ABSTRACT
Omadacycline is a novel aminomethylcycline antibiotic in clinical development for community-acquired bacterial pneumonia (CABP). We used a neutropenic murine pneumonia infection model to characterize the in vivo pharmacodynamic activity of omadacycline against Streptococcus pneumoniae. Four strains with various phenotypic resistances to other antimicrobials, including tetracyclines, were utilized. Drug concentration measurements were performed in the plasma and epithelial lining fluid (ELF) after administration of 0.5, 2, 8, and 32 mg/kg. Pharmacokinetic parameters were calculated using a noncompartmental model and were linear over the dose range. Penetration into ELF ranged from 72 to 102%. Omadacycline demonstrated net cidal activity in relation to the initial burden against all four strains. The pharmacokinetic/pharmacodynamic index AUC/MIC correlated well with efficacy (R2 = 0.74). The plasma 24-h static dose AUC/MIC values were 16 to 20 (24-h ELF AUC/MIC of 14 to 18). A 1-log10 kill was achieved at 24-h plasma AUC/MIC values of 6.1 to 180 (24-h ELF AUC/MIC values 6.0 to 200). A 2-log10 kill was achieved at 24-h plasma AUC/MIC values of 19 to 56 (24-h ELF AUC/MIC of 17 to 47). The targets identified in this study in combination with in vitro potency and favorable human pharmacokinetics make omadacycline an attractive candidate for further development and study in patients with CABP.
KEYWORDS: omadacycline, pharmacodynamics, pneumonia
INTRODUCTION
Lower respiratory tract infections are the second leading cause of morbidity and mortality worldwide (1). Despite vaccination efforts, Streptococcus pneumoniae continues to be the most common pathogen of community-acquired bacterial pneumonia (CABP) irrespective of age and geographical location (2–4). Morbidity and mortality remain unacceptably high in part due to increasing drug resistance and limited effective antimicrobial options. Indeed, macrolide resistance in S. pneumoniae has soared in recent years, rendering macrolides ineffective for many cases of S. pneumoniae CABP (5, 6). In addition, fluoroquinolones have recently undergone increased scrutiny regarding the potential risks of side effects or adverse effects during use (7, 8). Therefore, novel antibiotics effective against S. pneumoniae are urgently needed.
Omadacycline is a novel aminomethylcycline antibiotic in clinical development for CABP and skin and skin structure infections (9). The goal of our experiments was to characterize the in vivo pharmacokinetic/pharmacodynamic (PK/PD) properties of omadacycline. Specifically, we sought to determine (i) the serum and epithelial lining fluid (ELF) pharmacokinetics of omadacycline in the murine model and (ii) the magnitude of the PK/PD parameter AUC/MIC required for efficacy against S. pneumoniae in the murine neutropenic pneumonia model.
RESULTS
In vitro susceptibility studies.
The MICs of omadacycline for the selected strains are listed in Table 1. Also shown are MICs to other relevant antimicrobial agents (when known). The four organisms varied in MIC to omadacycline by only 4-fold despite phenotypic variation in susceptibility to other antimicrobials such as minocycline and tigecycline.
TABLE 1.
Study organisms and omadacycline susceptibility resultsa
| S. pneumoniae strainb | MIC (mg/liter) |
|||
|---|---|---|---|---|
| Omadacycline | Tigecycline | Minocycline | Erythromycin | |
| 1293* | 0.0625 | 0.06 | 4 | >8 (Erm) |
| ATCC 10813† | 0.0625 | 0.12 | 0.25 | 0.015 |
| 140† | 0.125 | NAc | NA | NA |
| ATCC 49619* | 0.03125 | 0.06 | 0.06 | 0.06 |
Where known, the antimicrobial susceptibility results for other relevant antimicrobial agents are also listed.
*, Penicillin resistant; †, penicillin susceptible.
NA, not available.
Pharmacokinetics.
