ABSTRACT
Coccidioidomycosis can be a chronic, systemic fungal infection requiring long-term to lifetime medication. Thus, there is a need for improved antifungal agents with greater efficacy and reduced toxicity. VT-1161 has a low affinity for mammalian cytochromes and potently inhibits fungal CYP51 with proven efficacy in murine models of central nervous system (CNS) and respiratory coccidioidomycosis. Dogs experience coccidioidomycosis similar to humans and are a useful preclinical model for naturally occurring disease. Twenty-four client-owned dogs diagnosed with respiratory coccidioidomycosis based on radiography, serology, clinical signs, and clinicopathologic abnormalities were treated with a loading dose of VT-1161 for 14 days, followed by 46 days of a lower maintenance dose. Twelve dogs received a high dose (29 mg/kg loading, 6 mg/kg maintenance) and 12 received a low dose (10 mg/kg loading, 1.6 mg/kg maintenance). Response to treatment was assessed by calculating the reduction in disease scores at exit compared to disease scores at enrollment. Overall, 20 of 24 (83%) dogs had ≥50% reduction in enrollment disease scores at exit (P < 0.001), with no difference between the high- and low-dose groups (P = 0.66). Time-weighted average plasma concentrations for the high- and low-dose groups were 39 ± 5 μg/ml and 19 ± 2 μg/ml, respectively. In this open-label study, VT-1161 was efficacious for the treatment of respiratory coccidioidomycosis in naturally infected dogs. Combined with previously reported murine data, this finding supports the further development of VT-1161 for the treatment of coccidioidomycosis in humans.
KEYWORDS: Coccidioides, VT-1161, antifungal agents, dogs
INTRODUCTION
Coccidioidomycosis is a fungal disease that is contracted primarily via inhalation of spores in regions of endemicity in the southwestern United States. The spectrum of clinical illness from inhaling spores of Coccidioides spp. ranges from mild, self-limiting symptoms to fulminant and fatal disease (1). Although most patients will recover without medical intervention, people with progressive or chronic disease and populations with various compromises of the immune system require antifungal therapy (2–4). The most severe consequence of coccidioidal infection is meningitis, which requires lifetime antifungal therapy (5, 6).
Current drugs for the treatment of chronic coccidioidal infection consist primarily of azoles, which have a range of adverse effects and drug-drug interactions (1, 7, 8). VT-1161 is a novel fungal CYP51 inhibitor that was rationally designed through the use of a tetrazole moiety for greater selectivity against binding mammalian cytochrome P450 enzymes while retaining the same or greater potency for the fungal CYP51 target (9, 10). VT-1161 shows a broad spectrum of in vitro and in vivo activity against fungal pathogens (11–13), and separate phase 2b studies on VT-1161 in onychomycosis (ClinicalTrials registration no. NCT02267356) and recurrent vulvovaginal candidiasis (NCT02267382) have recently been completed. VT-1161 has a favorable toxicity profile with minimal predicted drug-drug interactions and minimal toxicity in preclinical and clinical studies to date. We recently showed that it is efficacious in reducing brain and lung fungal burdens in murine models of central nervous system (CNS) and respiratory coccidioidomycosis, respectively, and significantly extends survival in both models (13).
Dogs living in the regions where coccidioidomycosis is endemic have a rate and range of clinical disease similar to those of humans, with respiratory illness being the most common presentation (14). Unlike laboratory models of infection, dogs present to veterinarians with established illness, the same way human patients present for medical care; therefore, naturally infected dogs are a useful species for preclinical assessment of drug candidates for the treatment of coccidioidomycosis in humans. In this study, naturally infected, client-owned dogs with primary respiratory coccidioidomycosis were enrolled and treated with VT-1161 for 60 days, with assessment of efficacy performed as described in a previous study (15). Twelve dogs were enrolled in each of two open-label study arms, with a starting dose selected based on pharmacokinetics (PK) and a second dose, 3-fold lower, based on concentrations in plasma in dogs in the first arm. Dogs were assessed clinically, radiographically, and with blood testing at enrollment and exit. Plasma concentrations of VT-1161 were measured at intervals during the study and at exit to assess relationships between blood drug levels and clinical responses.
