ABSTRACT
Antofloxacin is a novel broad-spectrum fluoroquinolone under development for the treatment of infections caused by a diverse group of bacterial species. We explored the pharmacodynamic (PD) profile and targets of antofloxacin against seven Klebsiella pneumoniae isolates by using a neutropenic murine lung infection model. Plasma and bronchopulmonary pharmacokinetic (PK) studies were conducted at single subcutaneous doses of 2.5, 10, 40, and 160 mg/kg of body weight. Mice were infected intratracheally with K. pneumoniae and treated using 2-fold-increasing total doses of antofloxacin ranging from 2.5 to 160 mg/kg/24 h administered in 1, 2, 3, or 4 doses. The Emax Hill equation was used to model the dose-response data. Antofloxacin could penetrate the lung epithelial lining fluid (ELF) with pharmacokinetics similar to those in plasma with linear elimination half-lives over the dose range. All study strains showed a 3-log10 or greater reduction in bacterial burden and prolonged postantibiotic effects (PAEs) ranging from 3.2 to 5.3 h. Dose fractionation response curves were steep, and the free-drug area under the concentration-time curve over 24 h (AUC0–24)/MIC ratio was the PD index most closely linked to efficacy (R2 = 0.96). The mean free-drug AUC0–24/MIC ratios required to achieve net bacterial stasis, a 1-log10 kill, and a 2-log10 kill for each isolate were 52.6, 89.9, and 164.9, respectively. When integrated with human PK data, these PD targets could provide a framework for further optimization of dosing regimens. This could make antofloxacin an attractive option for the treatment of respiratory tract infections involving K. pneumoniae.
KEYWORDS: antofloxacin, PK/PD, murine lung infection, Klebsiella pneumoniae
INTRODUCTION
Acute exacerbations of chronic bronchitis (AECB) represent an important health burden to patients, including increased morbidity, mortality, and health care costs worldwide (1, 2). Although viral infections have an important role in both development and progression of AECB, the pathophysiological mechanisms are not completely understood and seem to be a complex of many interrelated factors (3). Apart from exacerbations caused by viral infections and environmental irritants, approximately 40 to 50% of exacerbations are attributed to bacteria that include Streptococcus pneumoniae, Klebsiella pneumoniae, Haemophilus influenzae, and Staphylococcus spp. (4). It is a common practice to prescribe antibiotics for AECB patients with severe illness (5, 6). However, treatment of these bacterial infections is frequently challenging due to drug resistance and limited therapeutic options.
Antofloxacin is a novel fluoroquinolone antibiotic with a broad-spectrum antimicrobial activity against both Gram-positive and -negative bacteria. The Chinese Food and Drug Administration (CFDA) has approved it for the treatment of AECB caused by K. pneumoniae, acute pyelonephritis and cystitis due to Escherichia coli, and wound infection and multiple epifolliculitis due to Staphylococcus aureus or coagulase-negative staphylococci. The MIC50 and MIC90 values of antofloxacin against K. pneumoniae (53 strains) are 0.063 and 0.5 mg/liter (unpublished data), and the reported MIC90 values of antofloxacin against E. coli, methicillin-sensitive S. aureus, and Staphylococcus epidermidis are 2, 0.5 to 1, and 0.125 mg/liter, respectively (7, 8). Previous studies also demonstrated that antofloxacin possesses an excellent in vitro activity against Mycobacterium tuberculosis (9).
In this study, we described the pharmacokinetics (PK) and penetration of antofloxacin into lung epithelial lining fluid (ELF) in neutropenic murine lungs infected with seven K. pneumoniae strains. We also evaluated the impact of dose and dosing regimens on the in vivo drug efficacy. The objectives of our experiments were designed to (i) elucidate antofloxacin pharmacodynamic (PD) characteristics via in vivo postantibiotic effects (PAEs), (ii) investigate PK/PD indices that best correlated with the efficacy of antofloxacin via dose fractionation studies, and (iii) determine the magnitude of PK/PD index values required for various efficacy targets against K. pneumoniae isolates, including an extended-spectrum β-lactamase (ESBL)-producing strain.
RESULTS
Organism susceptibility testing.
The MICs of antofloxacin against K. pneumoniae isolates used in this study varied from 0.03 to 0.25 mg/liter (Table 1). The levofloxacin MIC was comparable to the antofloxacin MIC for the same isolate. However, two isolates (160211 and 700603) with cefotaxime MICs of ≥4 mg/liter were considered resistant types based on the CLSI MIC interpretive criteria (10). This indicated that β-lactam resistance did not impact antofloxacin potency.
TABLE 1.
Disease classification and in vitro antimicrobial susceptibilities of K. pneumoniae isolates selected for use in murine neutropenic lung infection studies with antofloxacin
| Organism | Diagnosisa | MIC (mg/liter) (susceptibility)c |
|||
|---|---|---|---|---|---|
| Antofloxacin | Amikacin | Cefotaxime | Levofloxacin | ||
| 160491 | AECB | 0.125 | 4 (S) | 1 (S) | 0.125 (S) |
| 160271 | AECB | 0.06 | 4 (S) | 1 (S) | 0.125 (S) |
| 160244 | COPD | 0.06 | 4 (S) | 0.06 (S) | 0.25 (S) |
| 160211 | Pneumonia | 0.06 | 8 (S) | 4 (R) | 0.25 (S) |
| 160430 | AECB | 0.03 | 2 (S) | 1 (S) | 0.125 (S) |
| ATCC 700603b | 0.25 | 2 (S) | >32 (R) | 0.5 (S) | |
| ATCC 35657 | 0.25 | 1 (S) | 1 (S) | 0.5 (S) | |
AECB, acute exacerbations of chronic bronchitis; COPD, chronic obstructive pulmonary disease.
