ABSTRACT
Campylobacter is a major foodborne pathogen, and previous studies revealed that Campylobacter isolates from food-producing animals are increasingly resistant to gentamicin in China. The molecular epidemiology and genetic mechanisms responsible for gentamicin resistance in China have not been well understood. In this study, 607 Campylobacter isolates of chicken and swine origins collected in 2014 were analyzed, revealing that 15.6% (25/160) of the Campylobacter jejuni isolates and 79.9% (357/447) of the Campylobacter coli isolates were resistant to gentamicin. PCR detection of the gentamicin resistance genes indicated that aph(2″)-If was more prevalent than the previously identified aacA/aphD gene and has become the dominant gentamicin resistance determinant in Campylobacter. Transformation and whole-genome sequencing as well as long-range PCR discovered that aph(2″)-If was located on a chromosomal segment inserted between two conserved genes, Cj0299 and panB. Cloning of aph(2″)-If into gentamicin-susceptible C. jejuni NCTC 11168 confirmed its function in conferring high-level resistance to gentamicin and kanamycin. Molecular typing by pulsed-field gel electrophoresis suggested that both regional expansion of a particular clone and horizontal transmission were involved in the dissemination of the aph(2″)-If gene in Campylobacter. To our knowledge, this is the first report describing the high prevalence of a chromosomally encoded aph(2″)-If gene in Campylobacter. The high prevalence and predominance of this gene might be driven by the use of aminoglycoside antibiotics in food animal production in China and potentially compromise the usefulness of gentamicin as a therapeutic agent for Campylobacter-associated systemic infection.
KEYWORDS: gentamicin resistance, aph(2″)-If, Campylobacter, food safety
INTRODUCTION
Campylobacter is a significant cause of foodborne diarrhea in humans, and Campylobacter jejuni and Campylobacter coli are the main species accounting for the majority of campylobacteriosis (1). Severe cases of the infection require antibiotic therapy, for which fluoroquinolones and macrolides are considered the drugs of choice (2). However, recent studies have indicated that Campylobacter isolates are increasingly resistant to these two clinically important classes of antibiotics, posing a serious threat to public health (3, 4). In addition to causing a localized infection in the intestine, Campylobacter is also able to induce systemic infections such as bacteremia, for which aminoglycosides (i.e., gentamicin) are the drugs of choice for treatment (2).
In general, gentamicin resistance rates in Campylobacter have been reported to be low and stable in most countries (5–8). In the United States, gentamicin resistance in Campylobacter was rarely reported before 2007 according to National Antimicrobial Resistance Monitoring System (NARMS) studies; however, an increasing trend of resistance to aminoglycosides was observed in recent years (9). In 2011, 12.2% of human isolates and 18.1% of retail isolates showed resistance to aminoglycosides (9). In China, the gentamicin resistance rate in Campylobacter has been at a much higher level than in other countries (10–12). Several mechanisms of gentamicin resistance have been reported in Campylobacter. The gentamicin resistance gene aacA4 was described in C. jejuni isolated from a broiler house (13). A multidrug-resistant plasmid, pCG8245, harboring resistance to gentamicin, was identified in a clinical strain of C. jejuni, which was isolated from a U.S. soldier deployed to Thailand (14). The gene conferring gentamicin resistance was initially annotated as aac(6′)-Ie/aph(2″)-Ia (also aacA/aphD), but it was found to encode the phosphotransferase activity only and thus was renamed aph(2″)-If (15). Subsequently, an aacA/aphD gene, which was contained in an aminoglycoside resistance genomic island, was reported in C. coli in China. The aacA/aphD gene encoding a bifunctional enzyme was associated with gentamicin resistance (16). Very recently, several variants of 2″-phosphotransferase accounting for gentamicin resistance were identified in Campylobacter in the United States (9, 17).
Here, we report the prevalence of gentamicin resistance and the associated resistance mechanisms in Campylobacter isolates of food animal origin in China. For the first time, we detected the aph(2″)-If gene in China. In addition, we found that this gene was located on the chromosome instead of plasmids. Molecular typing of the Campylobacter isolates indicated that the aph(2″)-If gene was disseminated by both clonal expansion and horizontal transmission. Additionally, the aph(2″)-If gene is more prevalent than aacA/aphD and has become the predominant determinant conferring gentamicin resistance in Campylobacter in China. These findings provide new insights into the epidemiology and spread of aminoglycoside resistance in Campylobacter.
