ABSTRACT
The fluoroquinolone resistance score (FQRS) predicts the probability of fluoroquinolone resistance with good discrimination. The score has been derived from patients with bloodstream infections caused by Gram-negative bacteria and is based on fluoroquinolone use within the past 6 months, among other clinical and health care exposure criteria. This study aims to examine the utility of the FQRS in patients with complicated urinary tract infections (cUTI) and determine whether extension of prior fluoroquinolone use to 12 months improves model discrimination. Adults with cUTI at Palmetto Health in central South Carolina, USA, from 1 April 2015 through 31 July 2015 were prospectively identified. Multivariate logistic regression was used to examine the association between prior fluoroquinolone use and resistance. Among 238 patients, 54 (23%) had cUTI due to fluoroquinolone-resistant bacteria. Overall, the median age was 66 years, 162 (68%) patients were women, and 137 (58%) patients had cUTI due to Escherichia coli. Prior exposure to fluoroquinolones within 3 months (adjusted odds ratio [aOR], 23.4; 95% confidence interval [CI], 8.2 to 76.8; P < 0.001) and within 3 to 12 months (aOR, 13.2; 95% CI, 3.1 to 68.4; P < 0.001) was independently associated with fluoroquinolone resistance compared to no prior use. The area under the receiver operating characteristic curve for the FQRS increased from 0.73 to 0.80 when prior fluoroquinolone use was extended from 6 to 12 months. FQRSs of ≥2 and ≥3 had negative predictive values of 91% and 90%, respectively. The modified FQRS stratifies patients with cUTI on the basis of the predicted probability of fluoroquinolone resistance with very good discrimination. Application of the modified FQRS may improve antimicrobial utilization in patients with acute pyelonephritis.
KEYWORDS: risk factors, ciprofloxacin, levofloxacin, pyelonephritis, sepsis, bacteremia, bloodstream infections
INTRODUCTION
Complicated urinary tract infections (cUTI) are a leading cause of hospitalization due to infections in the United States (1, 2). Increasing rates of antimicrobial resistance have limited the options for oral empirical therapy of cUTI (3–5). The rates of resistance to fluoroquinolones (FQ), trimethoprim-sulfamethoxazole, and amoxicillin-clavulanate among Escherichia coli isolates have exceeded 20% in multiple geographical settings in North America (6–8). Despite increasing rates of antimicrobial resistance, fluoroquinolones remain among the antimicrobial agents most commonly prescribed for the treatment of cUTI (9).
The fluoroquinolone resistance score (FQRS) was recently developed by our group to predict the probability of fluoroquinolone resistance in bloodstream Gram-negative isolates. The score is based on male sex (1 point), diabetes mellitus (1 point), residence at a skilled nursing facility (2 points), recent outpatient procedures (3 points), and prior fluoroquinolone use (within 3 months, 5 points; within 3 to 6 months, 3 points). The FQRS stratifies patients with bloodstream infections caused by Gram-negative bacteria according to the predicted risk of fluoroquinolone resistance with good discrimination on the basis of an area under a receiver operating characteristic curve (AUC) of 0.73 (10).
The aim of this study was to prospectively examine the utility of the FQRS in the management of patients with cUTI and examine whether extension of fluoroquinolone use up to 12 months prior to infection improves model discrimination.
(The preliminary results of this study were presented, in part, at IDWeek, 26 to 30 October 2016, New Orleans, LA, USA [11].)
RESULTS
During the 4-month study period, 238 patients with cUTI were included. Overall, the median age was 66 years, and 76 (32%) of the patients were men. Escherichia coli was the most common pathogen (n = 137 patients; 58%), followed by Klebsiella pneumoniae (n = 37; 16%), Pseudomonas aeruginosa (n = 14; 6%), Enterobacter cloacae (n = 13; 5%), Proteus mirabilis (n = 10; 4%), Serratia marcescens (n = 10; 4%), Enterobacter aerogenes (n = 7; 3%), and others (n = 10; 4%). Among the patients in this cohort, 54 (23%) had cUTI due to fluoroquinolone-nonsusceptible (FQ-NS) Gram-negative bacilli. The baseline demographics and clinical characteristics of the patients with cUTI due to FQ-NS and fluoroquinolone-susceptible (FQ-S) bacteria are shown in Table 1.