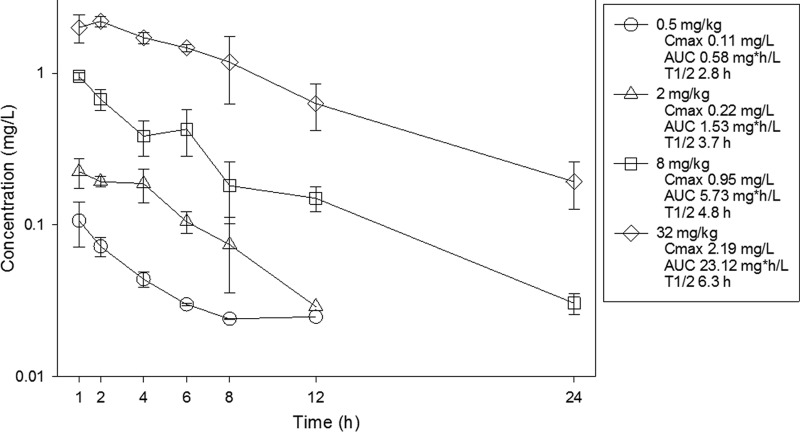
The time course of plasma concentrations of omadacycline in mice after subcutaneous doses of 0.5, 2, 8, and 32 mg/kg are shown in Fig. 1. Over the dose range, the pharmacokinetics were relatively linear (AUC R2 = 1.00, Cmax R2 = 0.97). Peak levels ranged from 0.11 to 2.19 mg/liter. AUC0–∞ values ranged from 0.58 to 23.12 mg·h/liter. The elimination half-life ranged from 2.8 to 6.3 h.
FIG 1.
Plasma concentrations of omadacycline in mice following single subcutaneous doses. Samples were obtained at seven time points over 24 h. Each symbol represents the mean and standard deviation from three mice. Cmax, peak concentration; t1/2, beta elimination half-life. The AUC is from 0 to infinity.
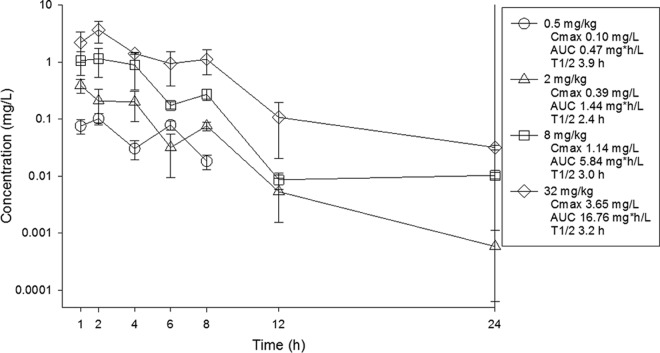
ELF concentrations were determined from BAL fluid concentrations utilizing the urea correction methodology described in methods above. Pharmacokinetic analysis of the data is presented in Fig. 2. Over the dose range once again the pharmacokinetics were linear (AUC R2 = 0.99, Cmax R2 = 0.99). Peak levels ranged from 0.10 to 3.65 mg/liter. AUC0–∞ values ranged from 0.47 to 16.76 mg·h/liter. The elimination half-life ranged from 2.4 to 3.9 h. The penetration of omadacycline into ELF relative to plasma drug concentrations was evaluated for each dose. Over the dose range, the percent penetrations based on AUC exposure were 80% at 0.5 mg/kg, 94% at 2 mg/kg, 102% at 8 mg/kg, and 72% at 32 mg/kg.
FIG 2.
ELF concentrations of omadacycline in mice following single subcutaneous doses. Samples were obtained at seven time points over 24 h. ELF concentrations were determined using urea concentration correction methods. Each symbol represents the mean and standard deviation from three mice. Cmax, peak concentration; t1/2, beta elimination half-life. The AUC is from 0 to infinity.
Relationship between PK/PD parameter AUC/MIC and efficacy.