RESULTS AND DISCUSSION
Gentamicin resistance in Campylobacter isolates.
In total, 62.9% (382/607) of Campylobacter isolates were resistant to gentamicin. Specifically, 15.6% (25/160) of C. jejuni isolates and 79.9% (357/447) of C. coli isolates were resistant to gentamicin. The proportions of gentamicin-resistant C. jejuni strains ranged from 0 to 37.5% among Guangdong, Ningxia, Shandong, Henan, and Shanghai, while the proportions of gentamicin-resistant C. coli strains ranged from 35.7% to 100% among the five regions (Table 1). The distributions of gentamicin MICs of C. jejuni and C. coli are shown in Fig. 1, which revealed that gentamicin resistance was much more prevalent in C. coli than in C. jejuni isolates (P < 0.0001, Fisher's exact test). Generally, the rate of resistance of Campylobacter to gentamicin is low (<2%) in other countries (8, 18, 19). However, recent studies performed in China suggested that the frequency of gentamicin resistance is high in Campylobacter, especially in C. coli isolated from swine and broiler chickens (23.2% to 95.4%) (10–12). The high prevalence of gentamicin resistance in Campylobacter found in this study is consistent with previous findings in China. According to the antimicrobial usage data collected in 2013, the total consumption of antibiotics was 162,000 tons in China, of which 83,200 tons was used in animals (20, 21). Additionally, antimicrobial usage records suggested that aminoglycoside agents, such as amikacin and neomycin, were commonly used to prevent and control bacterial diseases in food-producing animals in China (3). The exact reason for the high prevalence of gentamicin resistance in China is unknown, but it might be driven by the extensive use of aminoglycoside antibiotics in animal production.
TABLE 1.
Prevalence of gentamicin resistance and the associated resistance determinants aph(2″)-If and aacA/aphD
| Location of isolation | Host | No. of gentamicin-resistant isolates/total no. of isolates (%) |
No. of aph(2″)-If-positive isolates/total no. of isolates (%) |
No. of aacA/aphD-positive isolates/total no. of isolates (%) |
|||
|---|---|---|---|---|---|---|---|
| C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | ||
| Guangdong | Chicken | 6/16 (37.5) | 103/119 (86.6) | 3/16 (18.8) | 51/119 (42.9) | 1/16 (6.3) | 38/119 (31.9) |
| Ningxia | Chicken | 6/37 (16.2) | 23/23 (100) | 5/37 (13.5) | 11/23 (47.8) | 1/37 (2.7) | 8/23 (34.8) |
| Swine | 2/6 (33.3) | 11/20 (55.0) | 1/6 (16.7) | 9/20 (45.0) | 0/6 (0) | 0/20 (0) | |
| Shandong | Chicken | 0/1 (0) | 160/163 (98.2) | 0/1 (0) | 160/163 (98.2) | 0/1 (0) | 0/163 (0) |
| Shanghai | Chicken | 9/25 (36.0) | 25/52 (48.1) | 7/25 (28.0) | 2/52 (3.8) | 2/25 (8.0) | 17/52 (32.7) |
| Swine | 26/52 (50.0) | 20/52 (38.5) | 5/52 (9.6) | ||||
| Henan | Chicken | 2/75 (2.7) | 4/4 (100) | 0/75 (0) | 0/4 (0) | 0/75 (0) | 0/4 (0) |
| Swine | 5/14 (35.7) | 5/14 (35.7) | 0/14 (0) | ||||
| Total | 25/160 (15.6) | 357/447 (79.9) | 16/160 (10.0) | 258/447 (57.7) | 4/160 (2.5) | 68/447 (15.2) | |
FIG 1.
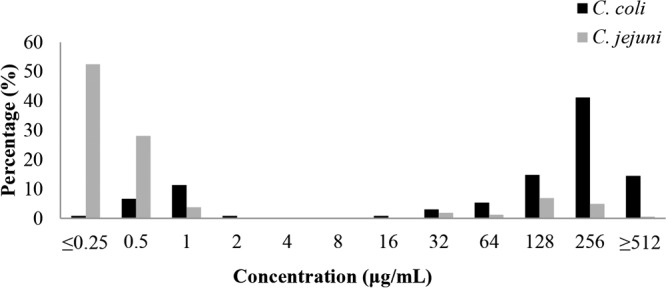
Distributions of gentamicin MICs for C. coli (n = 447) and C. jejuni (n = 160) isolates. The breakpoint for gentamicin resistance is 8 μg/ml.
