ABSTRACT
We reviewed data for almost 300,000 clinical Escherichia coli urinary isolates (collected in 2009 through 2013) from 127 inpatient and outpatient facilities, to assess antibiotic resistance among Veterans Affairs health care system patients using Clinical and Laboratory Standards Institute and Centers for Disease Control and Prevention National Healthcare Safety Network definitions or guidance. Rates of resistance to amoxicillin or ampicillin/β-lactamase inhibitors were approximately 40% and rates of resistance to fluoroquinolones and trimethoprim-sulfamethoxazole approached 30%. Rates of resistance to nitrofurantoin, antipseudomonal penicillin/β-lactamase inhibitors, and carbapenems remained less than 10%. The percentage of isolates that were considered multidrug resistant varied (4% to 37%), depending on the definitions used.
KEYWORDS: Escherichia coli, resistance, urinary tract infection
TEXT
Escherichia coli is the most clinically relevant and multiply-drug-resistant bacterial pathogen causing urinary tract infections (UTIs) (1, 2). Monitoring resistance is important, to support clinical decision-making and public health and safety. The Clinical and Laboratory Standards Institute (CLSI) guidelines for clinical laboratories provide standardized methodology for the preparation and presentation of cumulative susceptibility data through the use of an antibiogram (3–5). Data from the Centers for Diseases Control and Prevention (CDC) National Healthcare Safety Network (NHSN) are also of great value for tracking antimicrobial resistance (6). Limited data are available to provide a comprehensive description of E. coli resistance nationally in inpatient and outpatient settings.
The Veterans Affairs (VA) system is the nation's largest integrated health care system, providing care to over 9 million veterans in over 140 medical centers and 1,200 outpatient clinics throughout the United States (7). Antimicrobial susceptibility data are captured in VA electronic data sets and provide a unique opportunity to assess resistance nationally. Our intent is to describe national antimicrobial resistance rates among clinical E. coli urinary isolates and to highlight differences in resistance rates using CLSI and NHSN criteria.
We retrospectively evaluated data for adult VA patients (≥18 years of age) with urine cultures growing E. coli, between January 2009 and December 2013. We utilized three different criteria for assessing resistance, i.e., the CDC NHSN criteria, which captures the first isolate per patient per month (8); the CLSI guidance, which recommends including only the first isolate per patient per year for antibiogram presentation (3, 5); and a third method, which uses the most resistant isolate per person per facility per year, since the first two approaches may underestimate overall resistance rates (9). We removed all same-day duplicate antibiotic susceptibility test results (same patient, same isolate, and same day), keeping the most resistant result (8, 10).
To classify antibiotic resistance rates, individual antimicrobial agents were further categorized based on international standard definitions from the European Centre for Disease Prevention and Control (ECDC) and the CDC for Enterobacteriaceae and the CDC Antibiotic Resistance Patient Safety Atlas (AR Atlas) E. coli phenotype definitions (11, 12). The CDC AR Atlas includes data on health care-associated infections reported to the CDC NHSN. MDR was defined as nonsusceptibility to at least one drug in at least 3 categories, using the ECDC/CDC international standards and the CDC AR Atlas definitions (11, 12).
During the 5-year study period, 297,046 E. coli isolates were identified from 127 sites, in all 9 CDC regions, by using the NHSN methods (first isolate per month). Most isolates were obtained from white (75%) male (78%) patients in an outpatient setting (77%). Resistance rates were 40% for amoxicillin or ampicillin/β-lactamase inhibitors, 34% for fluoroquinolones, 28% for trimethoprim-sulfamethoxazole, and less than 10% for extended-spectrum cephalosporins (7%), nitrofurantoin (6%), antipseudomonal penicillin/β-lactamase inhibitors (5%), and carbapenems (<1%) (Table 1). Resistance rates were higher for inpatient versus outpatient isolates for all antibiotic categories assessed (Table 2) and varied by CDC region and treatment setting (Fig. 1 and 2).