At the start of therapy mice had 106.3 ± 0.3 log10 CFU/lungs, and this increased to 107.8 ± 0.6 log10 CFU/lungs. The growth in untreated controls for each strain are shown in Table 2. The in vivo dose-response curves for all four strains are shown in Fig. 3. Omadacycline was quite potent over the dose range studied. Bactericidal activity was noted at all doses for two strains (140 and ATCC 49619), and a 1-log kill was achieved for all four strains over the dose range. A ≥3-log kill was achieved with three of four strains. The relationship between the log10 CFU in lungs and the 24-h plasma AUC/MIC ratio are illustrated in Fig. 4. The relationship between plasma 24-h AUC/MIC and treatment effect was relatively robust, with an R2 of 0.74. Also shown in the figure is the maximum effect (Emax), 50% maximal effect point (ED50), and the slope (N) of the best fit line based on the sigmoid (Hill) Emax model. Results for the same PK/PD analysis for 24-h AUC/MIC are shown in Fig. 5 using ELF pharmacokinetic data.
TABLE 2.
24-h static and 1-log and 2-log kill doses and associated AUC/MIC values for each strain in the murine pneumonia model
| S. pneumoniae strain | 24-h growth in untreated control animals (log10 CFU/lungs) | MIC (mg/liter) | Stasis |
1-log10 kill |
2-log10 kill |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24-h total dose (mg/kg) | Plasma AUC/MIC | ELF AUC/MIC | 24-h total dose (mg/kg) | Plasma AUC/MIC | ELF AUC/MIC | 24-h total dose (mg/kg) | Plasma AUC/MIC | ELF AUC/MIC | |||
| 1293 | 2.34 | 0.06 | 1.28 | 19.83 | 17.80 | 18.24 | 179.98 | 200.64 | NAa | ||
| 10813 | 1.64 | 0.06 | 0.92 | 15.79 | 14.18 | 1.26 | 19.66 | 17.61 | 1.81 | 25.05 | 23.19 |
| 140 | 1.13 | 0.125 | NCb | 0.71 | 6.06 | 6.00 | 3.06 | 18.65 | 17.26 | ||
| 49619 | 0.85 | 0.03 | NC | 0.45 | 15.21 | 13.31 | 2.12 | 56.20 | 47.27 | ||
NA, endpoint not achieved.
NC, not calculated.
FIG 3.
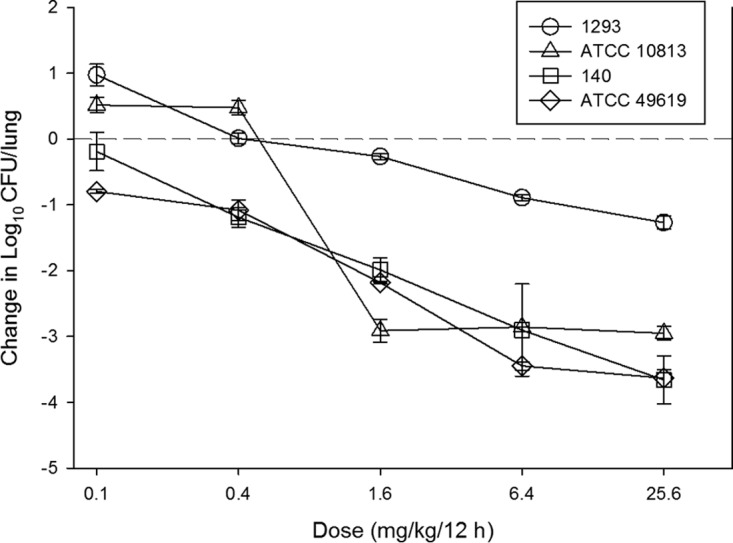
In vivo dose-response curves for omadacycline against select S. pneumoniae strains using a neutropenic murine pneumonia model. Each symbol represents the mean and standard deviation from three mice. Five total drug dose levels were administered by the subcutaneous route every 12 h. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal dashed-line at 0 represents the burden of organisms in the lungs of mice at the start of therapy. Data points below the line represent cidal activity and points above the line represent net growth.
FIG 4.