Prevalence of gentamicin resistance genes in Campylobacter isolates.
All 607 Campylobacter isolates (160 C. jejuni and 447 C. coli isolates; see Table S1 in the supplemental material) were examined by PCR for detecting various aminoglycoside resistance determinants. The aph(2″)-If gene was identified in 10.0% (16/160) of C. jejuni and 57.7% (258/447) of C. coli isolates (Table 1), indicating its common presence and higher prevalence in C. coli (P < 0.0001, Fisher's exact test). Notably, all the gentamicin-resistant C. coli isolates from Shandong province harbored the aph(2″)-If gene (Table 1), indicating that aph(2″)-If is responsible for gentamicin resistance in the C. coli isolates derived from this province. This finding is in contrast to a previous study conducted by us, in which the aacA/aphD-containing genomic island was found to account for gentamicin resistance in C. coli isolates derived during 2008 to 2009 from Shandong province (16). These results strongly suggest that aph(2″)-If recently emerged and became the predominant gentamicin resistance determinant in Campylobacter in Shandong province. The shift in the gentamicin resistance determinants during 2008 and 2009 to 2014 may be due to the rapid expansion of a C. coli clone containing aph(2″)-If (see the genotyping result).
The prevalence of the aacA/aphD gene was 2.5% (4/160) and 15.2% (68/447) in the C. jejuni isolates and C. coli isolates, respectively (Table 1). Similarly to the aph(2″)-If gene described above, aacA/aphD is more prevalent in C. coli than in C. jejuni (P < 0.0001, Fisher's exact test). In addition, we analyzed the gentamicin resistance genes reported in other countries, such as aacA4 and the variants of 2″-phosphotransferase, none of which were identified in the Campylobacter isolates examined in this study. However, neither aph(2″)-If nor aacA/aphD was found in 20.0% (5/25) of C. jejuni and 8.7% (31/357) of C. coli isolates that were resistant to gentamicin, suggesting that these isolates may harbor unknown gentamicin resistance mechanisms. These results indicate that aph(2″)-If and aacA/aphD genes are responsible for gentamicin resistance in most of the Campylobacter isolates in China, with aph(2″)-If as the predominant resistance determinant. Notably, aph(2″)-If and aacA/aphD coexisted in some Campylobacter isolates, but they were inserted in different sites of the chromosome (data not shown), which could facilitate the transmission of gentamicin resistance along with other antimicrobial resistance genes.
Transferability and the gene environment of aph(2″)-If.
The genomic DNAs of two aph(2″)-If-positive strains with high-level resistance to gentamicin, C. coli HS11B and C. jejuni CN9, were used as donors to transform C. jejuni NCTC 11168 by natural transformation. The transformants, NT-HS11B and NT-CN9, showed >512- and 512-fold increases in the MICs of gentamicin, respectively, compared with NCTC 11168 (Table 2). These findings indicated that the resistance to gentamicin in HS11B and CN9 can be transferred by natural transformation. PCR and DNA sequencing of the products revealed the existence of the aph(2″)-If gene in NT-HS11B and NT-CN9. Furthermore, the transformants NT-HS11B and NT-CN9 showed decreased susceptibility to kanamycin, neomycin, and amikacin (Table 2), which are commonly used for disease control in broiler chickens in China.
TABLE 2.
MICs of aminoglycoside antibiotics for various isolates and constructs
| Antimicrobial agent | MIC (μg/ml) for isolate or constructa: |
||||||
|---|---|---|---|---|---|---|---|
| ATCC 33560 | NCTC 11168 | HS11B | NT-HS11B | CN9 | NT-CN9 | 11168-aph(2″)-If | |
| Gentamicin | 1 | 1 | >512 | >512 | 512 | 512 | 512 |
| Kanamycin | 8 | 8 | >512 | >512 | >512 | >512 | >512 |
| Neomycin | 4 | 4 | >512 | 256 | 256 | 32 | 4 |
| Streptomycin | 4 | 4 | 128 | 4 | 128 | 4 | 4 |
| Amikacin | 2 | 4 | 256 | 64 | 128 | 32 | 4 |
| Apramycin | 2 | 4 | 4 | 4 | 4 | 4 | 4 |
NT-HS11B and NT-CN9 are transformants of NCTC 11168 with donor DNA from C. coli HS11B and C. jejuni CN9, respectively, while 11168-aph(2″)-If is the NCTC 11168 construct containing a cloned aph(2″)-If.
