ABSTRACT
In this study, we report a novel mcr-1 gene variant, named mcr-1.6, carried by an IncP plasmid in a colistin-resistant Salmonella enterica serovar Typhimurium isolate from a healthy person. Compared with mcr-1, the mcr-1.6 gene contains two single-nucleotide polymorphisms, one of which results in an arginine to histidine variation (Arg536→His). The plasmid carrying the mcr-1.6 gene was designated pMCR1.6_P053 and is similar to a recently discovered mcr-1-bearing plasmid found in Klebsiella pneumoniae.
KEYWORDS: colistin resistance, MCR-1.6, IncP plasmid, Salmonella enterica serovar Typhimurium
TEXT
Colistin is one of the last-resort antibacterial drugs and is increasingly used to treat carbapenem-resistant Enterobacteriaceae (CRE). Since the first report of plasmid pHNSHP45 carrying the mcr-1 gene (1), numerous retrospective studies have been performed worldwide to investigate the presence of this specific gene in strains isolated from environmental samples, food animals, food, and humans (2–11), and the earliest evidence for its presence dates back to the 1980s (12). The mcr-1 gene was recently found to be carried by different plasmid replicon types, such as IncI2, IncHI2, IncP, IncFIP, and IncX4 (4, 13–15). Moreover, the mcr-1 gene variant mcr-1.2 and the novel colistin resistance gene mcr-2 were discovered in isolates in Italy and Belgium, respectively (16, 17).
The mcr-1 gene product, MCR-1, is predicted to be an integral membrane protein with the catalytic activity of phosphoethanolamine transferases (18). The MCR-1 enzyme modifies the chemical structure of lipid A moiety on bacterial lipopolysaccharide by the addition of phosphoethanolamine, which in turn reduces the binding affinity to colistin (i.e., producing the colistin resistance) (1, 18, 19). To date, only one functional variant of MCR-1, MCR-1.2, has been reported (17). This recent variant had a Gln-to-Leu change in the N-terminal protein region (at position 3) compared with MCR-1.
Here, we report a new mcr-1 gene variant (named mcr-1.6) carried by an IncP plasmid in the colistin-resistant Salmonella enterica serovar Typhimurium strain YL14P053, which was isolated in 2014 from a rectal swab sample from a 46-year-old healthy woman who received a medical examination in the Yulin Center for Disease Control and Prevention.
The strain was subjected to 250-bp paired-end whole-genome sequencing with 150× coverage using the MiSeq sequencer (Illumina). A total of 3,051,328 reads and 762,832,000 clean bases were generated and were assembled into 311 contigs (171contigs of ≥1,000 bp) with an N50 length of 110,446 bp using the SOAPdenovo program (20). Plasmid finishing was achieved by a PCR-based strategy and Sanger sequencing. A 47,824-bp-long mcr-1.6-carrying plasmid, named pMCR1.6_P053, was obtained. Antimicrobial susceptibility was determined by reference broth microdilution using custom plates (PRCDCN2, Thermo Fisher) (21). EUCAST defines colistin resistance as >2 μg/ml for Enterobacteriaceae (22). Determinations of the multilocus sequence type (MLST), plasmid replicons, and resistance gene content were performed in silico using online tools (http://www.genomicepidemiology.org/).