RESULTS
VT-1161 pharmacokinetics in laboratory beagle dogs.
Single intravenous (i.v.) and oral dose pharmacokinetics of VT-1161 were determined in laboratory beagle dogs in order to estimate oral doses to treat client-owned dogs with coccidioidomycosis. VT-1161 was administered to dogs under fed and fasted conditions, and the data show that the extents of absorption were different (Fig. 1). Mean dose-normalized exposures (maximum concentration of drug in serum [Cmax] and area under the concentration-time curve [AUC]) were 2.6- to 3.1-fold greater when VT-1161 was given under fed conditions than under fasted conditions (Table 1), suggesting that food affects the oral bioavailability of VT-1161. VT-1161 exhibited a long elimination half-life (t1/2) in plasma, with mean t1/2 values greater for fed than fasted dogs and greater after oral administration than after intravenous administration (Table 1). The oral absorption of VT-1161 was variable between animals within each dose group. The absolute bioavailability of VT-1161 following administration under fed conditions ranged from 41% to 188% (mean, 132%). The absolute bioavailability of VT-1161 given under fasted conditions ranged from 23% to 63% (mean, 43%). Based on modeling of these and other data, the oral loading and maintenance doses of VT-1161 tablets were determined for the treatment of coccidioidomycosis in naturally infected dogs.
FIG 1.
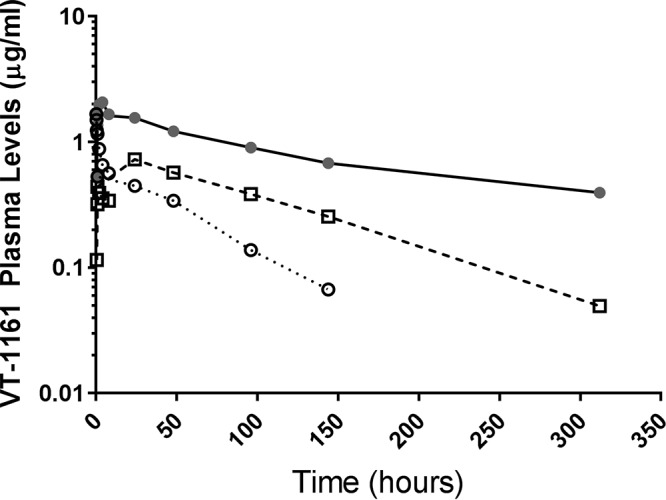
Group mean VT-1161 plasma concentration-time profiles in beagle dogs following single-dose intravenous and oral administration. Dogs were dosed with VT-1161 orally at 10 mg/kg in the fed state (filled circles and solid line), orally at 10 mg/kg in the fasted state (open squares and dashed line), or intravenously at 2 mg/kg (open circles and dotted line) (n = 3 dogs/group).
TABLE 1.
Pharmacokinetic parameters of VT-1161 in laboratory beagle dogsa
| Dog group | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUClast (h · μg/ml) | AUCinf (h · μg/ml) | CL (ml/h/kg) | Vz (liter/kg) | %F |
|---|---|---|---|---|---|---|---|---|
| Administered drug i.v. | 1.7 ± 0.2 | 0.17 | 41 ± 4 | 40 ± 3 | 44 ± 4 | 43 ± 8 | 2.5 ± 0.4 | |
| Administered drug orally, fasted | 0.81 ± 0.43 | 13 ± 11 | 77 ± 1 | 73 ± 32 | 94 ± 45 | 43 ± 20 | ||
| Administered drug orally, fed | 2.1 ± 1.0 | 3.3 ± 1.2 | 117 ± 37 | 234 ± 147 | 296 ± 177 | 132 ± 79 |
Values are means ± SD. Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; t1/2, terminal elimination half-life; AUClast, area under the plasma concentration-time curve from time zero to the last sampling time point with a measurable concentration; AUCinf, area under the plasma concentration-time curve from time zero to an extrapolated complete clearance of drug; CL, clearance; Vz, apparent volume of distribution; F, oral bioavailability.