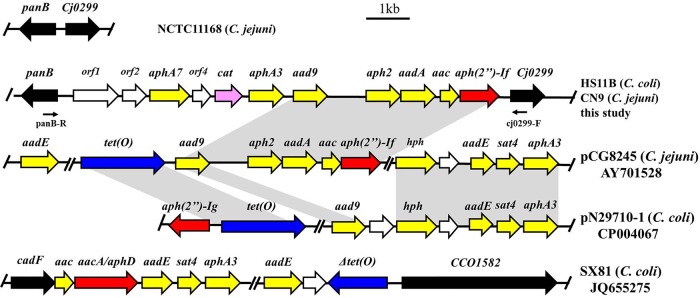
Subsequently, one of the NT-HS11B transformants was investigated by whole-genome sequencing. The draft genome of NT-HS11B was compared with NCTC 11168, revealing that the backbone of the transformant was NCTC 11168, but a 10,550-bp segment was inserted between Cj0299 and panB, which are highly conserved on C. jejuni and C. coli genomes. This inserted segment contained 11 open reading frames (ORFs), including 8 antimicrobial resistance genes, among which 7 [aph(2″)-If, aac, aadA, aph2, aad9, aphA3, and aphA7] encode aminoglycoside-modifying enzymes and one (cat) mediates chloramphenicol resistance. Thus, this genomic island can mediate resistance to multiple aminoglycoside antimicrobials, which was shown by the MICs of the transformants that had increased MICs of gentamicin, kanamycin, neomycin, and amikacin (Table 2). It is conceivable that use of either of the aminoglycosides should provide selection pressure for this genomic island. Comparative analysis suggested that most ORFs in this segment were highly similar to the ORFs located on plasmid pCG8245 (GenBank accession number AY701528) in C. jejuni but with additional genes, such as the chloramphenicol resistance gene cat (Fig. 2) (14). The aph(2″)-If gene was located immediately upstream of Cj0299 in NT-HS11B and showed 100% nucleotide identity to the “aph(2″)-If” in pCG8245 and 78% nucleotide identify to the APH(2″)-Ia domain with only phosphotransferase activity of the bifunctional enzyme AAC(6′)-Ie/APH(2″)-Ia (14, 16). In addition, the comparison with the previously reported aminoglycoside resistance genomic island (16) was also exhibited in the results shown in Fig. 2. In general, the novel multidrug resistance island identified in this study showed a very low similarity with the segments of pN29701 (CP004067) and SX81 (JQ655275) (16, 17) but was highly similar to the segment of pCG8245 (AY701528) (14).
FIG 2.
Chromosomal organization of the aph(2″)-If-carrying segment in C. coli HS11B and C. jejuni CN9 in comparison with plasmid pCG8245, pN29710-1, the multidrug resistance genomic island of C. coli SX81, and C. jejuni NCTC 11168. Arrows indicate the positions and directions of transcription of the genes. The locations of primers panB-F and cj0299-R used to detect the unique genomic island are indicated. The gray-shaded areas represent regions sharing 99% DNA identity. Gentamicin resistance genes are colored red, while other aminoglycoside resistance genes are colored yellow. Chloramphenicol and tetracycline genes are colored pink and blue, respectively.
S1 pulsed-field gel electrophoresis (S1-PFGE) and Southern hybridization verified that the aph(2″)-If gene was located on the chromosome instead of a plasmid (data not shown). Furthermore, long-range PCR was performed to determine the insertion of the segment in the field isolates using primers located in the conserved regions of the Cj0299 and panB genes, respectively. An amplicon of ∼10 kb was obtained from HS11B, CN9, and several other representative gentamicin-resistant Campylobacter isolates derived from different provinces (data not shown). The DNA sequencing results of the long-range PCR products revealed that the DNA segment in these isolates showed >99% nucleotide identity to that of NT-HS11B. These results indicate that the aph(2″)-If-containing segment is inserted in a conserved location on the chromosome and is widely spread in the gentamicin-resistant Campylobacter isolates in China.