K. pneumoniae ATCC 700603 is an extended-spectrum β-lactamase reference strain that produces the enzyme SHV-18.
Susceptible (S) and resistant (R) interpretations refer to the CLSI MIC interpretive criteria (10).
Drug pharmacokinetics.
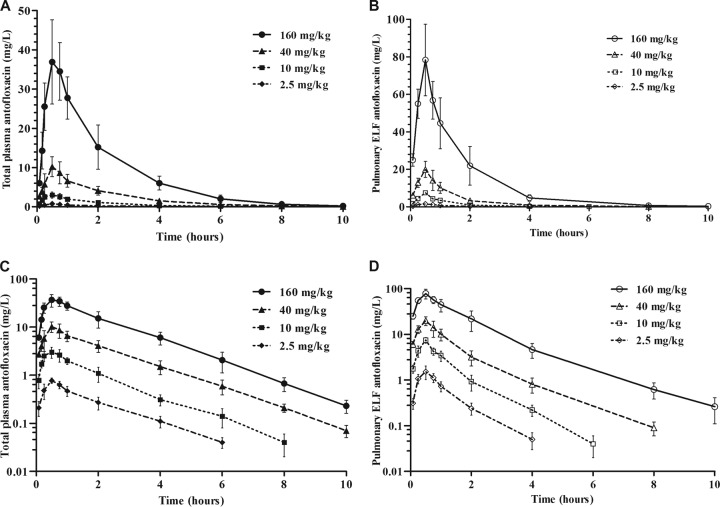
The elimination half-lives (t1/2s) of antofloxacin in plasma and ELF ranged from 1.21 to 1.43 h and 0.77 to 1.36 h, respectively (Table 2). The plasma pharmacokinetics were relatively linear for both the area under the concentration-time curve (AUC) and the maximum concentration of drug in plasma (Cmax) across the escalating dose range (AUC, R2 = 0.97; Cmax, R2 = 0.95). Similar to the pattern in plasma, there was linearity and dose proportionality for the pharmacokinetics in ELF. Of note, drug concentrations in ELF were nearly 2-fold higher than those in plasma (Fig. 1A and B).
TABLE 2.
PK parameters in plasma and ELF and ELF/plasma penetration ratios of antofloxacin after a single subcutaneous dose in neutropenic mice with lung infectionsa
| Dose (mg/kg) | ELF |
Plasma |
ELF/plasma ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (mg · h/liter) | AUC/dose (kg · h/liter) | t1/2 (h) | Cmax (mg/liter) | AUC (mg · h/liter) | AUC/dose (kg · h/liter) | t1/2 (h) | Cmax (mg/liter) | Total | Freeb | |
| 2.5 | 1.83 | 0.73 | 0.77 | 1.58 | 1.36 | 0.54 | 1.43 | 0.81 | 1.35 | 1.69 |
| 10 | 8.13 | 0.81 | 0.87 | 7.47 | 5.89 | 0.58 | 1.21 | 3.18 | 1.38 | 1.73 |
| 40 | 25.8 | 0.65 | 1.19 | 19.9 | 21.2 | 0.53 | 1.37 | 10.9 | 1.22 | 1.53 |
| 160 | 125.6 | 0.79 | 1.36 | 78.4 | 81.6 | 0.51 | 1.28 | 39.1 | 1.54 | 1.93 |
| Mean (SD) | NA | 0.74 (0.07) | 1.05 (0.23) | NA | NA | 0.53 (0.03) | 1.32 (0.08) | NA | 1.37 (0.11) | 1.72 (0.14) |
ELF, epithelial lining fluid; AUC, area under the concentration-time curve; t1/2, elimination half-life; Cmax, maximum concentration of drug in in plasma or ELF; NA, not applicable.
The proportion of protein binding for antofloxacin in murine plasma was 20.3%, and that in ELF was negligible.
FIG 1.
(A and B) Pharmacokinetic profiles of antofloxacin in plasma (A) and pulmonary ELF (B) after subcutaneous administration of single doses of 2.5, 10, 40, and 160 mg/kg in lung-infected neutropenic mice. (C and D) Data corresponding to panels A and B plotted on semilogarithmic coordinates. Filled and open symbols represent concentrations in plasma and ELF, respectively. Each symbol represents the mean ± standard deviation of the levels in the plasma or ELF of six mice.
Based on the overall AUC ratios in plasma and ELF, the penetration ratio of antofloxacin in ELF ranged from 1.22 to 1.54 for the total drug concentrations and was independent of the dose levels (Table 2). The binding degrees of antofloxacin in murine plasma varied from 18.6% to 22.3% in the range of 0.05 to 5 mg/liter, with a mean binding rate of 20.3% (see Table S1 in the supplemental material). Taking into account the protein binding of antofloxacin, the mean penetration ratio of free drug into ELF was 1.72 (standard deviation, 0.14), which further confirmed a considerable pulmonary distribution of antofloxacin.
