso A, Martinez JL. 1997. Multiple antibiotic resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 41:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]