TABLE 1.
Demographics and clinical characteristics of patients with cUTI and risk factors for fluoroquinolone resistance in univariate modela
| Variable | Value for patients infected with: |
OR | 95% CI | P value | |
|---|---|---|---|---|---|
| FQ-NS bacteria (n = 54) | FQ-S bacteria (n = 184) | ||||
| Median (IQR) age (yr) | 69 (59–81) | 65 (54–76) | 1.18b | 0.98–1.43 | 0.09 |
| No. (%) of male subjects | 21 (39) | 55 (30) | 1.49 | 0.79–2.94 | 0.22 |
| No. (%) of subjects of white race | 29 (53) | 83 (45) | 1.41 | 0.77–2.61 | 0.27 |
| No. (%) of subjects with: | |||||
| Diabetes mellitus | 21 (39) | 71 (39) | 1.01 | 0.54–1.88 | 0.97 |
| Cancer | 13 (24) | 33 (18) | 1.45 | 0.68–2.96 | 0.32 |
| Hospital-acquired infection | 9 (17) | 38 (21) | 0.77 | 0.33–1.65 | 0.51 |
| Recent hospitalizationc | 25 (46) | 61 (33) | 1.74 | 0.93–3.22 | 0.08 |
| Residence at a skilled nursing facility | 10 (19) | 18 (10) | 2.10 | 0.88–4.79 | 0.09 |
| Recent outpatient procedured | 6 (11) | 5 (3) | 4.48 | 1.30–16.13 | 0.02 |
| Prior fluoroquinolone use | |||||
| None | 21 (39) | 171 (93) | 1 | Referent | Referent |
| Within 3 mo | 18 (33) | 6 (3) | 24.43 | 9.18–73.91 | <0.001 |
| Within 3–12 mo | 15 (28) | 7 (4) | 17.45 | 6.60–50.47 | <0.001 |
cUTI, complicated urinary tract infections; OR, odds ratio; CI, confidence interval; IQR, interquartile range.
Per decade.
Within 3 months of infection.
Within 1 month of infection.
Univariate logistic regression results for risk factors for infection with FQ-NS bacteria are demonstrated in Table 1. In the multivariate model, male sex, residence at a skilled nursing facility, outpatient procedures within 1 month of the index infection, and prior fluoroquinolone use within 3 months and within 3 to 12 months of the index infection were independently associated with fluoroquinolone resistance (Table 2).
TABLE 2.
Independent risk factors for fluoroquinolone resistance in multivariate logistic regression modela
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Male sex | 2.22 | 1.00–5.34 | 0.049 |
| Diabetes mellitus | 1.48 | 0.71–3.14 | 0.29 |
| Residence at a skilled nursing facility | 2.80 | 1.02–7.25 | 0.046 |
| Outpatient GI/GU procedure within 1 mo | 6.65 | 1.47–29.78 | 0.01 |
| Prior fluoroquinolone use within 12 mo | |||
| None | 1 | Referent | Referent |
| Within 3 mo | 23.35 | 8.20–76.85 | <0.001 |
| Within 3–12 mo | 13.16 | 3.11–68.43 | <0.001 |
aOR, adjusted odds ratio; CI, confidence intervals; GI, gastrointestinal; GU, genitourinary.
On the basis of multivariate logistic regression results, the FQRS was modified by extending prior fluoroquinolone exposure from 6 to 12 months (Table 3). The AUC for the FQRS model increased from 0.73 to 0.80 when prior fluoroquinolone use was extended from 6 months (not shown) to 12 months (Fig. 1). The performance characteristics of the modified FQRS as a binary classification test are demonstrated in Table 4. The negative predictive values (NPVs) were 94%, 91%, and 90% when FQRS cutoff points of ≥1, ≥2, and ≥3 were used to indicate a high risk of fluoroquinolone resistance.