Signalment and assessment of disease in dogs treated with VT-1161.
Twenty-four dogs were enrolled in the study, 12 at a high dose (29 mg/kg loading, 6 mg/kg maintenance), followed by 12 at a low dose (10 mg/kg loading, 1.6 mg/kg maintenance). Fourteen (58%) of the dogs were castrated males, 6 (25%) were spayed females, and two each were intact males and females. Enrollment weights and ages for dogs in the study ranged from 4.9 to 29 kg (mean, 19 kg) and 6 months to 11 years (mean, 4.4 years), respectively, and there was a variety of breeds and mixes (Table 2). No statistical difference was found between the two groups in regard to age (P = 0.571), sex (P = 0.083), and weight (P = 0.33).
TABLE 2.
Signalment of dogs treated for 60 days with high (group 1) and low (group 2) doses of VT-1161
| Group 1 (29 mg/kg; 6 mg/kg)a |
Group 2 (10 mg/kg; 1.6 mg/kg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dog | Breed | Sexb | Age (yr) | Wt (kg) | Dog | Breed | Sex | Age (yr) | Wt (kg) |
| 1 | Cocker spaniel | CM | 8 | 14 | 13 | Miniature poodle | CM | 5 | 7 |
| 2 | Pitbull | SF | 5 | 24.3 | 14 | Mix | CM | 2 | 25 |
| 3 | Border collie | CM | 0.6 | 14.7 | 15 | Mix | CM | 3 | 4.9 |
| 4 | Boxer | SF | 0.5 | 19.3 | 16 | Mix | CM | 10 | 22.5 |
| 5 | Mix | CM | 2 | 26.9 | 17 | Border collie | CM | 9 | 16.8 |
| 6 | Havanese | SF | 3 | 7.2 | 18 | Rough collie | F | 4 | 20 |
| 7 | Border collie | SF | 9 | 16.6 | 19 | Mix | M | 3 | 26 |
| 8 | Mix | CM | 8 | 12.3 | 20 | Standard poodle | SF | 11 | 19.8 |
| 9 | Mix | CM | 2 | 22.5 | 21 | Pitbull | CM | 2 | 29 |
| 10 | Mix | SF | 5 | 29 | 22 | Rough collie | CM | 4 | 20 |
| 11 | Mix | CM | 2 | 5 | 23 | Akita | M | 2 | 29 |
| 12 | Beagle | CM | 3 | 13.6 | 24 | Rough collie | F | 2 | 22.3 |
| Mean | 4.0 | 17.1 | Mean | 4.8 | 20.2 | ||||
Loading and maintenance doses (in that order) are indicated in parentheses for both groups.
M, intact male; CM, castrated male; F, intact female; SF, spayed female.
At enrollment, all 24 dogs presented with coughing. Other clinical signs included anorexia or decreased appetite (n = 16), lethargy (n = 15), weight loss (n = 13), fever (n = 12), wheezing (n = 2), and reduced endurance (n = 1). Radiographic abnormalities included hilar lymphadenopathy as the most common finding (n = 21), followed by interstitial infiltrates (n = 17), nodules (n = 6), and lobar consolidation (n = 6). Clinicopathological abnormalities commonly included hyperglobulinemia (n = 19), monocytosis (n = 18), and neutrophilia (n = 11). Starting anticoccidioidal immunodiffusion antibody titers (IgG) ranged from negative to 1:128, with 23 seropositive dogs. The one seronegative dog had the diagnosis established by the cytologic presence of spherules in a lung aspirate. Another dog also had a lung aspirate to verify the diagnosis after showing no response to VT-1161 at the mid-study assessment, and spherules were identified.