MLST analysis indicated that the S. Typhimurium YL14P053 strain belonged to sequence type 34 (ST34). It was predicted to carry several resistance genes, e.g., strA, aph(3′)-Ia, aph(4)-Ia, aac(3)-IVa, aac(6′)Ib-cr, strB, blaTEM-1B, blaOXA-1, oqxA, oqxB, floR, catB3, cmlA1, arr-3, sul1, sul2, sul3, tet(B), dfrA12, and the mcr-1 gene variant, able to mediate resistance to aminoglycoside, β-lactam, fluoroquinolone, phenicol, rifampin, sulfonamide, tetracycline, trimethoprim, and colistin. The YL14P053 strain showed a multidrug-resistance phenotype, including resistance to colistin (MIC, 4 μg/ml), ampicillin (MIC, >64 μg/ml), tetracycline (MIC, >32 μg/ml), nalidixic acid (MIC, >64 μg/ml), erythromycin (MIC, >16 μg/ml), chloramphenicol (MIC, >64 μg/ml), and trimethoprim-sulfamethoxazole (MIC, >8/152 μg/ml), but was susceptible to imipenem (MIC, <0.25 μg/ml) and ciprofloxacin (MIC, 1 μg/ml). The absolute MIC values of ceftazidime, cefotaxime, cefazolin, and azithromycin were 1, 1, 1, and 4 μg/ml, respectively.
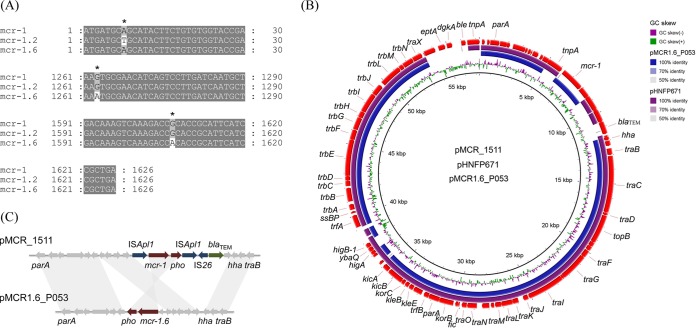
Interestingly, the mcr-1 gene variant harbored in this strain carried two single-nucleotide polymorphisms (SNPs), unlike the mcr-1 gene (confirmed by PCR and Sanger sequencing) (Fig. 1A). The first one was located at position 1263 (G-A), which was a synonymous mutation that did not cause amino acid change. The second one, at position 1607 (G-A), resulted in an arginine to histidine change (Arg536→His). The positions of these two SNPs were different from those in mcr-1.2. Therefore, we named this variant mcr-1.6. MCR-1 is organized into two domains: an N-terminal inner-membrane-bound domain predicted to contain 5 transmembrane α-helices and a soluble periplasmic domain containing the putative catalytic center (23). The catalytic domain of MCR-1 (cMCR-1, residues 215 to 541) is a globular protein with an overall hemispherical shape and a centrally located β-sheet composed of seven β-strands sandwiched between α-helical structures (24). Therefore, the amino acid change (Arg536→His) seen in this study was very close to that at the end of cMCR-1. To confirm and evaluate the colistin resistance activity of the mcr-1.6 gene, recombinant Escherichia coli DH5α strains harboring the mcr-1 and mcr-1.6 genes, respectively, were constructed by a previously reported method (25). Colistin susceptibility testing showed that the MIC values of strains carrying the recombinant plasmid increased from 0.125 μg/ml to 4 μg/ml, suggesting that the mutation did not obviously impact mcr-1 gene activity.
FIG 1.
Alignment of the mcr-1 gene with its variants and comparison of pMCR1.6_P053 with its closely related plasmids. (A) Nucleotide alignment of mcr-1, mcr-1.2, and mcr-1.6 genes. Only the alignment block containing the mutations is shown. Asterisks (*) indicate the positions of mutations. mcr-1.2: in nucleotide position 8, A was mutated to T, leading to a Gln to Leu change in amino acid position 3; mcr-1.6: in nucleotide position 1263, G was mutated to A, which is a synonymous mutation; in nucleotide position 1607, G was mutated to A, leading to an Arg to His change in amino acid position 536. (B) Circular comparison of plasmids pMCR_1511, pHNFP671, and pMCR1.6_P053. Plasmid pMCR_1511 was used as the reference genome sequence. Individual rings range from 1 (inner ring) to 4 (outer ring): ring 1, GC skew of plasmid pMCR_1511 reference genome; ring 2, plasmid pMCR1.6_P053 conservation plot; ring 3, plasmid pHNFP671 conservation plot; ring 4, gene arrangement in plasmid pMCR_1511. (C) Comparison of the genetic environments of mcr-1 and mcr-1.6 in plasmids pMCR_1511 and pMCR1.6_P053, respectively. Gray shading shows >99% nucleotide identity.