TABLE 3.
Modified FQRSa
| Risk factor for fluoroquinolone resistance | Point allocation |
|---|---|
| Male sex | 1 |
| Diabetes mellitus | 1 |
| Residence in a skilled nursing facility | 2 |
| Outpatient GI/GU procedure within past 1 mo | 3 |
| Prior fluoroquinolone use within past 12 mo | |
| None | 0 |
| Within 3 mo | 5 |
| Within 3–12 mo | 3 |
FQRS, fluoroquinolone resistance score; GI, gastrointestinal; GU, genitourinary.
FIG 1.
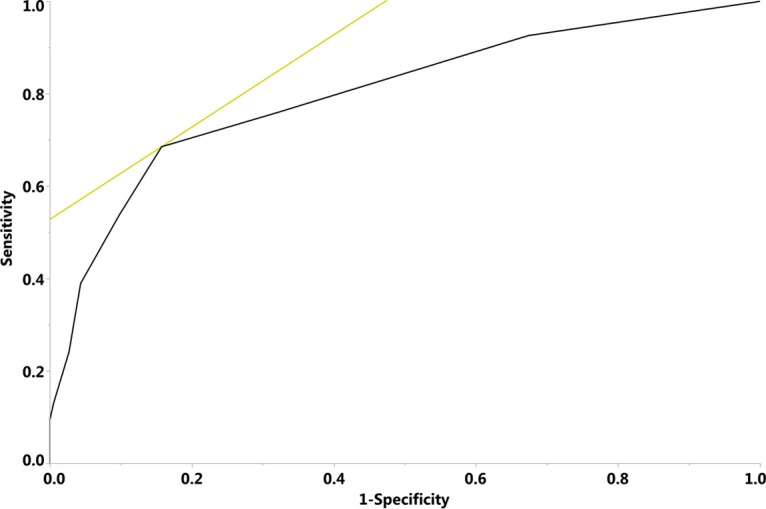
Receiver operating characteristic plot of the modified fluoroquinolone resistance score. The black line indicates the receiver operating characteristic curve. The light color tangent line highlights the point in the curve that represents the best performance of the model. The area under the curve was 0.80.
TABLE 4.
Performance of modified FQRS as a binary classification testa
| FQRS cutoff point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| ≥1 | 93 | 33 | 29 | 94 |
| ≥2 | 76 | 68 | 41 | 91 |
| ≥3b | 69 | 84 | 56 | 90 |
FQRS, fluoroquinolone resistance score; PPV, positive predictive value; NPV, negative predictive value.
The use of a cutoff score of ≥3 to indicate a high predicted risk of fluoroquinolone resistance provides the best performance in the statistical model.
The risk of fluoroquinolone resistance in patients with cUTI predicted using the modified FQRS is shown in Fig. 2. Patients with FQRSs of 0 and 1 have relatively low predicted probabilities of fluoroquinolone resistance of 6% and 10%, respectively. The estimated risk of fluoroquinolone resistance increases to 31% in patients with an FQRS of 3 and to 60% in patients with an FQRS of 5.
FIG 2.
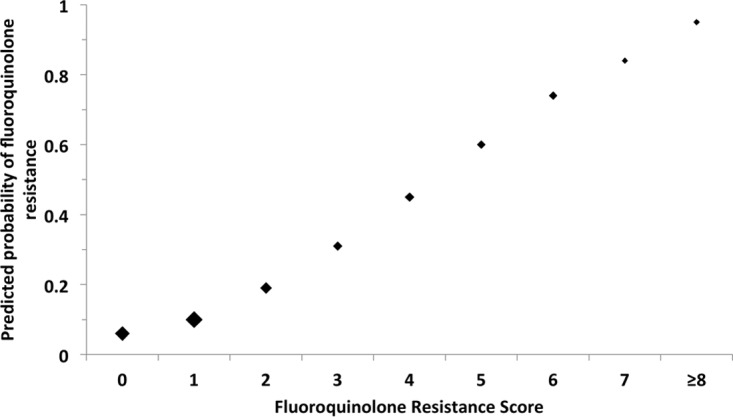
Predicted probability of fluoroquinolone resistance by modified fluoroquinolone resistance score. The size of the marker for point estimates is weighted approximately by the relative number of subjects with the corresponding score.