Table 3 shows the starting and exit disease scores and percent improvement for the dogs by dose group. Enrollment and exit scores were not significantly different for the two doses (P = 0.42 at start and P = 0.66 at exit), so the groups were combined for the remaining analyses. The mean enrollment score for all dogs was 13.8 (range, 8 to 19), and the mean exit score was 4.7 (range, 1 to 14). Twenty-three of 24 dogs completed the study, with one dog removed for worsening clinical signs and more extensive lung consolidation on day 32 of treatment. This dog was included in the statistical analysis of the data since plasma concentrations were analyzed using time-weighted averages. Overall, 20 of 24 (83%) dogs experienced some combination of clinical, radiographic, clinicopathologic, and serologic improvement that led to at least a 50% reduction (range, 53% to 90%) in disease score at exit, while four dogs, including the dog removed from the study, had poor responses (mean exit score, 10; improvement, <50%) (Table 3). Regarding radiographic improvement of lungs, 15 of 24 (62%) dogs had ≥50% improvement. Overall, VT-1161 resulted in a significant reduction in disease scores in dogs treated for 60 days (P ≤ 0.0001; 95% confidence interval [CI], 7.403, 10.847). Additionally, all owners of dogs considered responders reported ≥75% improvement in clinical signs compared to those at enrollment on the owner assessment questionnaire at exit.
TABLE 3.
Enrollment and exit coccidioidal disease scores and percent improvement for dogs treated with VT-1161
| Group 1 (29 mg/kg; 6 mg/kg)a |
Group 2 (10 mg/kg; 1.6 mg/kg) |
||||||
|---|---|---|---|---|---|---|---|
| Dog | Start score | Exit score | % improvement | Dog | Start score | Exit score | % improvement |
| 1 | 12 | 7 | 42b | 13 | 15 | 4 | 73 |
| 2 | 14 | 3 | 79 | 14 | 18 | 5 | 72 |
| 3 | 13 | 4 | 69 | 15 | 15 | 3 | 80 |
| 4 | 10 | 1 | 90 | 16 | 13 | 5 | 62 |
| 5 | 19 | 2 | 89 | 17 | 17 | 9 | 47b |
| 6 | 17 | 2 | 88 | 18 | 18 | 7 | 61 |
| 7 | 13 | 14 | −8b | 19 | 12 | 2 | 83 |
| 8 | 17 | 3 | 82 | 20 | 14 | 6 | 57 |
| 9 | 10 | 4 | 60 | 21 | 9 | 2 | 78 |
| 10 | 8 | 2 | 75 | 22 | 12 | 4 | 67 |
| 11 | 12 | 11 | 8b | 23 | 15 | 4 | 73 |
| 12 | 15 | 7 | 53 | 24 | 14 | 2 | 86 |
| Mean | 13.3 | 5 | Mean | 14.3 | 4.4 | ||
Loading and maintenance doses (in that order) are indicated in parentheses for both groups.
Poor responders, defined as having less than 50% improvement in scores from start to exit.
Drug plasma levels in VT-1161-treated dogs.
The time-weighted average (TWA) plasma level of VT-1161 for each dog was calculated from the 14-day, mid-study, and exit values. The mean TWA plasma levels of VT-1161 at steady state were 39 ± 5 μg/ml for the high-dose group and 19 ± 2 μg/ml for the low-dose group. These results show that the plasma concentration was dose dependent but not linearly proportionate. Plasma concentrations from both groups were well above the MIC90 for Coccidioides strains (13). To determine if a relationship existed between the plasma concentration of VT-1161 and the clinical response, the percent change in disease score at enrollment and exit was calculated for each dog, and linear regression was performed. Plasma levels were not predictive of the reduction in disease score (R2 = 0.017), and the relationship between percent improvement and plasma level was not significant (P = 0.547).