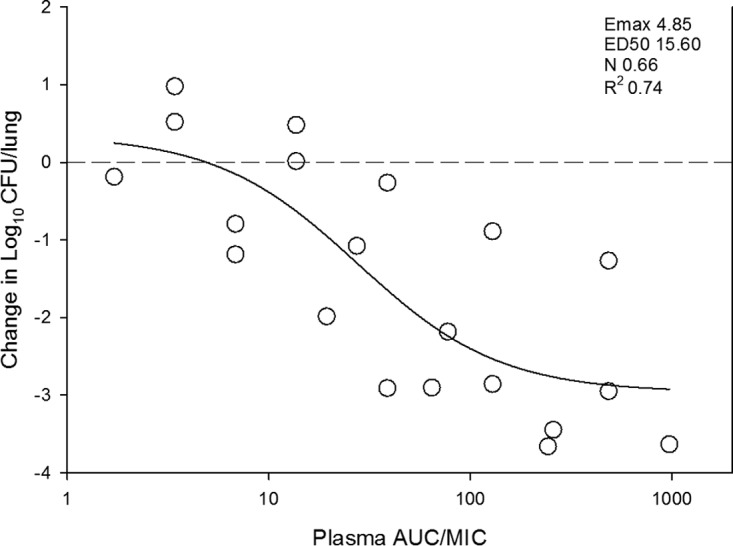
In vivo dose effect of omadacycline against select S. pneumoniae strains using a neutropenic murine pneumonia model. Each symbol represents the mean result from three mice. Five total drug dose levels were fractionated into an every-12-h regimen. The omadacycline exposure is expressed as the plasma 24 h AUC/MIC. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal dashed line at 0 represents the burden of organisms in the lungs of mice at the start of therapy. Data points below the line represent cidal activity and points above the line represent net growth. The R2 represents the coefficient of determination. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
FIG 5.
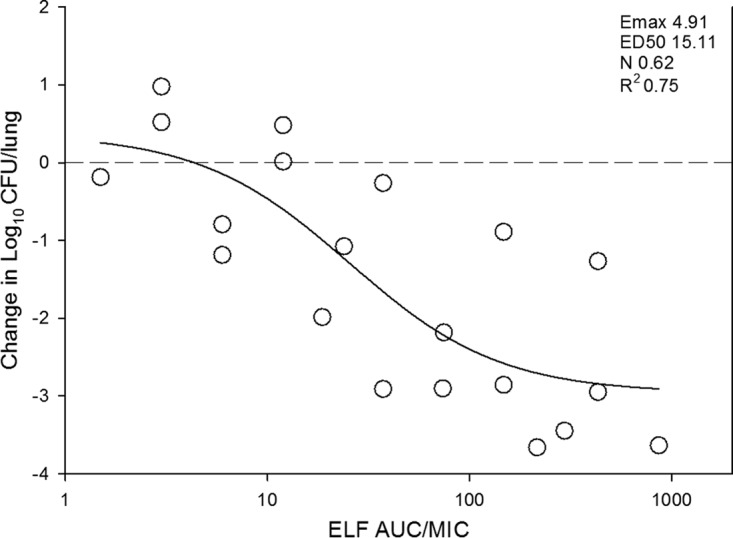
In vivo dose effect of omadacycline against select S. pneumoniae strains using a neutropenic murine pneumonia model. Each symbol represents the mean result from three mice. Five total drug dose levels were fractionated into an every-12-h regimen. The omadacycline exposure is expressed as the ELF 24-h AUC/MIC. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal dashed line at 0 represents the burden of organisms in the lungs of mice at the start of therapy. Data points below the line represent cidal activity, and points above the line represent net growth. The R2 represents the coefficient of determination. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
AUC/MIC magnitude associated with stasis and kill endpoints.