The pMCR1.6_P053 plasmid has no known antimicrobial resistance genes other than mcr-1.6, and the whole sequence was highly similar (98% coverage and 99% nucleotide identity) to that of the recently reported mcr-1-harboring IncP-type plasmid pMCR_1511 (KX377410), which was found in Klebsiella pneumoniae isolated from hospital sewage at West China Hospital (11). However, four fragments in pMCR_1511 were missing in pMCR1.6_P053. The first fragment (nucleotide [nt] 5015 to 6091) contained one complete ISApl1 mobile element (IRL [inverted repeat left], transposase, and IRR [inverted repeat right]). The second fragment (nt 8688 to 11753) included one ISApl1 mobile element interrupted by Tn3 and IS26 mobile elements and a blaTEM gene. The third fragment (nt 37198 to 37993) included higA, ybaQ, higB-1, and trfA genes. The fourth fragment (nt 53335 to 57278) contained one transposase of the Tn3 family, eptA, dgkA, ble, and one complete IS26 mobile element (Fig. 1B). In addition, a 2.6-kb fragment (nt 6091 to 8688 in pMCR1.6_P053) harboring the mcr-1 gene was inverted (Fig. 1C), making the orientation of the mcr-1 variant in pMCR1.6_P053 opposite to the orientation of the mcr-1 gene in pMCR_1511. In addition to plasmid from K. pneumoniae, a further BLAST search of the GenBank database showed that the pMCR1.6_P053 plasmid was similar to three plasmids from E. coli strains, i.e., pHNFP671 (KP324830), pJJ1886_4 (CP006788), and pHS102707 (KF701335) (80% coverage and 90% nucleotide identity). Among them, the pMCR1.6_P053 plasmid shared 92% coverage and 99% nucleotide identity to plasmid pHNFP671 (KP324830), which did not carry mcr-1 or its variants. The pHNFP671 plasmid was much longer than the pMCR1.6_P053 plasmid, which mainly lacked a long fragment (nt 48067 to 82807 in pHNFP671) flanked by two IS26 mobile elements and two ∼1,000-bp fragments (nt 21611 to 22775 and nt 37890 to 38859 in pHNFP671) containing one ISApl1 mobile element and the higB and higA genes, respectively. Interestingly, the pHNFP671 plasmid lost the 2,600-bp-long fragment (nt 3298 to 5903 in pMCR1.6_P053) containing the complete mcr-1 cassette (mcr-1 gene and orf723) (Fig. 1C).
To test the host range and transferability of pMCR1.6_P053, conjugation experiments using S. Typhi CT18, E. coli J53 AziR (met pro; azide resistant), and K. pneumoniae BJ1988 as recipients were performed. Transfer of the colistin resistance determinant by conjugation was assayed on LB agar plates (Oxoid) with an initial donor/recipient ratio of 1, using E. coli J53 as the recipient (18). After incubation at 37°C for 4 h, transconjugants were selected on LB agar supplemented with colistin (2 μg/ml) and sodium azide (100 μg/ml). When using S. Typhi CT18 or K. pneumoniae BJ1988 as recipients, the transformants were selected on LB agar with colistin (2 μg/ml) and streptomycin (5,000 μg/ml). The transfer frequency was expressed as the number of transconjugants per recipient. Positive transconjugants were confirmed by PCR targeting of the mcr gene (26). The results demonstrated that plasmid pMCR1.6_P053 was transferred from S. Typhimurium YL14P053 to E. coli J53, S. Typhi CT18, and K. pneumoniae BJ1988 with relatively high frequencies of 6.4 × 10−1, 6.8 × 10−1, and 8.2 × 10−2, respectively. The colistin MIC of the transconjugants showed a 32-fold increase (from 0.125 to 4 μg/ml). These results confirmed that this IncP-type mcr-1.6-harboring plasmid pMCR1.6_P053 is a broad-host-range plasmid and bears great potential to disseminate the mcr-1.6 gene.