TABLE 1.
Escherichia coli antibiotic resistance among VA inpatient and outpatient facilities nationally, by method used to describe rates (2009 to 2013)
| Antibiotic categorya | % nonsusceptible (no. of isolates tested) |
||
|---|---|---|---|
| First isolate per patient per facility per month (n = 297,046)b | First isolate per patient per facility per year (n = 244,411)c | Most resistant isolate per patient per facility per year (n = 244,411) | |
| Aminoglycoside | 12.6 (296,022) | 10.9 (243,577) | 11.5 (243,590) |
| Antipseudomonal penicillin/β-lactamase inhibitor | 5.3 (206,707) | 4.7 (170,013) | 5.5 (170,342) |
| Carbapenem | 0.4 (231,153) | 0.4 (189,809) | 0.4 (190,017) |
| Extended-spectrum cephalosporin | 6.9 (264,519) | 6.0 (217,513) | 6.5 (217,886) |
| Fluoroquinolone | 34.3 (291,674) | 29.5 (240,005) | 30.4 (240,086) |
| Nitrofurantoin | 6.2 (249,096) | 5.4 (204,526) | 6.1 (204,611) |
| Amoxicillin or ampicillin/β-lactamase inhibitor | 39.6 (238,738) | 37.2 (196,203) | 39.0 (196,450) |
| Trimethoprim-sulfamethoxazole | 28.2 (296,501) | 25.2 (243,957) | 26.3 (243,982) |
The aminoglycoside category included amikacin, gentamicin, and tobramycin. The antipseudomonal penicillin/β-lactamase inhibitor category included piperacillin-tazobactam and ticarcillin-clavulanic acid. The carbapenem category included imipenem, meropenem, doripenem, and ertapenem. The extended-spectrum cephalosporin category included ceftriaxone, ceftazidime, cefotaxime, and cefepime. The fluoroquinolone category included levofloxacin and ciprofloxacin. The amoxicillin or ampicillin/β-lactamase inhibitor category included amoxicillin-clavulanic acid and ampicillin-sulbactam.
National Healthcare Safety Network method.
Clinical and Laboratory Standards Institute method.
TABLE 2.
Escherichia coli antibiotic resistance among VA inpatient and outpatient facilities nationally, by health care setting (2009 to 2013)
| Antibiotic categorya | % nonsusceptible (no. of isolates tested)b |
||
|---|---|---|---|
| Overall (n = 297,046) | Inpatient (n = 70,101) | Outpatient (n = 226,945) | |
| Aminoglycoside | 12.6 (296,022) | 17.4 (69,824) | 11.1 (226,198) |
| Antipseudomonal penicillin/β-lactamase inhibitor | 5.3 (206,707) | 8.0 (50,795) | 4.5 (155,912) |
| Carbapenem | 0.4 (231,153) | 0.5 (55,643) | 0.4 (175,510) |
| Extended-spectrum cephalosporin | 6.9 (264,519) | 11.3 (63,706) | 5.4 (200,813) |
| Fluoroquinolone | 34.3 (291,674) | 46.5 (68,659) | 30.5 (223,015) |
| Nitrofurantoin | 6.2 (249,096) | 7.0 (56,025) | 6.0 (193,071) |
| Amoxicillin or ampicillin/β-lactamase inhibitor | 39.6 (238,738) | 47.7 (56,168) | 37.0 (182,570) |
| Trimethoprim-sulfamethoxazole | 28.2 (296,501) | 35.6 (69,958) | 26.0 (226,543) |
The aminoglycoside category included amikacin, gentamicin, and tobramycin. The antipseudomonal penicillin/β-lactamase inhibitor category included piperacillin-tazobactam and ticarcillin-clavulanic acid. The carbapenem category included imipenem, meropenem, doripenem, and ertapenem. The extended-spectrum cephalosporin category included ceftriaxone, ceftazidime, cefotaxime, and cefepime. The fluoroquinolone category included levofloxacin and ciprofloxacin. The amoxicillin or ampicillin/β-lactamase inhibitor category included amoxicillin-clavulanic acid and ampicillin-sulbactam.