The doses necessary to produce a bacteriostatic effect and a 1-log and 2-log kill are shown in Table 2. The corresponding 24-h AUC/MIC for these doses are also presented utilizing both plasma and ELF pharmacokinetic data. The static doses were 0.92 mg/kg/24 h and 1.28 mg/kg/24 h against the two strains of S. pneumoniae for which this endpoint was achieved. The corresponding plasma 24 h AUC/MIC values were 16 and 20. The corresponding ELF 24-h AUC/MIC values were 14 and 18. A 1-log10 kill was achieved for all S. pneumoniae strains with 24-h dose range of 0.45 to 18.2 mg/kg. The corresponding plasma and ELF AUC/MIC values were 6.1 to 180 and 6.0 to 200, respectively. The relatively larger range for 1 log kill targets was driven by a single isolate (strain 1293) in which the target increased to a much larger degree when comparing stasis and 1-log kill AUC/MIC targets. The reason for this is not completely clear, but it could be due to inherent variability in the model and/or strain variability. Future studies utilizing more isolates would test whether this is an uncommon outlier. A 2-log10 kill was achieved for three strains with a 24-h dose range of 1.8 to 3.1 mg/kg. The corresponding plasma and ELF 24-h AUC/MIC values were 19 to 56 and 17 to 47, respectively. The AUC/MIC values are relatively similar given the relative penetration of drug into ELF ranged from 72 to 102% over the dose range.
DISCUSSION
The tetracycline class of antibiotics has been around for more than 70 years; however, there has been growing antibacterial resistance, especially in recent years. Advances in synthetic chemistry has created renewed interest in derivatives of tetracycline that maintain antimicrobial activity despite acquired tetracycline resistance mechanisms such as efflux pumps and ribosomal protection. This has been successfully applied in the generation of glycylcyclines, fluorocyclines, and aminomethylcyclines, such as omadacycline which was the focus of these studies. Omadacycline is a novel, first-in-class aminomethylcycline antibiotic in development for the treatment of CABP and skin and skin structure infection. Omadacycline is a broad-spectrum agent with potency against a variety of pathogens, including gram-positives (including organisms such as methicillin-resistant Staphylococcus aureus, which is resistant to beta-lactams, and S. pneumoniae, which is resistant to macrolides), gram-negatives, anaerobes, and atypical pathogens (9–11). Importantly, omadacycline maintains excellent activity against organisms that have acquired resistance to older tetracyclines (tetracycline, minocycline, and doxycycline) (12, 13).
Pharmacodynamic assessment of antimicrobial efficacy is a critical step in drug development to determine the optimal dosing strategy for clinical studies as well as set preliminary susceptibility breakpoints. We present here the results of a pharmacodynamic assessment of omadacycline activity in a preclinical animal model of S. pneumoniae pneumonia. Omadacycline demonstrated in vitro and in vivo potency against a select group of S. pneumoniae strains, including those resistant to other antibiotics such as beta-lactams, macrolides, and earlier generations of the tetracycline class. In vivo we observed potent bactericidal activity for all four strains with a ≥3-log10 kill in three of four strains tested. We also examined the drug exposures associated with efficacy in terms of plasma pharmacokinetic and ELF pharmacokinetic exposures. Previous unpublished data had suggested favorable pharmacokinetics in lung penetration, and we affirmed these preliminary results. Almost 100% of the drug in plasma penetrated into the ELF compartment based on drug concentration measurements in both compartments. This translated into similar PK/PD targets when one compared plasma to ELF AUC/MIC targets for stasis or bactericidal endpoints. In addition, the exposure response curves were quite steep, such that small increases in drug exposure led to increasing cidal activity.