Previously, aph(2″)-If together with other aminoglycoside resistance genes was detected on a plasmid in C. jejuni (14), and the genomic islands harboring aminoglycoside resistance and multidrug resistance were identified between cadF and CCO1582 on the chromosome in C. coli (16, 22). In the present study, multiple aminoglycoside resistance genes and a cat gene are found to be located in a novel spot between Cj0299 and panB, which has never been reported previously. This novel integration site raises the possibility of homologous recombination through natural transformation. In addition, the multidrug resistance island is not associated with any known mobile element, such as insertion elements (ISs) and transposons, suggesting that homologous recombination mediated by natural transformation is likely involved in the dissemination of the genomic islands described in this study.
Functional confirmation of the aph(2″)-If gene.
Previously, the aph(2″)-If gene on plasmid pCG8245 was interrupted by insertional mutagenesis to demonstrate its function in conferring gentamicin resistance (14). To formally demonstrate that aph(2″)-If alone was responsible for the high-level gentamicin resistance, this gene was cloned from HS11B into C. jejuni NCTC 11168 and inserted between Cj0299 and panB on the chromosome. This construct, 11168-aph(2″)-If, showed 512-fold and >64-fold increases in the MICs of gentamicin and kanamycin, respectively, compared with NCTC 11168 (Table 2). This result indicated that aph(2″)-If alone confers high-level resistance to both gentamicin and kanamycin in Campylobacter. The aph2 gene was also contained in the segment between Cj0299 and panB. Although it was predicted to be active, it did not confer gentamicin resistance in either Escherichia coli or C. jejuni as determined in a previous study (14). Therefore, we did not further analyze the aph2 gene by functional cloning in this study. Interestingly, the cloned aph(2″)-If gene alone did not affect the MICs of neomycin and amikacin (Table 2), indicating that the elevated MICs of neomycin and amikacin in NT-HS11B and NT-CN9 were due to other aminoglycoside resistance genes in the genomic island.
Molecular typing and phylogenetic analysis of aph(2″)-If-carrying C. jejuni and C. coli isolates.
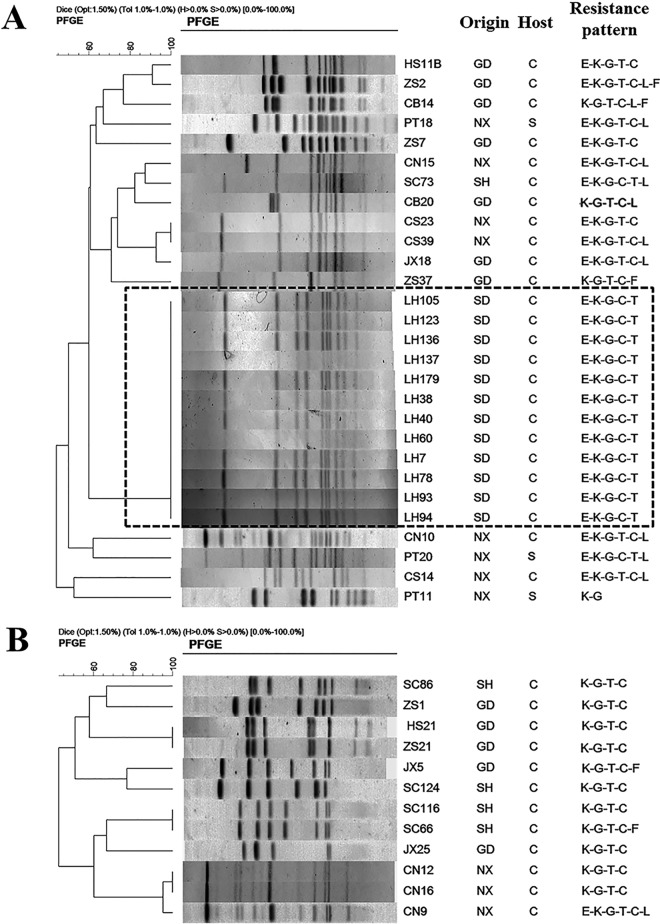
To understand if the aph(2″)-If-carrying Campylobacter isolates were genetically related, 40 isolates (28 of C. coli and 12 of C. jejuni), representing different regions and antimicrobial susceptibility patterns, were selected to perform pulsed-field gel electrophoresis (PFGE) analysis with SmaI digestion (Fig. 3). Using 80% genetic similarity as a cutoff, the 28 C. coli isolates were grouped into 4 clusters (PFGE patterns represented by multiple strains) and 16 unique PFGE patterns (PFGE patterns represented by a single strain) (Fig. 3A). The 12 C. jejuni isolates were grouped into 3 clusters and 9 unique PFGE patterns (Fig. 3B). In general, the C. coli and C. jejuni isolates from different regions were genetically diverse; however, the 12 C. coli isolates originating from Shandong (LH strains) showed identical PFGE patterns, suggesting that these isolates are clonally related. This finding supports the notion that the predominance of aph(2″)-If in gentamicin-resistant C. coli isolates in Shandong province is related to regional expansion of a particular clone. Overall, findings in this study revealed both genetic diversity and regional clonality of aph(2″)-If-carrying isolates, suggesting that both horizontal transmission and clonal expansion were involved in dissemination of aph(2″)-If in China.