In vivo PAEs.
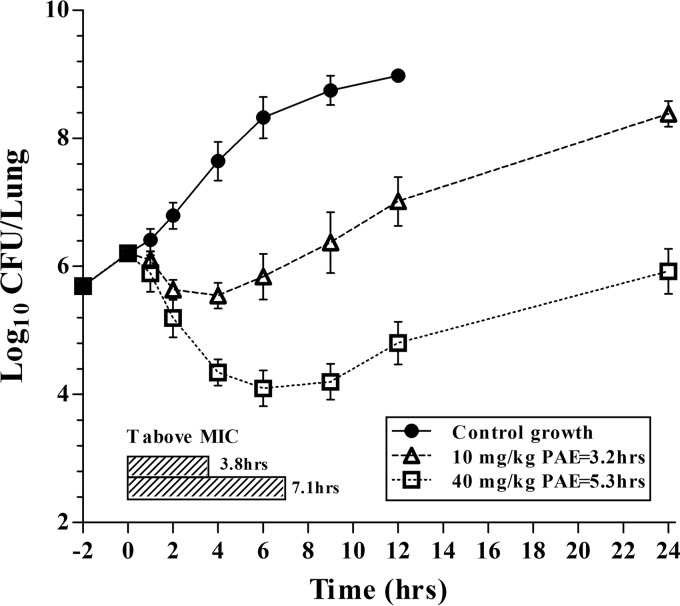
PAEs were calculated after allowing bacterial regrowth following administration of single doses of antofloxacin at 10 and 40 mg/kg of body weight. The lung burden of K. pneumoniae ATCC 35657 in untreated mice increased by 2.77 log10 CFU/lung over 12 h. A 1-log10 CFU/lung growth increase occurred at 3.0 h. Persistent growth inhibition or rapid killing was observed following administration of the two drug levels in a dose-dependent manner. Maximal killing was >2 log10 CFU/lung and occurred at the highest dose level (Fig. 2).
FIG 2.
In vivo postantibiotic effect estimates after a single subcutaneous dose of antofloxacin using a neutropenic mouse lung model. Each symbol represents the mean and standard deviation of data from four lungs infected with K. pneumoniae ATCC 35657. The black horizontal bars represent the time that plasma antofloxacin concentrations remained above the MIC for the infecting organism.
Based on the above pharmacokinetics data, single doses of antofloxacin at 10 and 40 mg/kg produced free drug concentrations that remained above the MIC at 3.8 and 7.1 h, respectively. However, after the drug levels fell below the MIC, the antofloxacin-treated organism still required around 6.2 and 8.3 h to increase by 1 log10 CFU/lung, resulting in in vivo PAEs of 3.2 and 5.3 h, respectively (Fig. 2). In fact, the bacterial load in lungs after dosing with 40 mg/kg antofloxacin was still less than the value at the initiation of drug therapy.
PK/PD index determination.
PK/PD indices were calculated using dose-response curves for antofloxacin administered every 6, 8, 12, and 24 h in neutropenic mice with K. pneumoniae ATCC 35657 lung infections. In untreated mice, the initial lung bacterial density ranged from 6.01 to 6.43 log10 CFU/lung and increased by a mean of 1.95 log10 CFU/lung after 24 h. The curves for the fractionated dose regimens were congruent, suggesting that the free-drug AUC from 0 to 24 h (AUC0–24)/MIC ratio is the predictive pharmacodynamic index (Fig. 3).
FIG 3.
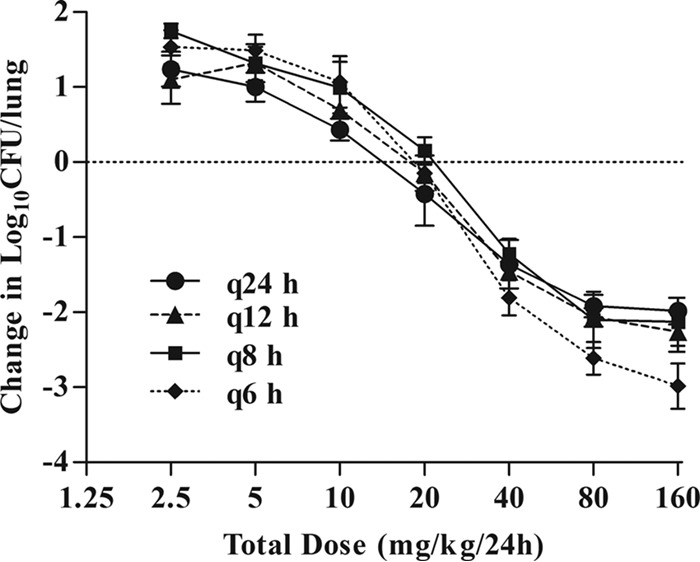
In vivo dose fractionation with antofloxacin using the murine neutropenic lung infection model. Each symbol represents the mean of data from four lungs infected with K. pneumoniae ATCC 35657. Seven total antofloxacin dose levels (mg/kg/24 h) were fractionated into each of four dose regimens. The microbial burden was measured at the start and after 24 h of therapy.