There is a paucity of pharmacodynamic studies for the tetracycline class, including new synthetic congeners, to provide a comparison to the current studies. Previous studies have demonstrated tetracyclines exhibit time dependent activity with prolonged postantibiotic effects (14–16). Therefore, the predictive PK/PD index has often been found to be AUC/MIC. This has been demonstrated in dose fractionation studies with two other synthetic tetracycline derivatives: tigecycline (16) and eravacycline (unpublished data). Pharmacodynamic studies evaluating the PK/PD target exposures in in vivo animal model studies are even more sparse. Utilizing four S. pneumoniae strains in a murine neutropenic thigh model, Christianson et al. demonstrated that a 24-h free-drug AUC/MIC target of 24 for doxycycline was associated with net stasis and that a value of 120 was associated with a 2-log10 kill (17). Tigecycline PK/PD studies have also demonstrated the importance of AUC/MIC as the PK/PD driver of efficacy with net stasis targets that have ranged from 2 to 5 for a variety of pathogens (18–20). The AUC/MIC exposures associated with efficacy in the current preclinical model for omadacycline are similar to other tetracycline class antibiotic PK/PD studies. Importantly, in the case of tigecycline, clinical PK/PD analyses in patients with skin and skin structure infection and community-acquired pneumonia have confirmed the relevance of the PK/PD targets identified in preclinical models on treatment outcome (21, 22).
Human pharmacokinetic studies have evaluated the plasma pharmacokinetics of omadacycline after 100-mg intravenous and 300-mg oral tablet administration (11). The AUC0–∞ is nearly identical at 10.0 and 10.3 mg·h/liter, respectively. In 2014, surveillance antimicrobial susceptibility results for omadacycline against S. pneumoniae (>1,800 strains) demonstrated an MIC90 of 0.06 mg/liter (range, 0.015 to 0.12 mg/liter) (11). Likewise, in 2015 the in vitro activity of omadacycline was examined against tetracycline-resistant strains and demonstrated an MIC90 of 0.25 mg/liter (range, 0.015 to 0.25 mg/liter) (11). Utilizing the median AUC/MIC drug exposure targets identified in this study for stasis and kill endpoints, the AUC exposures in humans using the dosing regimens described above would be expected to produce efficacy against most S. pneumoniae strains, including those with tetracycline resistance. Given the favorable PK, including low protein binding and penetration into ELF, the in vitro potency that included tetracycline-resistant S. pneumoniae strains, and the in vivo efficacy observed in our animal model study, omadacycline is a promising novel agent for community-acquired pneumonia due to S. pneumoniae. These studies should prove beneficial in optimizing clinical dosing regimen design for CABP and setting preliminary susceptibility breakpoints.
MATERIALS AND METHODS
Organisms, media, and antibiotic.
Four S. pneumoniae strains were used in the studies and are listed in Table 1. Unless specified by American Type Culture Collection (ATCC) labeling, the strains are clinical isolates from invasive infections. Strains were chosen that varied in phenotypic resistance patterns to a number of relevant antimicrobials, including penicillin, minocycline, tigecycline, and erythromycin. All organisms were grown, subcultured, and quantified using sheep blood agar (Remel, Milwaukee, WI). The drug compounds used for in vitro and in vivo studies were supplied by Paratek Pharmaceuticals (Boston, MA).
In vitro susceptibility studies.
The MICs of each compound for the various strains were determined using Clinical and Laboratory Standards Institute microdilution methods (23). All MIC assays were performed in duplicate on three separate occasions. The median MIC of replicate assays is reported and utilized in PK/PD analyses.
Murine model.
Animals were maintained in accordance with American Association for Accreditation of Laboratory Animal Care criteria (24). All animal studies were approved by the Animal Research Committees of the William S. Middleton Memorial VA Hospital and the University of Wisconsin. Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 24 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, IN). Mice were rendered neutropenic (neutrophils < 100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) intraperitoneally 4 days (150 mg/kg) and 1 day (100 mg/kg) before lung infection. S. pneumoniae strains were grown overnight on sheep blood agar. A sterile loop was then used to transfer organism to sterile saline and absorbance adjusted to 0.3 at 580 nm using a Spectronic 88 spectrophotometer (Bausch and Lomb, Rochester, NY). After a 1:10 dilution, bacterial counts of the inoculum ranged from 108.4–9.1 CFU/ml. Lung infections with each of the strains were produced by the administration of 50 μl of inoculum into the nares of isoflurane-anesthetized mice. Mice were then held upright to allow for aspiration into the lungs. Therapy with omadacycline was initiated 2 h after induction of infection. No treatment controls and zero-hour controls were included in all experiments. After 24 h, organism burden was quantified by CFU counts from whole-organ homogenates.