FIG 3.
PFGE analysis of aph(2″)-If-positive C. coli (A) and C. jejuni (B) isolates. SmaI was used for digestion of the genomic DNA. Isolation sources include Guangdong (GD), Shandong (SD), Ningxia (NX), and Shanghai (SH). Hosts include chicken (C) and swine (S). Abbreviations of antimicrobial agents: C, ciprofloxacin; T, tetracycline; E, erythromycin; F, florfenicol; L, clindamycin; K, kanamycin; G, gentamicin.
In summary, this study reports the high prevalence of the aph(2″)-If gene conferring high-level gentamicin resistance in C. coli in China. This gene is widely disseminated among Campylobacter isolates derived from chickens and swine. Horizontal transmission and clonal expansion are likely involved in the dissemination of aph(2″)-If. These findings provide new insights into the epidemiology of aminoglycoside resistance in Campylobacter and highlight the tremendous adaptability of this major foodborne pathogen to antibiotic selection pressure. Considering the usefulness of gentamicin in treating systemic infections caused by Campylobacter, the rising resistance to this class of antibiotics should be concerning, and measures should be taken to reduce the prevalence of antibiotic-resistant Campylobacter.
MATERIALS AND METHODS
Campylobacter isolates and susceptibility testing.
In total, 607 Campylobacter isolates (160 C. jejuni and 447 C. coli isolates) were investigated in this study. These strains were isolated from cecal contents of chickens and feces of swine from Guangdong, Ningxia, Henan, and Shandong provinces and Shanghai (see Table S1 in the supplemental material) in 2014. All the Campylobacter strains were grown on Mueller-Hinton (MH) agar (Sigma-Aldrich, MO, USA) at 42°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2). The standard agar dilution method was used to determine the MICs of gentamicin for Campylobacter according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (23). Those isolates that showed resistance to gentamicin were further examined for their susceptibility to kanamycin, neomycin, streptomycin, amikacin, and apramycin. The reference strain C. jejuni ATCC 33560 was used as a quality control strain. The resistance of antimicrobial agents was interpreted according to the criteria of CLSI (23). Antimicrobial agents were obtained from the China Institute of Veterinary Drug Control (Beijing, China).
PCR detection of gentamicin resistance genes in Campylobacter isolates.
The known gentamicin resistance genes, including aph(2″)-If, aacA/aphD, aph(2″)-Ig, and aacA4, were detected in Campylobacter isolates by PCR and sequencing. The primers used in this study are listed in Table S2. The PCR mixture was composed of 12.5 μl of Ex-Taq (TaKaRa, Dalian, China), 0.5 μl of each primer, 0.5 μl of chromosomal DNA template prepared by boiling as described previously (24), and 11 μl of sterile distilled water. The PCR was conducted in a Veriti 96-well Thermal Cycler (Applied Biosystems, Foster City, CA, USA) with the conditions described in Table S2.
Natural transformation and whole-genome sequencing.
The natural transformation assay was performed in accordance with the method described previously with minor modifications (25). The whole-genomic DNA of high-level-gentamicin-resistant Campylobacter isolates was used as the donor, while the reference strain of C. jejuni NCTC 11168 served as the recipient. Briefly, the recipient cells were spread on MH agar, and then, 1 μg of genome DNA of the donor was added onto the cells followed by culture for 7 h at 42°C under microaerobic conditions. The cells were collected and plated on the selective plate containing gentamicin (10 μg/ml) and then incubated for 72 h at 42°C under microaerobic conditions. Transformation without donor DNA was used as a negative control. Single colonies of transformants were picked and subcultured on gentamicin-containing plates for purity. The antimicrobial susceptibility of the transformants was determined to confirm the phenotype of gentamicin resistance. Subsequently, the transformants were subjected to whole-genome sequencing using an Illumina HiSeq 2500 sequencer (Berry Genomics Company, Beijing, China).