In conclusion, a new mcr-1 gene variant, mcr-1.6, bearing two SNPs was found in S. Typhimurium isolated from healthy human gut. The mutations in mcr-1.6 did not impact the gene's colistin resistance activity. The mcr-1.6 gene was found on a variant of a broad-host-range IncP-type mcr-1-harboring plasmid recently discovered in K. pneumoniae. To our knowledge, this plasmid and its variants have not been found in S. Typhimurium. The antibiotic resistance genes are frequently exchanged among E. coli, K. pneumoniae, and S. enterica serovars (27), and the conjugation experiments in this study showed that the plasmid carrying mcr-1.6 can transfer from S. Typhimurium to E. coli and K. pneumoniae. Therefore, the presence of mcr-1.6 in clinical E. coli and K. pneumoniae strains needs to be a focus in public health surveillance.
Accession number(s).
The sequence of pMCR1.6_P053 was deposited into GenBank under accession number KY352406.
ACKNOWLEDGMENTS
This work was supported by the Priority Project on Infectious Disease Control and Prevention (2015CB554201 and 2016YFC1200103) from the Ministry of Health, China.
Y.H. is a member of the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2015069).
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Ohnishi M, Kawanishi M, Akiba M, Kuroda M. 2016. Investigation of a plasmid genome database for colistin-resistance gene mcr-1. Lancet Infect Dis 16:284–285. doi: 10.1016/S1473-3099(16)00008-6. [DOI] [PubMed] [Google Scholar]
- 3.Perrin-Guyomard A, Bruneau M, Houee P, Deleurme K, Legrandois P, Poirier C, Soumet C, Sanders P. 2016. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Euro Surveill 21 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21380. [DOI] [PubMed] [Google Scholar]
- 4.Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, Day M, Muller-Pebody B, Ellington MJ, de Pinna E, Johnson AP, Hopkins KL, Woodford N. 2016. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother 71:2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 5.Khalifa HO, Ahmed AM, Oreiby AF, Eid AM, Shimamoto T, Shimamoto T. 2016. Characterisation of the plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli isolated from animals in Egypt. Int J Antimicrob Agents 47:413–414. doi: 10.1016/j.ijantimicag.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Olaitan AO, Chabou S, Okdah L, Morand S, Rolain JM. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147. doi: 10.1016/S1473-3099(15)00540-X. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes MR, Moura Q, Sartori L, Silva KC, Cunha MP, Esposito F, Lopes R, Otutumi LK, Goncalves DD, Dropa M, Matte MH, Monte DF, Landgraf M, Francisco GR, Bueno MF, de Oliveira Garcia D, Knobl T, Moreno AM, Lincopan N. 2016. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill 21 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22458. [DOI] [PubMed] [Google Scholar]
- 8.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Liu F, Lin IY, Gao GF, Zhu B. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:146–147. doi: 10.1016/S1473-3099(15)00533-2. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi OJ, Kuenzli E, Pires J, Tinguely R, Carattoli A, Hatz C, Perreten V, Endimiani A. 2016. Travelers can import colistin-resistant Enterobacteriaceae, including those possessing the plasmid-mediated mcr-1 gene. Antimicrob Agents Chemother 60:5080–5084. doi: 10.1128/AAC.00731-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao F, Feng Y, Lu X, McNally A, Zong Z. 2017. IncP plasmid carrying colistin resistance gene mcr-1 in Klebsiella pneumoniae from hospital sewage. Antimicrob Agents Chemother 61: pii=e02229-16. doi: 10.1128/AAC.02229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Z, Wang Y, Shen Y, Shen J, Wu C. 2016. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis 16:293. doi: 10.1016/S1473-3099(16)00061-X. [DOI] [PubMed] [Google Scholar]