Drug pharmacokinetics.
Single dose plasma pharmacokinetics of omadacycline were performed in mice. Dose levels of 0.5, 2, 8, and 32 mg/kg were administered subcutaneously. Groups of three mice were sampled for drug concentration determination at 1, 2, 4, 6, 8, 12, and 24 h. Both plasma and bronchoalveolar lavage (BAL) fluid was obtained for pharmacokinetic analysis. Plasma was obtained from each animal by centrifugation of anticoagulated blood obtained by cardiac puncture. BAL fluid was obtained by instillation of 1 ml of sterile saline into the lungs of each animal, followed by immediate removal. The BAL fluid was centrifuged to remove blood and cellular debris, and the supernatant was collected. Plasma and BAL supernatant was stored at −70°C. All drug concentrations were determined by liquid chromatography-tandem mass spectrometry methods by the sponsor. ELF concentrations were calculated from BAL fluid concentrations by urea correction methodology (25) according to the following formula: [drug]ELF = [drug]BAL × ([urea]plasma/[urea]BAL).
Pharmacokinetic parameters (mean ± the standard deviation), including the elimination half-life (t1/2), AUC0–∞, and Cmax, were calculated using a noncompartmental model using mean concentration values from each group of mice. The half-life was determined by linear least-squares regression. The AUC was calculated from the mean concentrations using the trapezoidal rule. Pharmacokinetic estimates for dose levels that were not directly measured were calculated using linear interpolation for dose levels between those with measured kinetics and linear extrapolation for dose levels above or below the highest and lowest dose levels with kinetic measurements. Protein binding of omadacycline is very low, and therefore total drug concentrations were utilized in all PK/PD calculations (26).
Relationship between PK/PD parameter AUC/MIC and efficacy.
AUC/MIC was chosen as the pharmacodynamic parameter for omadacycline based on previous studies demonstrating this PK/PD index to be predictive of treatment efficacy for the tetracycline class (14–16). In vivo treatment studies were performed in the murine pneumonia model for each strain. Groups of three mice per dosing regimen and control group were utilized. Dose-response studies consisted of 4-fold increasing doses (range, 0.1 to 25.6 mg/kg/12 h) administered subcutaneously. The dose-response effect was determined as described above by measurement of CFU in lung homogenates. The correlation between efficacy and the PK/PD parameter AUC/MIC was determined by nonlinear least-squares multivariate regression (SigmaPlot version 12.3; Systat Software, San Jose, CA). The mathematical model used was derived from the Hill equation E = (Emax × AUC/MICN)/(ED50N – AUC/MICN), where E is the effector, in this case, the log change in CFU per lung between treated mice and untreated controls after the 24-h period of study, Emax is the maximum effect, D is the 24-h total AUC/MIC, ED50 is the AUC/MIC required to achieve 50% of the Emax, and N is the slope of the dose-effect curve. The values for the indices Emax, ED50, and N were calculated using nonlinear least-squares regression. The coefficient of determination (R2) was used to estimate the variance that might be due to regression with the PK/PD parameter AUC/MIC.
AUC/MIC magnitude associated with stasis and kill endpoints.
Using the Sigmoid Emax model described above, the dose required to produce net static effect (static dose) and the 1- and 2-log10 kill compared to the start of therapy were calculated for each drug-organism combination. The plasma and ELF pharmacokinetic results were then used to estimate the AUC/MIC exposure associated with each of the endpoints for each organism. The associated 24-h total drug AUC/MIC targets were calculated.
ACKNOWLEDGMENT
This study was funded by Paratek Pharmaceuticals.