Potential adverse effects.
The majority of dogs (17 of 23) had elevated serum cholesterol at the exit visit that ranged from 331 to 700 mg/dl (normal, 90 to 324 mg/dl), although 23 dogs had normal serum cholesterol at enrollment and one had mildly elevated serum cholesterol (365 mg/dl). However, there were no clinical signs associated with hypercholesterolemia in the dogs. Triglycerides were not included in the chemistry panel performed at exit, and dogs were not fasted prior to testing.
Owners reported few clinical adverse effects in dogs taking VT-1161. These included decreased appetite and soft stools as treatment progressed (n = 1), vomiting up the dose during the loading phase (n = 1), increased frequency of urination (n = 1), and epistaxis (n = 1). The dog that vomited immediately after administration of the pills required seven tablets for a loading dose, and when the owner divided the number of tablets in half and administered them approximately 12 h apart, the vomiting stopped. The same dog with emesis had increased urination reported by the owner. The owner reported that the dog had also experienced increased urination while taking fluconazole. It is unknown if the epistaxis in one dog was causally related to VT-1161, but it began a week after the first dose was taken and the last episode of bleeding occurred 2 weeks after stopping it. This dog did not have an unusually high plasma level of VT-1161 (TWA, 21.1 μg/ml).
DISCUSSION
Dogs experience a range of coccidioidal illnesses similar to those in humans, with pulmonary coccidioidomycosis being the most common presentation (14, 16). Owners seek medical care for dogs when they develop coughing, inappetance, lethargy, fever, and weight loss (17), as was typical of the dogs in this study. In this open-label study of VT-1161, 83% of the dogs clinically ill with coccidioidomycosis that were treated for 60 days had at least 50% improvement in their scored disease parameters by the end of the study. Time-weighted average plasma concentrations of VT-1161 showed that all the dogs had levels much greater than the MIC90 for Coccidioides spp. (2 μg/ml for 52 isolates) (13) and that there was no difference in clinical responses for dogs with a mean plasma TWA of 39 μg/ml (high dose) and those with a mean plasma TWA of 19 μg/ml (low dose). In addition, there was no significant relationship between the plasma level and the percent reduction in disease score, most likely because all plasma concentrations were well above the MIC for Coccidioides spp. and at maximum effect for treating coccidioidomycosis in dogs. Further dose reductions would be required to determine a dose dependency and the minimum effective dose.
Some dogs had been receiving fluconazole prior to enrollment in the study and were discontinued from this treatment 48 to 96 h before enrollment. These dogs had detectable levels of fluconazole in plasma at enrollment (mean estimated concentration, 52 μg/ml), as determined after the fact, but all dogs had undetectable or subtherapeutic levels (≤0.1 μg/ml) by day 14. These data are consistent with the 15-h half-life for fluconazole in dogs (18), which would result in mean plasma levels of 2 to 3 μg/ml after only 3 days off the drug, which is less than the geometric mean MIC of fluconazole against Coccidioides spp. of 8 μg/ml (19). Therefore, we conclude that fluconazole did not contribute to efficacy in this study, both because of its intrinsic half-life and MIC potency and because the dogs previously treated with fluconazole enrolled in this study due to a poor response. However, we recognize that the presence of fluconazole in plasma at enrollment is a limitation of the study and a potential confounder.
Because of the long half-life of VT-1161, a loading dose/maintenance dose (LD/MD) strategy was employed in this study. PK parameters from a single dose of VT-1161 capsules in laboratory beagle dogs were used to model PK for dosing VT-1161 tablets in an LD/MD strategy in dogs of various breeds, weights, ages, and sex. Given this complexity, along with the variations in eating patterns of the ill dogs, the variability in the plasma levels at the two doses was quite acceptable, e.g., standard deviations of about 20%. Separately, it is noted that the clinical development of VT-1161 has also used an LD/MD strategy (e.g., ClinicalTrials registration no. NCT02267356), with the maintenance dose being a relatively higher dose given once weekly rather than the lower dose given once daily used in this study. An LD/MD strategy could prove an attractive feature in treating a chronic disease for long periods of time.