Results were determined as the first isolate per patient per facility per month (National Healthcare Safety Network method).
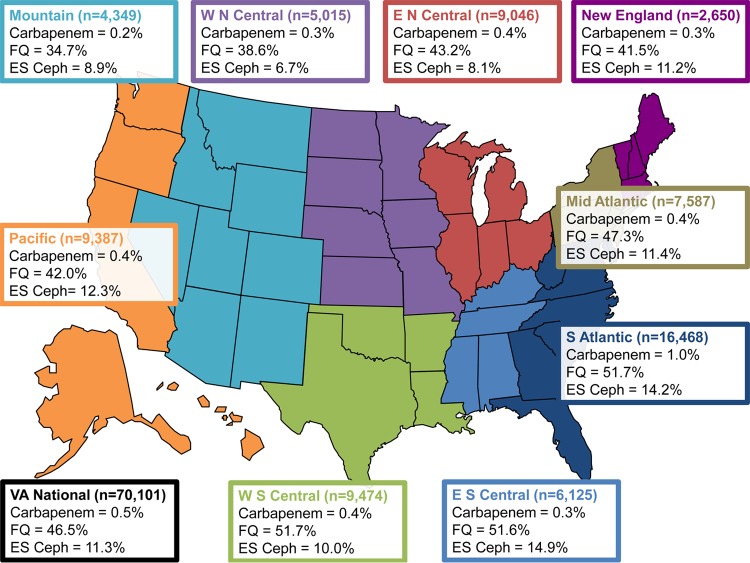
FIG 1.
Escherichia coli antibiotic resistance among Veterans Affairs inpatient facilities nationally, by CDC region, in 2009 to 2013. Results by CDC region represent the first isolate per patient per facility per month (CDC NHSN method). E N Central, East North Central Region; E S Central, East South Central Region; ES Ceph, extended-spectrum cephalosporin; FQ, fluoroquinolone; Mid Atlantic, Middle Atlantic Region; Mountain, Mountain Region; New England, New England Region; Pacific, Pacific Region; S Atlantic, South Atlantic Region; W N Central, West North Central Region; W S Central, West South Central Region. Data are the percent nonsusceptible (total number of isolates tested). Not every antibiotic category was tested for every isolate tested. The carbapenem category included imipenem, meropenem, doripenem, and ertapenem. The extended-spectrum cephalosporin category included ceftriaxone, ceftazidime, cefotaxime, and cefepime. The fluoroquinolone category included levofloxacin and ciprofloxacin.
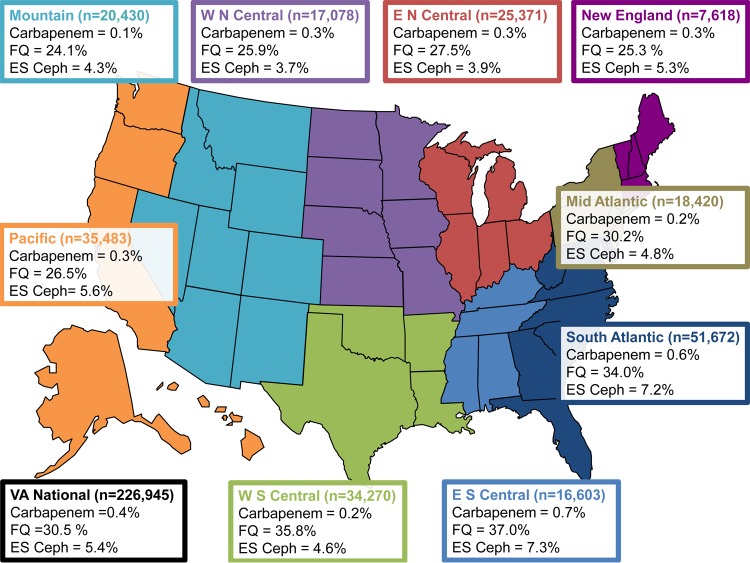
FIG 2.