Consistent with the above finding, the AUC0–24/MIC ratio was the index that most strongly correlated with treatment efficacy (R2 = 0.96). Correlation to the other indices was not nearly as strong (R2 = 0.87 for Cmax/MIC, and 77% for the percentage of time that free-drug levels are above the MIC [%fT>MIC]) (Fig. 4B and C).
FIG 4.
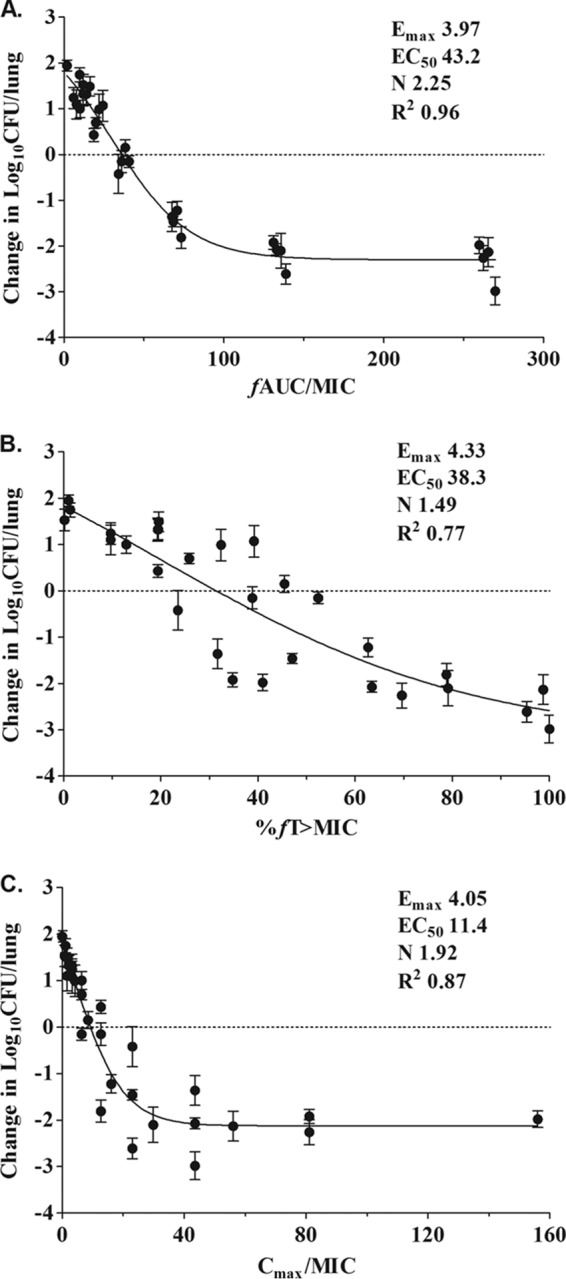
Impact of pharmacodynamic regression in the in vivo dose fractionation study with antofloxacin against K. pneumoniae ATCC 35657. Each symbol represents the mean of data from four thighs. The dose data are expressed as the free-drug AUC/MIC (A), the percentage of time that drug levels are above the MIC (%fT>MIC) (B), and Cmax/MIC (C). The R2 values are the coefficients of determination. The EC50 represents the PK/PD index associated with 50% of the maximal effect (Emax), and N is the Hill coefficient. The line drawn through the data points is the best-fitting line based upon the sigmoid Emax model.
Magnitudes of PK/PD index target for efficacy.
We examined the dose-effect relationship for each of the seven K. pneumoniae isolates to determine the impact of exposure indices on treatment effects. The bacterial burden of untreated control mice increased similarly with all bacterial strains over the 24-h study period (Table 3). In treated mice, antofloxacin demonstrated potent efficacy against K. pneumoniae. The highest dose level resulted in a reduction of 2.82 to 3.81 log10 CFU/lung compared with the initial bacterial burden (Fig. 5).
TABLE 3.
In vitro and in vivo efficacies of antofloxacin against selected K. pneumoniae isolates using the fAUC0–24/MIC ratio as a predictive PD indexa
| Strain | Antofloxacin MIC (mg/liter) | Growth after 24 h in untreated animals (log10 CFU/lung) |
fAUC0–24/MIC ratio of antofloxacin required to achieve: |
||
|---|---|---|---|---|---|
| Static effect | 1-log10 kill | 2-log10 kill | |||
| 160491 | 0.13 | 1.73 | 37.8 | 67.3 | 115.3 |
| 160211 | 0.06 | 1.56 | 55.2 | 104.5 | 212.3 |
| 160271 | 0.06 | 2.03 | 57.7 | 85.3 | 126.1 |
| 160244 | 0.06 | 2.16 | 86.4 | 163.3 | 345.1 |
| 160430 | 0.03 | 2.01 | 68.5 | 105.7 | 164.6 |
| ATCC 35657 | 0.25 | 1.95 | 33.6 | 55.6 | 109.3 |
| ATCC 700603 | 0.25 | 2.19 | 28.8 | 47.4 | 81.3 |
| Mean | NA | 1.95 | 52.6 | 89.9 | 164.9 |
| SD | NA | 0.23 | 19.2 | 36.6 | 83.5 |
fAUC0–24, AUC for unbound fraction (not protein bound) from 0 to 24 h; NA, not applicable.
FIG 5.