There were no major adverse events in dogs taking VT-1161, and there were very few minor ones. Since the dogs did not have similar adverse effects, it is difficult to speculate on the relationship between the reported events and the administration of VT-1161. Because the vomiting ceased when the large number of tablets administered to one dog was divided, we surmise that the amount of medication caused local gastric irritation that induced vomiting. The mechanism for elevated cholesterol in many of the dogs is not known but appears to be unrelated to humans. To date, elevated cholesterol has not been measured in any of the more than 600 patients and healthy volunteers that have been exposed to therapeutic levels of VT-1161 (T. Degenhardt, A. Tavakkol, and R. Schotzinger, unpublished data). Overall, VT-1161 was well tolerated in this group of dogs taking doses that yielded plasma levels significantly above the MIC for the infectious agent.
Although VT-1161 penetrates CNS tissues and has shown efficacy for treatment of CNS coccidioidomycosis in a murine infection model (13), only pulmonary cases were selected for assessment of VT-1161 in naturally infected dogs because of the ease of enrollment and monitoring and the ability to assess responses in a short time period (60 days). Pulmonary coccidioidomycosis is common, while CNS manifestations in dogs are infrequent, similar to those in humans, and quantitative assessment would require serial imaging performed under anesthesia, as well as a long enrollment period to collect sufficient cases. Also due to constraints on the time to enroll dogs, we accepted a wide variety of breeds, ages, and status regarding prior treatment of disease as long as the dog met the enrollment criteria. This, along with the lack of uniformity of fluconazole withdrawal time and verification of subtherapeutic blood levels prior to instituting VT-1161, can be considered a limitation of the study.
In summary, VT-1161 demonstrated utility in treating coccidioidomycosis in a clinical setting in dogs and showed minimal toxicity. VT-1161 bears further development and has the potential to broaden the spectrum of less toxic antifungal therapies for treating coccidioidomycosis in humans.
MATERIALS AND METHODS
VT-1161.
VT-1161 was provided by Viamet Pharmaceuticals, Inc. (Durham, NC), as 20-, 50-, or 150-mg tablets or as 50-mg tablets encapsulated into 100-mg and 150-mg doses.
Naturally infected dogs.
Client-owned dogs were enrolled with informed consent, and all procedures were approved by the University of Arizona Institutional Animal Care and Use Committee. Dogs were enrolled and all examinations and procedures were performed at the Veterinary Specialty Center of Tucson (Tucson, AZ). Dogs were eligible for enrollment if they had lung radiographs consistent with a diagnosis of coccidioidomycosis and were seropositive with consistent clinical signs or if they had cytologically demonstrated Coccidioides spherules in the lungs. Both naive dogs and those responding inadequately to antifungal treatment were enrolled. Dogs on antifungal treatment (fluconazole) were discontinued from that treatment 48 to 96 h before the start of the study. Exclusions were other underlying diseases, such as liver or renal disease, secondary infections, steroid therapy, or significant serum biochemical abnormalities not considered related to coccidioidomycosis.
Clinical study procedures.
All dogs had lung radiographs, serum chemistries, a complete blood cell count (CBC), and a positive anticoccidioidal antibody serology or definitive cytology within 30 days prior to enrollment. Twelve dogs were enrolled to receive a loading dose of 29 mg/kg of VT-1161 once daily for 14 days, followed by a maintenance dose of 6 mg/kg for 46 days (60 days total of treatment). The second 12 dogs enrolled received a loading dose of 10 mg/kg for 14 days, followed by 46 days of maintenance treatment at 1.6 mg/kg. Due to the fixed drug amount in the VT-1161 tablets and various weights of the dogs, combinations of available tablets were used to achieve the dose closest to the target. The greatest variance of the actual dose to the target dose was ±15%. Owners were instructed to give the medication with a meal and to keep a log of the time of treatment, time of feeding, and how much of the meal was eaten (all, partial, or none).