S1-PFGE and Southern blotting.
S1 nuclease pulsed-field gel electrophoresis (PFGE) and Southern blotting were performed to determine the location of gentamicin resistance gene aph(2″)-If on plasmids or on the chromosome. S1 nuclease (TaKaRa, Dalian, China) was used to digest the agarose gel plugs containing the cells of gentamicin-resistant isolates and then separated by PFGE under the following conditions: 0.5× Tris-borate-EDTA, 1% agarose solution for 11 h at 6 V/cm and 14°C, with a pulse angle of 120° and the initial and final switch of 2 s and 55 s, respectively, as described previously with minor modifications (26). Southern blotting was performed to detect the location of aph(2″)-If. The probe was amplified using specific primers (If-F, 5′-TTGGTGAGGGTTATGACAGC, and If-R, 5′-CACTTCCTTAATTTTTCATCTTTGC) for the aph(2″)-If gene and then labeled with a digoxigenin (DIG) High Prime I DNA labeling and detection starter kit for hybridization. Hybridization was conducted at 42°C for 16 h. Membranes were washed twice at room temperature (22 to 25°C) in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS for 5 min and twice at 68°C in 0.1× SSC-0.1% SDS for 15 min. The signals from the bands were visualized using a nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) color detection kit (Roche Diagnostics, Mannheim, Germany) according to the recommendations of the supplier.
Functional cloning of the aph(2″)-If gene.
In order to demonstrate the function of the aph(2″)-If gene, we cloned it into aminoglycoside-susceptible Campylobacter NCTC 11168. Briefly, several pairs of primers were designed to amplify the entire aph(2″)-If gene together with its upstream Cj0299 gene (primers P1 and P2), the cat gene (primers P3 and P4) in HS11B, and the panB (primers P5 and P6) gene in NCTC 11168 using the online assembly tool NEBuilder (New England Biolabs, Ipswich, MA). These primers are listed in Table S2. Then, the 3 fragments were assembled with NEBuilder HiFi DNA assembly master mix (New England Biolabs, Beverly, MA, USA) according to the protocol provided by the manufacturer. Subsequently, the assembled 3 fragments were used as the template, the primers P1 and P6 were used to amplify the donor DNA, and C. jejuni NCTC 11168 was used as the recipient strain for natural transformation according to the method mentioned above. The transformants were selected with 8 μg/ml chloramphenicol.
PFGE.
PFGE analysis of Campylobacter strains was performed using SmaI as the restriction endonuclease according to the protocol for Campylobacter (27). Salmonella strain H9812 digested by Xba was used as the reference marker. PFGE results were analyzed by using the InfoQuest software, version 4.5 (Bio-Rad Laboratories).
Accession number(s).
The sequence described in this paper has been deposited at GenBank under accession number KX272768.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Basic Research Program of China and the National Natural Science Foundation of China (2013CB127200) and the Special Fund for Agro-scientific Research in the Public Interest (201203040).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00112-17.