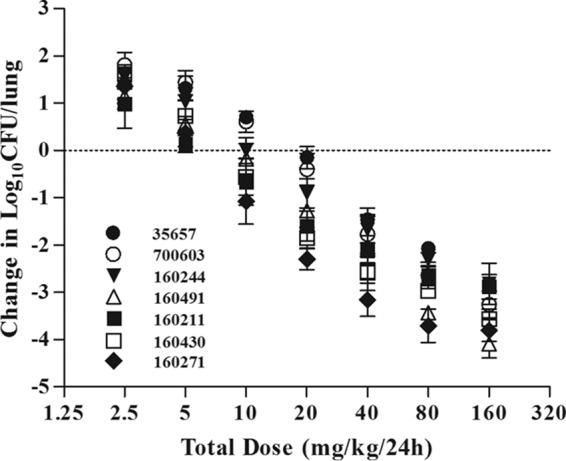
In vivo dose effect of antofloxacin against seven selected K. pneumoniae isolates using the murine neutropenic lung infection model. Each symbol represents the mean of data from four thighs. Seven total antofloxacin dose levels were divided into a regimen of administration every 12 h. Microbial burdens were measured at the start and after 24 h of therapy. The horizontal dashed line at 0 represents the burden of organisms in the lungs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth.
The free-drug AUC0–24/MIC ratios required for the various efficacy targets as derived from sigmoid Emax profiles are shown in Table 3. The PD targets producing a 1-log10 kill endpoint were about 2-fold higher than those associated with stasis. The free-drug AUC0–24/MIC ratios varied from 28.8 to 86.4 for a net bacteriostatic effect, 47.4 to 163.3 for a 1-log10 reduction, and 81.3 to 345.1 for a 2-log10 reduction, respectively.
DISCUSSION
In this study, we investigated the PK properties of antofloxacin, including in vivo PAEs and the PK/PD relationships with drug exposure, in vitro potency (MIC), and treatment outcomes. Consistent with other fluoroquinolones, antofloxacin dose fractionation experiments demonstrated that the free-drug AUC0–24/MIC ratio was most closely linked to the therapeutic efficacy against K. pneumoniae strains. This finding is supported by a similar result from a previous study in a neutropenic murine thigh infection model, where a total AUC0–24/MIC ratio of 65.7 was required to achieve a net bactericidal activity against S. aureus (11). Our results demonstrated that a mean free-drug AUC0–24/MIC ratio of 89.9 was necessary to achieve a 1-log10 kill effect against all K. pneumoniae isolates. This microbiological response in the animal model was analogous to, or in excess of, that of the currently available “respiratory” fluoroquinolones levofloxacin and moxifloxacin, which required an AUC0–24/MIC ratio of >100 to produce a significant bactericidal effect (12). Once more, an early clinical trial showed that antofloxacin had clinical and bacteriological outcomes for K. pneumoniae (30 strains) and E. coli (33 strains) similar to those of levofloxacin (13).
In general, fluoroquinolones have good penetration into ELF, and our study confirms this observation (14, 15). Of note, the measurement methodology may potentially confound the interpretation of antibiotic concentrations in ELF (14). The bronchoalveolar lavage (BAL)-derived fluid is a mixture of components containing fluids and white cells, especially macrophages. The released cellular components could artificially increase the measured antibiotic concentrations in ELF, and the degree of contamination may vary with the amount of lysed cells. Therefore, the efficient removal of macrophages and cellular debris from BAL fluids is essential for the accurate measurement of antibiotic concentrations in ELF. Nevertheless, bronchopulmonary experiments with antofloxacin have not been conducted in humans. The current study is the first to demonstrate the considerable pulmonary distribution of antofloxacin in neutropenic animals. The mouse-derived results of good ELF penetration are considered good models of penetration into human lung tissue (16). In addition, the increase in drug concentrations was linear and dose proportional in both plasma and ELF, potentially reflecting passive diffusion from plasma to ELF. However, because lung infections can disrupt alveolar walls and invade the interstitial space (14), the ELF levels of antibiotics determined in infected models may not be an accurate measure of drug concentration at the actual site of lung infections, and in fact ELF may represent the antibiotic concentrations for some specific infections (17). In this case, it may still be best to choose plasma levels as a target in relation to MIC to predict outcomes in lung infections.
Our treatment studies demonstrated a marked dose-dependent bactericidal activity, with prolonged growth suppression (PAEs) that ranged from 3.2 to 5.3 h. The duration of in vivo PAEs of antofloxacin for K. pneumoniae was longer than those measured with in vitro techniques (1.2 to 3.5 h) (unpublished data). The longer-lasting PAEs in vivo may be due to postantibiotic sub-MIC effects (PA-SME), the effect of serum factors, or slower growth in vivo than in the high-nutrient environment of broth (18, 19). In fact, the PAEs derived from in vitro testing may be nullified by various in vivo factors that are intrinsic to drug, host, and bacterium (20). Since fluoroquinolones inhibit the bacterial DNA synthesis, the in vitro PAEs induced by antofloxacin may represent the time required for the drug to dissociate from receptor binding sites and to diffuse out of the bacterium. The clinical significance of in vivo PAEs remains unclear; however, the presence of PAEs could suppress bacterial regrowth while permitting drug levels to fall below the MIC for considerable periods of time without loss of efficacy (21). Although antibiotic treatment may benefit from a long in vivo PAE, the PK/PD targets should be the major index correlating with the in vivo efficacy of antofloxacin.