Escherichia coli antibiotic resistance among Veterans Affairs outpatient facilities nationally, by CDC region, in 2009 to 2013. Results by CDC region represent the first isolate per patient per facility per month (CDC NHSN method). E N Central, East North Central Region; E S Central, East South Central Region; ES Ceph, extended-spectrum cephalosporin; FQ, fluoroquinolone; Mid Atlantic, Middle Atlantic Region; Mountain, Mountain Region; New England, New England Region; Pacific, Pacific Region; S Atlantic, South Atlantic Region; W N Central, West North Central Region; W S Central, West South Central Region. Data are the percent nonsusceptible (total number of isolates tested). Not every antibiotic category was tested for every isolate tested. The carbapenem category included imipenem, meropenem, doripenem, and ertapenem. The extended-spectrum cephalosporin category included ceftriaxone, ceftazidime, cefotaxime, and cefepime. The fluoroquinolone category included levofloxacin and ciprofloxacin.
We identified 297,046 E. coli isolates when we included only the first (per CLSI recommendations) or most resistant isolate per patient per facility per year (Table 1). Resistance rates were similar with the two methods (first isolate versus most resistant).
In a subanalysis, we overlaid the two global MDR definitions (11, 12). The percentages of MDR isolates were 37% (108,500/297,046 isolates) using the ECDC/CDC international standard and 4% (12,293/297,046 isolates) using the CDC AR Atlas definitions. We further classified the prevalence of MDR for inpatient and outpatient isolates using both methods (ECDC/CDC, 47% and 33%, respectively; CDC AR Atlas, 7% and 3%, respectively).
Antimicrobial resistance among E. coli urinary isolates is increasing in the United States (6, 13). Confusion exists when local facilities compare their CLSI-based antibiograms with national surveillance data. We identified high rates of antimicrobial resistance to several commonly used E. coli UTI treatment options. The overall rate of fluoroquinolone resistance using NHSN methods was 34%, with resistance reaching almost 50% among inpatients and being 30% for outpatients, similar to previous studies (6, 13–16). These findings are concerning, as fluoroquinolones are frequently used empirically to treat UTIs, especially complicated infections.
Our study also demonstrated trimethoprim-sulfamethoxazole resistance rates approaching 30%. Several studies with E. coli urinary isolates from U.S. outpatients have reported >20% resistance to trimethoprim-sulfamethoxazole (15, 16). Trimethoprim-sulfamethoxazole should not be used for empirical treatment of acute cystitis when local antibiograms reveal ≥20% resistance, according to Infectious Diseases Society of America (IDSA) guidelines (2). Similar to previous findings, we demonstrated that rates of resistance to nitrofurantoin remain low, and this is an appropriate option for patients with uncomplicated cystitis (15, 16). For empirical inpatient treatment options, our data suggest that antipseudomonal penicillin/β-lactamase inhibitors and carbapenems remain among the most active agents, similar to recent nationwide surveillance data (6, 13).
We found vast differences in the numbers of isolates considered MDR, depending on the definition used. According to the CDC AR Atlas definition, 7% of our inpatient isolates were MDR. Similarly, 5.5 to 8.1% of E. coli isolates causing catheter-associated UTIs that were reported to the CDC NHSN in 2011 to 2014 were MDR (6). Using the international standard MDR definition, over 45% and 30% of inpatient and outpatient isolates, respectively, were considered MDR. Our results suggest that these definitions may overestimate resistance rates, compared to the methods used by the CDC AR Atlas.