DISCUSSION
This study supports the use of the FQRS for the management of patients with cUTI. On the basis of prior and current results, the FQRS predicts the risk of fluoroquinolone resistance with good discrimination both in patients with bloodstream infections caused by Gram-negative bacteria and in patients with cUTI, respectively (AUC = 0.73 for both) (10). Modification of the FQRS by extension of fluoroquinolone use up to 12 months prior to infection improves the discriminative ability of the model (AUC = 0.80).
It is alarming that fluoroquinolone use in patients with cUTI predisposes these patients to an increased risk of fluoroquinolone resistance up to 1 year after exposure. This is supported by the results of a recent study which demonstrated the persistent impact of antimicrobials on the intestinal flora up to 1 year after exposure (12). This highlights the significance of antimicrobial stewardship efforts to reduce the unnecessary use of fluoroquinolones for the treatment of mild infections, e.g., upper respiratory tract infections and uncomplicated cystitis. The risks of fluoroquinolone therapy, including the induction of antimicrobial resistance, in patients with these minor infections exceed the potential benefits (13). Fluoroquinolones are potentially life-saving antimicrobial agents. Due to recent safety concerns and updates to fluoroquinolone labels by the U.S. Food and Drug Administration, fluoroquinolone use should be reserved for patients with potentially life-threatening infections (13). Narrower-spectrum antimicrobial agents are more appropriate for less serious infections. Fluoroquinolone therapy is not appropriate in patients with uncomplicated cystitis when other treatment options (e.g., nitrofurantoin, fosfomycin, and trimethoprim-sulfamethoxazole) are available (2, 14).
In the era of increasing antimicrobial resistance, creative solutions to maintain the adequacy of antimicrobial therapy in patients with serious infections by optimizing the utilization of currently available resources are of paramount importance. Fluoroquinolones are among the most effective oral antimicrobial agents for the treatment of acute pyelonephritis due to their excellent oral bioavailability and high urinary and tissue concentrations (15). However, increasing rates of fluoroquinolone resistance have complicated the management of acute pyelonephritis, particularly in outpatient settings. The current national guidelines recommend using oral fluoroquinolones in patients with acute pyelonephritis not requiring hospitalization in settings with a <10% overall prevalence of fluoroquinolone resistance among uropathogens (2). However, geographical locations with a <10% overall prevalence of resistance to fluoroquinolones hardly exist these days in North America or elsewhere (3–8). This forces the administration of intravenous ceftriaxone to most patients with acute pyelonephritis prior to discharge from the emergency room or other outpatient settings on oral fluoroquinolones (2). The FQRS presents health care providers with opportunities to improve antimicrobial utilization in acute pyelonephritis by categorizing patients on the basis of the predicted probability of fluoroquinolone resistance into 3 groups: low, moderate, and high risk (Fig. 3). These categories are based on the performance characteristics of the modified FQRS using cutoff points of ≥2 and ≥3 to optimize the NPV and positive predictive value (PPV), respectively. Patients with an FQRS of 0 and 1 have a 6% and 10% predicted probability of fluoroquinolone resistance, respectively (Fig. 2), corresponding to a NPV of 91% for an FQRS of ≥2. Since the predicted rate of resistance to fluoroquinolones is <10% in this low-risk group, patients with an FQRS of <2 are eligible for empirical oral fluoroquinolone therapy without the need for administration of intravenous ceftriaxone, in concordance with the national management guidelines (2). Application of the FQRS may simplify antimicrobial management in the majority of patients with acute pyelonephritis, since 60% of patients in the current study met the criteria for the low-risk category (no prior fluoroquinolone use, outpatient procedures, or residence at a skilled nursing facility). The management of patients in the moderate-risk group (FQRS = 2) remains unchanged from current guidelines since the predicted probability of fluoroquinolone resistance in this group is comparable to the overall resistance rate in the study (19% and 23%, respectively). The FQRS also identifies patients in whom fluoroquinolone therapy is most likely inadequate. If fluoroquinolones were initially prescribed to patients with acute pyelonephritis in the high-risk category, the majority would undergo revision of antimicrobial therapy when in vitro antimicrobial susceptibility testing results become available, given a PPV of 56% for an FQRS of ≥3. Unfortunately, current data are insufficient to recommend alternative oral antimicrobial agents in this group. It would be valuable to develop clinical scores to predict resistance to other oral antimicrobial agents with a high efficacy in the treatment of cUTI, e.g., trimethoprim-sulfamethoxazole, in future studies. That would help identify other potential oral options for patients with a high risk of fluoroquinolone resistance and may diversify antimicrobial prescriptions overall.