REFERENCES
- 1.GBD Study 2013 Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drijkoningen JJ, Rohde GG. 2014. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 20(Suppl 5):S45–S51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 3.Howard LS, Sillis M, Pasteur MC, Kamath AV, Harrison BD. 2005. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect 50:107–113. doi: 10.1016/j.jinf.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Welte T, Torres A, Nathwani D. 2012. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins SG, Farrell DJ. 2009. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis 15:1260–1264. doi: 10.3201/eid1508.081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RN, Sader HS, Mendes RE, Flamm RK. 2013. Update on antimicrobial susceptibility trends among Streptococcus pneumoniae in the United States: report of ceftaroline activity from the SENTRY Antimicrobial Surveillance Program (1998-2011). Diagn Microbiol Infect Dis 75:107–109. doi: 10.1016/j.diagmicrobio.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Tillotson GS. 2016. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis 16:e11–12. doi: 10.1016/S1473-3099(16)00051-7. [DOI] [PubMed] [Google Scholar]
- 8.Aschenbrenner DS. 2016. The FDA revises boxed warning for fluoroquinolones—again. Am J Nurs 116:22–23. doi: 10.1097/01.NAJ.0000494687.10004.61. [DOI] [PubMed] [Google Scholar]
- 9.Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, Mechiche R, Ohemeng K, Verma AK, Cannon EP, Macone A, Tanaka SK, Levy S. 2015. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother 59:7044–7053. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macone AB, Caruso BK, Leahy RG, Donatelli J, Weir S, Draper MP, Tanaka SK, Levy SB. 2014. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother 58:1127–1135. doi: 10.1128/AAC.01242-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villano S, Steenbergen J, Loh E. 2016. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol 11:1421–1434. doi: 10.2217/fmb-2016-0100. [DOI] [PubMed] [Google Scholar]
- 12.Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, Levy SB. 2014. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 58:1279–1283. doi: 10.1128/AAC.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka SK, Steenbergen J, Villano S. 2016. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 24:6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 15.Andes D, Craig W. 2007. Pharmacokinetics and pharmacodynamics of tetracyclines, p 267–278. In Nightingale CH, Ambrose PG, Drusano GL, Murakawa T (ed), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed Informa Healthcare USA, New York, NY. [Google Scholar]
- 16.van Ogtrop ML, Andes D, Stamstad TJ, Conklin B, Weiss WJ, Craig WA, Vesga O. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob Agents Chemother 44:943–949. doi: 10.1128/AAC.44.4.943-949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christianson J, Andes D, Craig W. 2001. Magnitude of the 24-h AUC/MIC required for efficacy of doxycycline (doxy) against Streptococcus pneumoniae (SP) in a murine thigh-infection model, abstr 475 39th Infectious Diseases Society of America Annual Meeting. Infectious Disease Society of America, San Francisco, CA. [Google Scholar]
- 18.Crandon JL, Banevicius MA, Nicolau DP. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob Agents Chemother 53:1165–1169. doi: 10.1128/AAC.00647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob Agents Chemother 53:5060–5063. doi: 10.1128/AAC.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicasio AM, Crandon JL, Nicolau DP. 2009. In vivo pharmacodynamic profile of tigecycline against phenotypically diverse Escherichia coli and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 53:2756–2761. doi: 10.1128/AAC.01678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meagher AK, Passarell JA, Cirincione BB, Van Wart SA, Liolios K, Babinchak T, Ellis-Grosse EJ, Ambrose PG. 2007. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother 51:1939–1945. doi: 10.1128/AAC.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino CM, Bhavnani SM, Forrest A, Dukart G, Dartois N, Cooper A, Korth-Bradley J, Ambrose PG. 2012. Pharmacokinetics-pharmacodynamics of tigecycline in patients with community-acquired pneumonia. Antimicrob Agents Chemother 56:130–136. doi: 10.1128/AAC.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed Document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 25.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi P, Esposito C, Koroma J, Cannon EP, Tanaka SK. 2003. In vitro assessment of plasma protein binding and metabolic stability of PTK.0796, abstr 2675. 43rd Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]