There are several limitations to our study. We did not distinguish colonization from symptomatic infection. Our data represent all positive microbiological E. coli urine cultures and thus represent full ecological resistance among all cultures in the VA system. The heterogeneity among VA microbiology laboratories and the antibiotics tested also affects our data. The CLSI MIC susceptibility breakpoints for Enterobacteriaceae have changed over time, and the changes might have been applied at different times by individual laboratories. Therefore, we applied the 2014 CLSI breakpoints to our data when MIC data were available. Finally, the generalizability of our results may be limited to the VA population.
In conclusion, among almost 300,000 urinary E. coli isolates collected from a predominately male VA outpatient population, the rates of resistance to amoxicillin or ampicillin/β-lactamase inhibitors were approximately 40% and rates of resistance to fluoroquinolones and trimethoprim-sulfamethoxazole approached 30%. The rates of resistance to extended-spectrum cephalosporins, nitrofurantoin, antipseudomonal penicillin/β-lactamase inhibitors, and carbapenems remained low. Of note, the prevalence of isolates considered to be MDR varied considerably, depending on the definitions used.
ACKNOWLEDGMENTS
This material is based upon work supported, in part, by the Office of Research and Development, Department of Veterans Affairs.
H.J.M. is supported in part by a career development award from the Department of Veterans Affairs and has received research funding from Merck (Cubist). A.R.C. has received research funding from Pfizer, Merck (Cubist), and the Medicines Company. D.D. is a Veterans Affairs government employee; he has received research funding through the VA system, the West Foundation, and the National Institute on Aging. L.A.M. has served as a consultant for the Medicines Company and has received research support from Bard. K.L.L. has received research funding or acted as an advisor or consultant for Bard/Davol, Merck (Cubist), Forest, Pfizer, and the Medicines Company. J.B.M. and L.J. have no conflicts of interest.
The views expressed are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 3.Hindler JF, Stelling J. 2007. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 44:867–873. doi: 10.1086/511864. [DOI] [PubMed] [Google Scholar]
- 4.Bax R, Bywater R, Cornaglia G, Goossens H, Hunter P, Isham V, Jarlier V, Jones R, Phillips I, Sahm D, Senn S, Struelens M, Taylor D, White A. 2001. Surveillance of antimicrobial resistance–what, how and whither? Clin Microbiol Infect 7:316–325. doi: 10.1046/j.1198-743x.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2014. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline—4th ed. CLSI document M39-A4 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Veterans Analysis and Statistics. Department of Veterans Affairs statistics at a glance (updated 06/30/2015.). U.S. Department of Veterans Affairs, Washington, DC: http://www.va.gov/vetdata/docs/Quickfacts/Stats_at_a_glance_08_27_15.pdf. [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2014. Antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf. [Google Scholar]
- 9.Fridkin SK, Edwards JR, Tenover FC, Gaynes RP, McGowan JE Jr. 2001. Antimicrobial resistance prevalence rates in hospital antibiograms reflect prevalence rates among pathogens associated with hospital-acquired infections. Clin Infect Dis 33:324–330. doi: 10.1086/321893. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2016. Antibiotic resistance patient safety atlas: phenotype definitions. Centers for Disease Control and Prevention, Atlanta, GA: http://gis.cdc.gov/grasp/PSA/Downloads/ARPatientSafetyAtlas-PhenotypeDefinitions.pdf. [Google Scholar]
- 12.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. 2016. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis 85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Bidell MR, Palchak M, Mohr J, Lodise TP. 2016. Fluoroquinolone and third-generation cephalosporin resistance among hospitalized patients with urinary tract infections due to Escherichia coli: do rates vary by hospital characteristics and geographic region? Antimicrob Agents Chemother 60:3170–3173. doi: 10.1128/AAC.02505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez GV, Master RN, Karlowsky JA, Bordon JM. 2012. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 56:2181–2183. doi: 10.1128/AAC.06060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. 2016. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother 60:2680–2683. doi: 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]