FIG 3.
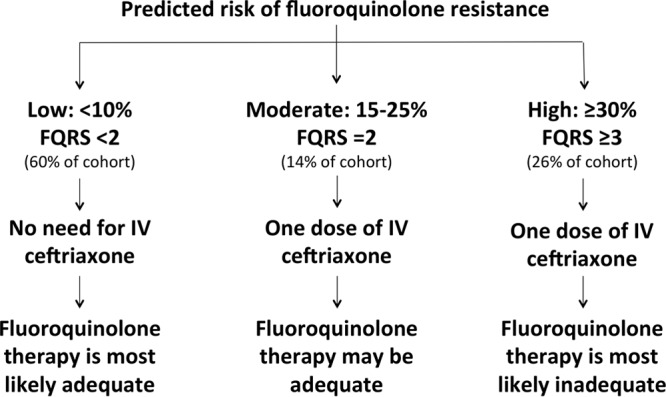
Proposed algorithm for application of the modified FQRS in the outpatient management of acute pyelonephritis. FQRS, fluoroquinolone resistance score; IV, intravenous.
In the inpatient setting, utilization of the FQRS may allow the early discharge of hospitalized patients with cUTI who demonstrate clinical improvement prior to the availability of urine culture and in vitro antimicrobial susceptibility testing results. If used properly, this may confer significant cost savings for the health care system. However, the FQRS should not replace in vitro antimicrobial susceptibility testing. Antimicrobial therapy should be adjusted, if needed, on the basis of organism identification and in vitro antimicrobial susceptibility testing results.
In addition to its clinical applications, the FQRS may also be used in clinical research studies. Increasing rates of fluoroquinolone resistance limited the utility of fluoroquinolones as comparators to a new antimicrobial agent for cUTI in a recent clinical trial (16). Exclusion of patients with a high predicted risk of fluoroquinolone resistance, such as those with an FQRS of ≥3, would make comparisons between fluoroquinolones and new antimicrobial agents more meaningful.
This study has strengths and limitations. The identification of cases and risk factors for antimicrobial resistance was facilitated by having a centralized microbiology laboratory and access to both inpatient and outpatient records from several affiliated medical facilities in the region. The patients' prior antimicrobial treatment histories were available, for the most part, in electronic medical records, including medication administration records and electronic prescriptions. Utilization of the FRQS in settings that lack access to prior records would heavily depend on patients' recall of their prior use of antimicrobial agents and providers' efforts to ascertain medical records. In addition, the case-control section of the current study was not powered to demonstrate an association between fluoroquinolone resistance and each individual component of the FQRS. Rather, it was focused on examining the risk of fluoroquinolone resistance on the basis of fluoroquinolone exposure within the past 12 months. Four of the variables in the FQRS were identified as independent risk factors for fluoroquinolone resistance in the current study. The role of diabetes mellitus in predicting fluoroquinolone resistance remains unclear on the basis of the current results. Moreover, although the impact of gender on antimicrobial resistance was statistically significant, it warrants further explanations in populations with different antimicrobial consumption and dietary habits.