Based on the pharmacokinetics study of a single 400-mg oral dose of antofloxacin in healthy human volunteers, the mean total AUC is 66.6 mg · h/liter. This translates to a free-drug AUC of 54.9 mg · h/liter using a reported protein binding ratio of 17.5% in humans (8, 22). In our laboratory, previous antimicrobial susceptibility results for antofloxacin against K. pneumoniae (53 strains) demonstrated a MIC90 of 0.5 mg/liter (range of 0.015 to 1 mg/liter) (unpublished data). In light of the reported PK study in humans, the current PD targets, and MIC data, a 5,000-subject Monte Carlo simulation (Oracle Crystal Ball software) showed that the probabilities of target attainment (PTA) of 100% and 91.2% could be achieved for 1-log10 and 2-log10 killing effects, respectively, against K. pneumoniae isolates having MICs of ≤0.5 mg/liter. These indicated that the antofloxacin dose of 400 mg would be successful for the treatment of pulmonary infections caused by K. pneumoniae. In addition, a randomized, multicenter, double-blind phase II clinical trial of antofloxacin in 140 AECB patients from 6 hospitals in China was conducted using an oral 400-mg loading dose, followed by a 200-mg/day maintenance dose (7). At the end of the two-regimen treatment, the observed effectiveness rate was 90.3% for AECB, with a 100% bacterial eradication of K. pneumoniae and 95.9% overall microbiological eradication.
In conclusion, the current study demonstrated that antofloxacin has potent efficacy against K. pneumoniae with a prolonged PAE and considerable pulmonary penetration. The PD index of AUC0–24/MIC was a strong predictor describing the therapeutic efficacy. The data from the murine model suggested that the antofloxacin dose regimen should produce a free-drug AUC0–24/MIC ratio of roughly 50 to 90 for K. pneumoniae to achieve net stasis and bactericidal endpoints. The integration of these PD targets with human PK data will provide a framework for further optimization of antofloxacin dosing regimens in the treatment of respiratory tract infections.
MATERIALS AND METHODS
Bacteria, media, and antibiotic.
Five clinical K. pneumoniae strains isolated from chronic bronchitis and pneumonia patients and K. pneumoniae strains ATCC 35657 and ATCC 700603 were used in this study. All K. pneumoniae isolates were identified using matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry (Shimadzu-Biotech). Bacteria were cultured and maintained in Mueller-Hinton broth and agar (Oxoid Ltd., Basingstoke, United Kingdom) for testing. Antofloxacin hydrochloride (purity, 90.9%; lot no. NJPW-5HA1) for in vitro and in vivo experiments was obtained from the Chinese National Institutes for Food and Drug Control (Beijing, China). For in vitro studies, amikacin, cefotaxime, and levofloxacin analytical standard powders (Sigma-Aldrich, St. Louis, MO) were utilized.
In vitro susceptibility testing.
The MICs of antofloxacin, amikacin, cefotaxime, and levofloxacin against the various isolates were determined using Clinical and Laboratory Standards Institute (CLSI) microdilution methods (23). All isolates were tested in triplicate to ensure reproducibility, and S. aureus ATCC 29213 served as a quality control strain.
Murine lung infection model.
Six-week-old, specific-pathogen-free female ICR mice (25 to 27 g) were obtained from Guangdong Medical Lab Animal Center (Guangzhou, China). Animals were maintained in accordance with the National Standards for Laboratory Animals of China (GB 14925-2010). All experiments were performed under authorization from the Animal Research Committees of South China Agricultural University. Neutropenia (neutrophils, ≤100/mm3) was induced using intraperitoneal cyclophosphamide (Yuanye Biotechnology, Shanghai, China) injections at 4 days (150 mg/kg) and 1 day (100 mg/kg) prior to lung infection. Broth cultures of freshly plated K. pneumoniae bacteria were grown to logarithmic phase overnight to an absorbance of 0.3 at 630 nm and diluted to 107.0 to 107.5 CFU/ml. Mice were infected using an intratracheal injection of 0.05 ml of a bacterial suspension with a tracheal cannula under light anesthesia with isoflurane as previously described (24). Therapy was initiated 2 h after lung inoculation. The mice were sacrificed by CO2 asphyxiation at 24 h of therapy, and the lungs were aseptically removed, homogenized, and processed for bacterial CFU determination.
Drug pharmacokinetics and penetration into ELF.
Single-dose pharmacokinetics of antofloxacin was performed in neutropenic infected mice following administration of single subcutaneous doses of 2.5, 10, 40, and 160 mg/kg. Concomitant samples of plasma and BAL fluid were collected from each of six mice at 0.08, 0.25, 0.5, 0.75, 1, 2, 4, 6, and 8 h after drug administration. Blood was sampled by retro-orbital puncture into heparin-containing tubes, and plasma was separated by centrifugation at 3,000 × g for 10 min at 4°C. BAL fluid was obtained using a previously reported technique (17, 25). In short, mice were humanely sacrificed under isoflurane anesthesia, followed by cervical dislocation. Subsequently, a 1-cm neck incision was made on the ventral neck skin to expose the trachea. A flexible cannula (22GA, BD Venflon; Becton, Dickinson) was inserted into the trachea and sutured in place. The lungs were flushed 4 times with 0.5 ml of sterile 0.9% saline, and the fluid was immediately aspirated and pooled per mouse. Immediately, the BAL fluid was centrifuged at 400 × g for 10 min to remove blood, macrophages, and cellular debris, and supernatant was collected and stored at −80°C until being shipped for urea and antofloxacin concentration analysis.