At enrollment, dogs were examined and serum was collected and frozen for coccidioidal serology to pair with the exit serum. Dogs were examined at mid-study (∼day 28) and at the exit visit, which occurred within 1 week of the last dose of medication. At the exit visit, lung radiographs, serum chemistries, CBC, and coccidioidal serology were repeated for comparison with enrollment data. Sera drawn at enrollment and exit were submitted together to the laboratory for paired coccidioidal serology. Enrollment and exit radiographs were compared by the same individual (M.E.R.), and the percent improvement was estimated. At the mid-study visit and the exit visit, owners completed a questionnaire estimating improvement in valley fever clinical signs (0, 25%, 50%, 100%) and reporting perceived adverse effects of the medication, e.g., vomiting, diarrhea, and lack of appetite.
VT-1161 pharmacokinetics in beagle dogs and plasma levels in dogs with coccidioidomycosis.
Single-dose PK studies were conducted in male beagle dogs (Ricerca Biosciences, Concord, OH). VT-1161 was dosed i.v. at 2 mg/kg in 10% Cremophor EL in sterile water and orally at 10 mg/kg, using 20-mg VT-1161 capsules, under fed and fasted conditions (n = 3 dogs/dose group). Fed dogs had access to food prior to dosing and through the time of collection of the last blood sample. Fasted dogs were fasted overnight prior to the day of dosing and were fed 4 h after dosing. Blood samples were collected from all animals at the following time points: predosing and 0.5, 1, 2, 4, 8, 12, 24, 48, 96, 144, and 312 h after dosing.
During the coccidioidomycosis VT-1161 treatment study, blood was collected prior to the initiation of treatment and on day 14, on approximately day 28 (at the mid-study visit), and at the exit visit, which was within 1 week of the last dose of medication. Plasma was stored frozen until analysis.
For all analyses, VT-1161 was extracted from plasma samples and analyzed using high-performance liquid chromatography with tandem mass spectrometry using electrospray ionization (Tandem Labs, Durham, NC, for the PK study, and OpAns, LLC, Durham, NC, for the coccidioidomycosis study). Quantification was achieved against an external calibration curve generated in the same matrix and using the signal response ratio of the sample to the internal standard. PK parameters were determined for individual animals from plasma concentration-time data using noncompartmental modeling (NCA Model 201 for intravenous administration or NCA Model 200) in WinNonlin Professional version 5.3 (Pharsight Corp., Mountain View, CA).
Data scoring and statistical analysis.
Dogs were scored on clinical signs (coughing, lethargy, anorexia, and weight loss, etc.) (2 points for each clinical finding), serology (1 point for each 2-dilution increase in titer from 1:4), CBC/serum biochemistry changes, radiographic findings (pneumonia, hilar lymphadenopathy, nodules), and cytology (1 point for each abnormality). The scores were modeled on the Mycosis Study Group scoring system as used in a previous clinical study in dogs (15). Time-weighted mean plasma levels of VT-1161 were calculated from the three data points for each dog, and these were used for statistical analysis of the relationships of plasma level to disease score. Data analysis was conducted in SPSS (IBM Analytics, version 24). Comparison of signalment characteristics between groups was conducted using Student's t test or χ2 test, as appropriate. A paired t test was used to calculate the significance between disease scores at entrance and exit. Linear regression was used to evaluate the relationship between disease score and drug plasma levels.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases (5R33AI101497) and Viamet Pharmaceuticals, Inc. (Durham, NC).
We thank Christine Butkiewicz for statistical assistance in analyzing the data.
Edward P. Garvey, William J. Hoekstra, and Robert J. Schotzinger have potential conflicting interests as employees of Viamet Pharmaceuticals, Inc.