In summary, the modified FQRS stratifies patients with cUTI on the basis of the predicted probability of fluoroquinolone resistance with very good discrimination. Application of the modified FQRS may simplify antimicrobial management in patients with acute pyelonephritis and improve the design of future clinical trials.
MATERIALS AND METHODS
Setting.
The study was conducted at Palmetto Health inpatient and outpatient facilities in central South Carolina, USA. These facilities included three large community hospitals with a combined capacity of nearly 1,200 licensed beds, three emergency rooms, several affiliated urgent treatment centers, and ambulatory care clinics. The Institutional Review Board at Palmetto Health approved the study.
Definitions.
Patients were considered to have cUTI if they had urinary symptoms or signs and monomicrobial growth of Gram-negative bacilli in urine cultures in the presence of complications such as obstructive uropathy, indwelling urinary catheters, urinary retention from neurologic disease, or bladder outlet obstruction (17, 18). Patients with clinical manifestations of acute pyelonephritis (temperature of >38°C, unilateral flank pain, or costovertebral angle tenderness) and monomicrobial growth of Gram-negative bacilli in urine cultures were also included among those with cUTI (19). Outpatient procedures included both invasive procedures (e.g., bladder, colon, and prostate biopsies) and noninvasive procedures (e.g., colonoscopy, cystoscopy, and duodenoscopy) (10). Fluoroquinolone-nonsusceptible (FQ-NS) isolates were defined as Gram-negative bacilli that had intermediate in vitro susceptibility to ciprofloxacin (MIC = 2 μg/ml) or were resistant in vitro to ciprofloxacin (MIC ≥ 4 μg/ml) according to Clinical and Laboratory Standards Institute guidelines.
Case ascertainment.
All adult patients ≥18 years old with growth of Gram-negative bacilli in urine cultures from 1 April 2015 to 31 July 2015 were prospectively identified through a real-time alert system which interfaced with the central microbiology laboratory at Palmetto Health (n = 582). Patients with polymicrobial growth of bacteria in urine cultures (n = 56) or recurrent episodes of positive urine cultures during the study period (n = 11) and those with positive urine cultures who did not meet the predefined criteria for cUTI (n = 277) were excluded. Patients with recurrences and polymicrobial growth of bacteria in urine cultures were excluded to avoid enrolling the same patient as a case and a control. A total of 238 unique adults with cUTI were included in the study. Demographic, microbiology, and clinical data, including potential risk factors for antimicrobial resistance, were collected from electronic medical records. The receipt of fluoroquinolones in the 12 months preceding the cUTI was ascertained from medication administration records, electronic prescriptions, and clinical notes from current and prior hospitalizations and visits to affiliated emergency rooms or other ambulatory care settings.
Statistical analysis.
Logistic regression was used to examine the association between fluoroquinolone resistance and fluoroquinolone exposure up to 12 months prior to the index cUTI in a case-control design. For the simplification of statistical analysis, Gram-negative bacilli that were resistant or intermediate to ciprofloxacin in vitro were analyzed as FQ-NS isolates. Demographics and clinical variables were collected for patients with cUTI due to FQ-NS (cases) and fluoroquinolone-susceptible (FQ-S) Gram-negative bacilli (controls). Variables included age, sex, ethnicity, diabetes mellitus, cancer, nosocomial acquisition, recent hospitalization within 3 months, residence in a skilled nursing facility, and outpatient procedures within 1 month. Fluoroquinolone use within 12 months prior to the cUTI was analyzed as a categorical variable: none and within 3 months and within 3 to 12 months of the time of the index infection. The individual components of the FQRS, in addition to other variables associated with fluoroquinolone resistance in the univariate logistic regression model with a P value of <0.05, if any, were included in the multivariate logistic regression model to identify independent risk factors for fluoroquinolone resistance. Adjusted odds ratios (aOR) with 95% confidence intervals (CI) were reported to demonstrate the strength of the association between each variable and fluoroquinolone resistance.