- 13.Zhi C, Lv L, Yu LF, Doi Y, Liu JH. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:292–293. doi: 10.1016/S1473-3099(16)00063-3. [DOI] [PubMed] [Google Scholar]
- 14.Nordmann P, Lienhard R, Kieffer N, Clerc O, Poirel L. 2016. Plasmid-mediated colistin-resistant Escherichia coli in bacteremia in Switzerland. Clin Infect Dis 62:1322–1323. doi: 10.1093/cid/ciw124. [DOI] [PubMed] [Google Scholar]
- 15.Li A, Yang Y, Miao M, Chavda KD, Mediavilla JR, Xie X, Feng P, Tang YW, Kreiswirth BN, Chen L, Du H. 2016. Complete sequences of mcr-1-harboring plasmids from extended-spectrum-beta-lactamase- and carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4351–4354. doi: 10.1128/AAC.00550-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill 21 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22525. [DOI] [PubMed] [Google Scholar]
- 17.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, Giani T, Menichetti F, Rossolini GM. 2016. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother 60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye H, Li Y, Li Z, Gao R, Zhang H, Wen R, Gao GF, Hu Q, Feng Y. 2016. Diversified mcr-1-harbouring plasmid reservoirs confer resistance to colistin in human gut microbiota. mBio 7:e00177. doi: 10.1128/mBio.00177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao R, Hu Y, Li Z, Sun J, Wang Q, Lin J, Ye H, Liu F, Srinivas S, Li D, Zhu B, Liu YH, Tian GB, Feng Y. 2016. Dissemination and mechanism for the MCR-1 colistin resistance. PLoS Pathog 12:e1005957. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, Tang J, Wu G, Zhang H, Shi Y, Liu Y, Yu C, Wang B, Lu Y, Han C, Cheung DW, Yiu SM, Peng S, Xiaoqian Z, Liu G, Liao X, Li Y, Yang H, Wang J, Lam TW, Wang J. 2015. Erratum: SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience 4:30. doi: 10.1186/s13742-015-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing; approved standard—27th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. http://aurosan.de/wp-content/uploads/2015/05/v_6.0_Breakpoint_table.pdf.
- 23.Wanty C, Anandan A, Piek S, Walshe J, Ganguly J, Carlson RW, Stubbs KA, Kahler CM, Vrielink A. 2013. The structure of the neisserial lipooligosaccharide phosphoethanolamine transferase A (LptA) required for resistance to polymyxin. J Mol Biol 425:3389–3402. doi: 10.1016/j.jmb.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Stojanoski V, Sankaran B, Prasad BV, Poirel L, Nordmann P, Palzkill T. 2016. Structure of the catalytic domain of the colistin resistance enzyme MCR-1. BMC Biol 14:81. doi: 10.1186/s12915-016-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zurfluh K, Kieffer N, Poirel L, Nordmann P, Stephan R. 2016. Features of the mcr-1 cassette related to colistin resistance. Antimicrob Agents Chemother 60:6438–6439. doi: 10.1128/AAC.01519-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chabou S, Leangapichart T, Okdah L, Le Page S, Hadjadj L, Rolain JM. 2016. Real-time quantitative PCR assay with Taqman(R) probe for rapid detection of MCR-1 plasmid-mediated colistin resistance. New Microbes New Infect 13:71–74. doi: 10.1016/j.nmni.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Yang X, Li J, Lv N, Liu F, Wu J, Lin IY, Wu N, Weimer BC, Gao GF, Liu Y, Zhu B. 2016. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl Environ Microbiol 82:6672–6681. doi: 10.1128/AEM.01802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]