REFERENCES
- 1.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, Pappagianis D, Edwards JE, Wasserman RB, Clark RJ, Antoniskis D, Larsen RA, Englender SJ, Petersen EA. 1990. Coccidioidomycosis during human immunodeficiency virus infection. Medicine 69:384–391. doi: 10.1097/00005792-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Logan JL, Blair JE, Galgiani JN. 2001. Coccidioidomycosis complicating solid organ transplantation. Semin Respir Infect 16:251–256. doi: 10.1053/srin.2001.29318. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom L, Yocum DE, Ampel NM, Villanueva I, Lisse J, Gluck O, Tesser J, Posever J, Miller M, Araujo J, Kageyama DM, Berry M, Karl L, Yung CM. 2004. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum 50:1959–1966. doi: 10.1002/art.20454. [DOI] [PubMed] [Google Scholar]
- 5.Blair JE. 2009. Coccidioidal meningitis: update on epidemiology, clinical features, diagnosis, and management. Curr Infect Dis Rep 11:289–295. doi: 10.1007/s11908-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 6.Galgiani JN. 1997. Coccidioides immitis meningitis, p 227–238. In Peterson PK, Remington JS (ed), Defense of the brain: current concepts in the immunopathogenesis and clinical aspects of CNS infections. Blackwell Science, Inc., Malden, MA. [Google Scholar]
- 7.Fromtling RA. 1988. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1:187–217. doi: 10.1128/CMR.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nivoix Y, Leveque D, Herbrecht R, Koffel JC, Beretz L, Ubeaud-Sequier G. 2008. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin Pharmacokinet 47:779–792. doi: 10.2165/0003088-200847120-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 10.Warrilow AG, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Long L, Ghannoum MA. 2015. VT-1161 dosed once daily or once weekly exhibits potent efficacy in treatment of dermatophytosis in a guinea pig model. Antimicrob Agents Chemother 59:1992–1997. doi: 10.1128/AAC.04902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvey EP, Hoekstra WJ, Schotzinger RJ, Sobel JD, Lilly EA, Fidel PL Jr. 2015. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother 59:5567–5573. doi: 10.1128/AAC.00185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shubitz LF, Trinh HT, Galgiani JN, Lewis ML, Fothergill AW, Wiederhold NP, Barker BM, Lewis ER, Doyle AL, Hoekstra WJ, Schotzinger RJ, Garvey EP. 2015. Evaluation of VT-1161 for treatment of coccidioidomycosis in murine infection models. Antimicrob Agents Chemother 59:7249–7254. doi: 10.1128/AAC.00593-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shubitz LF. 2007. Comparative aspects of coccidioidomycosis in animals and humans. Ann N Y Acad Sci 1111:395–403. doi: 10.1196/annals.1406.007. [DOI] [PubMed] [Google Scholar]
- 15.Shubitz LF, Roy ME, Nix DE, Galgiani JN. 2013. Efficacy of nikkomycin Z for respiratory coccidioidomycosis in naturally infected dogs. Med Mycol 51:747–754. doi: 10.3109/13693786.2013.770610. [DOI] [PubMed] [Google Scholar]
- 16.Graupmann-Kuzma A, Valentine BA, Shubitz LF, Dial SM, Watrous B, Tornquist SJ. 2008. Coccidioidomycosis in dogs and cats: a review. J Am Anim Hosp Assoc 44:226–235. doi: 10.5326/0440226. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LR, Herrgesell EJ, Davidson AP, Pappagianis D. 2003. Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995-2000). J Am Vet Med Assoc 222:461–466. doi: 10.2460/javma.2003.222.461. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey MJ, Jevons S, Tarbit MH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother 28:648–653. doi: 10.1128/AAC.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramani R, Chaturvedi V. 2007. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia 163:315–319. doi: 10.1007/s11046-007-9018-7. [DOI] [PubMed] [Google Scholar]