The area under a receiver operating characteristic curve (AUC) was used to quantify the discriminative ability of the FQRS using fluoroquinolone exposure up to 6 and 12 months prior to the cUTI. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for fluoroquinolone nonsusceptibility were calculated from the receiver operating characteristic curve for the best cutoff points in the FQRS.
JMP Pro (version 12.1; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. The level of significance for statistical testing was defined as a P value of <0.05 (2-sided) unless otherwise specified.
ACKNOWLEDGMENTS
We thank the Palmetto Health Antimicrobial Stewardship and Support Team and Microbiology Laboratory in South Carolina, USA, for their help in facilitating the conduct of this study.
A.S. and M.N.A.-H. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
A.S., J.A.J., J.K., and H.A. have no conflicts of interest. M.N.A-H. is on the Continuing Medical Education Steering Committee for Rockpointe Corporation, Columbia, MD, USA. P.B.B. is on the Research Advisory Board for Actavis Pharmaceuticals (now Allergan), has received research funding from Actavis Pharmaceuticals (now Allergan), and is on the Continuing Medical Education Steering Committee for Rockpointe Corporation, Columbia, MD, USA.
REFERENCES
- 1.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 3.Prais D, Straussberg R, Avitzur Y, Nussinovitch M, Harel L, Amir J. 2003. Bacterial susceptibility to oral antibiotics in community-acquired urinary tract infection. Arch Dis Child 88:215–218. doi: 10.1136/adc.88.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. 2009. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1998-2007. J Antimicrob Chemother 64:169–174. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigmon M, Bookstaver BP, Kohn J, Albrecht H, Al-Hasan MN. 2015. Impact of fluoroquinolone resistance on community-onset gram-negative bloodstream infections. Clin Microbiol Infect 21:843–849. doi: 10.1016/j.cmi.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez GV, Master RN, Karlowsky JA, Bordon JM. 2012. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 56:2181–2183. doi: 10.1128/AAC.06060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peirano G, Pitout JD. 2014. Fluoroquinolone-resistant Escherichia coli sequence type 131 isolates causing bloodstream infections in a Canadian region with a centralized laboratory system: rapid emergence of the H30-Rx sublineage. Antimicrob Agents Chemother 58:2699–2703. doi: 10.1128/AAC.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawker JI, Smith S, Smith GE, Morbey R, Johnson AP, Fleming DM, Shallcross L, Hayward AC. 2014. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 69:3423–3430. doi: 10.1093/jac/dku291. [DOI] [PubMed] [Google Scholar]
- 10.Dan S, Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2016. Prediction of fluoroquinolone resistance in Gram-negative bacteria causing bloodstream infections. Antimicrob Agents Chemother 60:2265–2272. doi: 10.1128/AAC.02728-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2016. IDWeek, New Orleans, LA, USA, abstr 57597. [Google Scholar]
- 12.Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU, Weintraub A, Nord CE, Savell A, Hu Y, Coates AR, Hubank M, Spratt DA, Wilson M, Keijser BJ, Crielaard W. 2015. Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. mBio 6:e01693-15. doi: 10.1128/mBio.01693-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. 26 July 2016. FDA updates warnings for fluoroquinolone antibiotics. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513183.htm Accessed 11 October 2016. [Google Scholar]
- 14.Grigoryan L, Trautner BW, Gupta K. 2014. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 312:1677–1684. doi: 10.1001/jama.2014.12842. [DOI] [PubMed] [Google Scholar]
- 15.Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 283:1583–1590. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 16.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. 2015. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0. [DOI] [PubMed] [Google Scholar]
- 17.Johansen TE, Botto H, Cek M, Grabe M, Tenke P, Wagenlehner FM, Naber KG. 2011. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents 38:S64–S70. doi: 10.1016/j.ijantimicag.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Levison ME, Kaye D. 2013. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep 15:109–115. doi: 10.1007/s11908-013-0315-7. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Drug Evaluation and Research. 2012. Complicated urinary tract infections and pyelonephritis: developing antimicrobial drugs for treatment. Guidance for industry. U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070981.pdf Accessed 11 October 2016. [Google Scholar]