The antofloxacin concentrations in plasma and BAL fluids were determined using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (details are given in the supplemental material). The values of PK parameters, including the elimination half-life (t1/2), the area under concentration-time curve over 24 h (AUC0–24), the peak drug concentration (Cmax), and the time of maximum concentration (Tmax), were calculated using a noncompartmental model in WinNonlin software (version 6.1; Pharsight, St. Louis, MO, USA). The PK estimates for treatment doses that were not directly determined were calculated using linear extrapolation for dose levels between those with measured kinetics (e.g., between 2.5 and 10 mg/kg).
The antofloxacin concentrations in ELF were measured by the ratio of the urea concentration in BAL fluid to that in plasma (26), as follows: drug concentrationELF = drug concentrationBAL fluids × (urea concentrationplasma/urea concentrationBAL fluids). Urea in BAL fluid and plasma was determined utilizing a commercial urea assay kit (MLBIO Biotechnology, Shanghai, China). The urea assay was linear with an R2 of ≥0.999 for both plasma and BAL fluid samples in the range of 0.05 to 3.0 mg/dl. The intraday coefficients of variation for quality control samples varied from 1.9 to 3.6%, and the interday coefficients of variation ranged from 3.2 to 6.5%.
The protein binding of antofloxacin in murine plasma was determined at spiked concentrations of 0.05, 0.5, and 5 μg/ml using ultrafiltration methods as previously reported (27). In ELF, the drug protein binding was considered negligible (14).
In vivo PAE.
K. pneumoniae strain ATCC 35657 was used to produce a lung infection for determining PAE. The infected mice were treated with single subcutaneous doses of antofloxacin (10 and 40 mg/kg) at 2 h postinfection. The treated and untreated mice were sacrificed at 1, 2, 4, 6, 8, 12, and 24 h, four mice per time point, over a 24-h study period. Bacterial burden was quantified by CFU determination from whole-lung homogenates. The PAE was calculated by the formula PAE = T − C, where C is the time for the growth of 1 log10 CFU/lung in control growth and T is the time for the growth of 1 log10 CFU/lung in treated mice after free-drug levels in plasma have fallen below the MIC (28).
Pharmacokinetic/pharmacodynamic index determination.
Neutropenic mice were infected with the standard strain of K. pneumoniae ATCC 35657 for a dose fractionation experiment. Treatment with antofloxacin was initiated 2 h after infection. Dose regimens included seven total dose levels (2.5, 5, 10, 20, 40, 80, and 160 mg/kg) administered subcutaneously over a 24-h study period using 4-, 8-, 12-, and 24-h dosing intervals. Groups of four mice were included in each dose regimen. The mice were sacrificed after 24 h of therapy, and the lung bacterial burden was measured by viable plate counts of lung tissue homogenates (CFU/lung). Untreated control mice were similarly sacrificed before treatment and at 24 h after treatment.
To determine which PK/PD index was most closely linked with efficacy, the log change of the number of CFU in the lung homogenate over the 24-h treatment period was correlated with (i) the AUC0–24/MIC ratio, (ii) the Cmax/MIC ratio, and (iii) the percentage of time that drug levels are above the MIC (%fT>MIC). The relationship between efficacy and the three PK/PD indices was determined using a sigmoid Emax model (29). Data were analyzed using the nonlinear WinNonlin regression program. The PD index that best correlated with efficacy was determined by comparing the coefficients of determination (R2) for the three different indices.
Pharmacodynamic index target for efficacy.
Similar treatment studies were performed using the lung model as described above against the six additional strains of K. pneumoniae. Antofloxacin treatment was initiated 2 h after infection and administered following 2-fold-increasing single subcutaneous doses from 2.5 to 160 mg/kg every 12 h. At the end of the study, the mice were euthanized and the lungs were immediately processed for CFU determinations. The sigmoid Emax profile was applied to calculate the AUC0–24/MIC target of antofloxacin that produced a net bacteriostatic effect, a 1-log10 kill effect, or a 2-log10 kill effect.
Supplementary Material
ACKNOWLEDGMENTS
We thank Guangdong Second Traditional Chinese Medicine Hospital for providing clinical K. pneumoniae isolates.
This work was supported by the National Key Research and Development Program of China (2016YFD0501300), the Program for Changjiang Scholars and the Innovative Research Team in the University of Ministry of Education of China (IRT13063), and the Natural Science Foundation of Guangdong Province (S2012030006590).
No conflict of interest exists in the submission of the manuscript, and the manuscript is approved by all authors for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02691-16.