REFERENCES
- 1.Ruiz-Palacios GM. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Engberg J. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections, p 99–121. In Nachamkin I, Szymanski CM, Blaser MJ (ed), Campylobacter, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 3.Wang Y, Dong Y, Deng F, Liu D, Yao H, Zhang Q, Shen J, Liu Z, Gao Y, Wu C, Shen Z. 2016. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008-14. J Antimicrob Chemother 71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- 4.Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su C, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J. 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 7:e01543-16. doi: 10.1128/mBio.01543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Adawy H, Ahmed MF, Hotzel H, Tomaso H, Tenhagen BA, Hartung J, Neubauer H, Hafez HM. 2015. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli recovered from organic turkey farms in Germany. Poult Sci 94:2831–2837. doi: 10.3382/ps/pev259. [DOI] [PubMed] [Google Scholar]
- 6.Ronner AC, Engvall EO, Andersson L, Kaijser B. 2004. Species identification by genotyping and determination of antibiotic resistance in Campylobacter jejuni and Campylobacter coli from humans and chickens in Sweden. Int J Food Microbiol 96:173–179. doi: 10.1016/j.ijfoodmicro.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TN, Hotzel H, Njeru J, Mwituria J, El-Adawy H, Tomaso H, Neubauer H, Hafez HM. 2016. Antimicrobial resistance of Campylobacter isolates from small scale and backyard chicken in Kenya. Gut Pathog 8:39. doi: 10.1186/s13099-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashoma IP, Kassem II, Kumar A, Kessy BM, Gebreyes W, Kazwala RR, Rajashekara G. 2015. Antimicrobial resistance and genotypic diversity of Campylobacter isolated from pigs, dairy, and beef cattle in Tanzania. Front Microbiol 6:1240. doi: 10.3389/fmicb.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S, Mukherjee S, Chen Y, Li C, Young S, Warren M, Abbott J, Friedman S, Kabera C, Karlsson M, McDermott PF. 2015. Novel gentamicin resistance genes in Campylobacter isolated from humans and retail meats in the USA. J Antimicrob Chemother 70:1314–1321. doi: 10.1093/jac/dkv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Naren GW, Wu CM, Wang Y, Dai L, Xia LN, Luo PJ, Zhang Q, Shen JZ. 2010. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol 144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Qin SS, Wu CM, Wang Y, Jeon B, Shen ZQ, Wang Y, Zhang Q, Shen JZ. 2011. Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int J Food Microbiol 146:94–98. doi: 10.1016/j.ijfoodmicro.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Wang Y, Shen J, Zhang Q, Wu C. 2014. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int J Food Microbiol 181:77–84. doi: 10.1016/j.ijfoodmicro.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Lee MD, Sanchez S, Zimmer M, Idris U, Berrang ME, McDermott PF. 2002. Class 1 integron-associated tobramycin-gentamicin resistance in Campylobacter jejuni isolated from the broiler chicken house environment. Antimicrob Agents Chemother 46:3660–3664. doi: 10.1128/AAC.46.11.3660-3664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nirdnoy W, Mason CJ, Guerry P. 2005. Mosaic structure of a multiple-drug-resistant, conjugative plasmid from Campylobacter jejuni. Antimicrob Agents Chemother 49:2454–2459. doi: 10.1128/AAC.49.6.2454-2459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth M, Frase H, Antunes NT, Vakulenko SB. 2013. Novel aminoglycoside 2″-phosphotransferase identified in a Gram-negative pathogen. Antimicrob Agents Chemother 57:452–457. doi: 10.1128/AAC.02049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C, Shen J. 2012. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob Agents Chemother 56:5332–5339. doi: 10.1128/AAC.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Mukherjee S, Hoffmann M, Kotewicz ML, Young S, Abbott J, Luo Y, Davidson MK, Allard M, McDermott P, Zhao S. 2013. Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from U.S. retail meats reveals novel plasmid-mediated aminoglycoside resistance genes. Antimicrob Agents Chemother 57:5398–5405. doi: 10.1128/AAC.00669-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wieczorek K, Kania I, Osek J. 2013. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from poultry carcasses in Poland. J Food Prot 76:1451–1455. doi: 10.4315/0362-028X.JFP-13-035. [DOI] [PubMed] [Google Scholar]
- 19.Guyard-Nicodeme M, Rivoal K, Houard E, Rose V, Quesne S, Mourand G, Rouxel S, Kempf I, Guillier L, Gauchard F, Chemaly M. 2015. Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. Int J Food Microbiol 203:8–14. doi: 10.1016/j.ijfoodmicro.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. 2015. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 21.Ying GG, He LY, Ying AJ, Zhang QQ, Liu YS, Zhao JL. 2017. China must reduce its antibiotic use. Environ Sci Technol 51:1072–1073. doi: 10.1021/acs.est.6b06424. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q, Shen J. 2014. Emergence of multidrug-resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrob Agents Chemother 58:5405–5412. doi: 10.1128/AAC.03039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disc susceptibility testing of infrequently isolated or fastidious bacteria. M45, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 24.Bachoual R, Ouabdesselam S, Mory F, Lascols C, Soussy CJ, Tankovic J. 2001. Single or double mutational alterations of gyrA associated with fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Microb Drug Resist 7:257–261. doi: 10.1089/10766290152652800. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Taylor DE. 1990. Natural transformation in Campylobacter species. J Bacteriol 172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 27.Ribot EM, Fitzgerald C, Kubota K, Swaminathan B, Barrett TJ. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J Clin Microbiol 39:1889–1894. doi: 10.1128/JCM.39.5.1889-1894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.