REFERENCES
- 1.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. 1998. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. 2007. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S. 2000. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 117:380S–385S. doi: 10.1378/chest.117.5_suppl_2.380S. [DOI] [PubMed] [Google Scholar]
- 4.Liu KX, Xu B, Wang J, Zhang J, Ding HB, Ariani F, Qu JM, Lin QC. 2014. Efficacy and safety of moxifloxacin in acute exacerbations of chronic bronchitis and COPD: a systematic review and meta-analysis. J Thorac Dis 6:221–229. doi: 10.3978/j.issn.2072-1439.2013.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starakis I, Gogos CA, Bassaris H. 2004. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents 23:129–137. doi: 10.1016/j.ijantimicag.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Balter M, Weiss K. 2006. Treating acute exacerbations of chronic bronchitis and community-acquired pneumonia: how effective are respiratory fluoroquinolones? Can Fam Physician 52:1236–1242. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Xiao Y, Huang W, Xu N, Bai C, Xiu Q, Mei C, Zheng Q. 2010. A phase II study of antofloxacin hydrochloride, a novel fluoroquinolone, for the treatment of acute bacterial infections. Chemotherapy 56:378–385. doi: 10.1159/000317581. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Y, Lu Y, Kang Z, Zhang M, Liu Y, Zhang M, Li T. 2008. Pharmacokinetics of antofloxacin hydrochloride, a new fluoroquinolone antibiotic, after single oral dose administration in Chinese healthy male volunteers. Biopharm Drug Dispos 29:167–172. doi: 10.1002/bdd.600. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Wang G, Chen S, Wei G, Shang Y, Dong L, Schon T, Moradigaravand D, Parkhill J, Peacock SJ, Koser CU, Huang H. 2016. Wild-type and non-wild-type Mycobacterium tuberculosis MIC distributions for the novel fluoroquinolone antofloxacin compared with those for ofloxacin, levofloxacin, and moxifloxacin. Antimicrob Agents Chemother 60:5232–5237. doi: 10.1128/AAC.00393-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Xiao XM, Xiao YH. 2008. Pharmacokinetics/pharmacodynamics of antofloxacin hydrochloride in a neutropenic murine thigh model of Staphylococcus aureus infection. Acta Pharmacol Sin 29:1253–1260. doi: 10.1111/j.1745-7254.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 12.Odenholt I, Cars O. 2006. Pharmacodynamics of moxifloxacin and levofloxacin against Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli: simulation of human plasma concentrations after intravenous dosage in an in vitro kinetic model. J Antimicrob Chemother 58:960–965. doi: 10.1093/jac/dkl356. [DOI] [PubMed] [Google Scholar]
- 13.Li YF, Wang K, Yin F, He YC, Huang JH, Zheng QS. 2012. Dose findings of antofloxacin hydrochloride for treating bacterial infections in an early clinical trial using PK-PD parameters in healthy volunteers. Acta Pharmacol Sin 33:1424–1430. doi: 10.1038/aps.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, Smith H, Hoban DJ. 2002. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs 62:13–59. doi: 10.2165/00003495-200262010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ishida Y, Kurosaka Y, Murakami Y, Otani T, Yamaguchi K. 1999. Therapeutic effect of oral levofloxacin, ciprofloxacin, and ampicillin on experimental murine pneumonia caused by penicillin intermediate Streptococcus pneumoniae for which the minimum inhibitory concentrations of the quinolones are similar. Chemotherapy 45:183–191. doi: 10.1159/000007181. [DOI] [PubMed] [Google Scholar]
- 17.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrobial Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 19.Pankuch GA, Jacobs MR, Appelbaum PC. 1998. Postantibiotic effect and postantibiotic sub-MIC effect of quinupristin-dalfopristin against gram-positive and -negative organisms. Antimicrob Agents Chemother 42:3028–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10; quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 21.Carbone M, Pennisi MG, Masucci M, De Sarro A, Giannone M, Fera MT. 2001. Activity and postantibiotic effect of marbofloxacin, enrofloxacin, difloxacin and ciprofloxacin against feline Bordetella bronchiseptica isolates. Vet Microbiol 81:79–84. doi: 10.1016/S0378-1135(01)00349-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Pan X, Liu HY, Liu XD, Yang HW, Xie L, Cheng JL, Fan HW, Xiao DW. 2011. Modulation of pharmacokinetics of theophylline by antofloxacin, a novel 8-amino-fluoroquinolone, in humans. Acta Pharmacol Sin 32:1285–1293. doi: 10.1038/aps.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Qu Y, Qiu Z, Cao C, Lu Y, Sun M, Liang C, Zeng Z. 2015. Pharmacokinetics/pharmacodynamics of marbofloxacin in a Pasteurella multocida serious murine lung infection model. BMC Vet Res 11:294. doi: 10.1186/s12917-015-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2014. Intrapulmonary posaconazole penetration at the infection site in an immunosuppressed murine model of invasive pulmonary aspergillosis receiving oral prophylactic regimens. Antimicrob Agents Chemother 58:2964–2967. doi: 10.1128/AAC.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YF, Zhao DH, Yu Y, Yang X, Shi W, Peng YB, Liu YH. 2015. Pharmacokinetics, bioavailability and PK/PD relationship of cefquinome for Escherichia coli in Beagle dogs. J Vet Pharmacol Ther 38:543–548. doi: 10.1111/jvp.12225. [DOI] [PubMed] [Google Scholar]
- 28.Vogelman BS, Craig WA. 1985. Postantibiotic effects. J Antimicrob Chemother 15(Suppl A):37–46. doi: 10.1093/jac/15.suppl_A.37. [DOI] [PubMed] [Google Scholar]
- 29.Lepak AJ, Andes DR. 2016. In vivo pharmacodynamic target assessment of delafloxacin against Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 60:4764–4769. doi: 10.1128/AAC.00647-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.