ABSTRACT
Unlike vancomycin trough concentrations, data on the utility of vancomycin pharmacokinetic (PK) parameters, namely, the area under the concentration-time curve from 0 to 24 h (AUC0–24), in predicting acute kidney injury (AKI) are limited. Our aim was to investigate this relationship in patients receiving vancomycin therapy for methicillin-resistant Staphylococcus aureus bacteremia (MRSA-B). A single-center retrospective observational cohort study involving 127 consecutive MRSA-B patients was conducted to examine the incidence of AKI (defined as serum creatinine of ≥0.5 mg/liter and a 50% increase from baseline) and vancomycin exposure parameters associated with nephrotoxicity. Bayesian estimation was used to predict individual vancomycin AUC0–24. All patients received vancomycin monotherapy for a minimum of 14 days following the diagnosis of MRSA-B. AKI was observed in 15.7% of patients (20/127). Clinical characteristics were similar between patients with and without AKI. At steady state, higher vancomycin trough concentrations were associated with AKI (17.2 mg/liter versus 13.1 mg/liter; P = 0.003). A vancomycin AUC0–24 threshold for AKI of >563 mg · h/liter was detected by classification and regression tree (CART) analysis; patients with exposures above this threshold were significantly more likely to experience AKI than patients with lower vancomycin exposures (40% [8/20] versus 11.2% [12/107]; P = 0.002). This parameter remained an independent predictor of AKI on multivariate logistic regression (odds ratio [OR], 5.07; 95% confidence interval [CI], 1.57 to 16.29; P = 0.006) and was a better predictor of nephrotoxicity than vancomycin trough concentrations. Overall, AKI is associated with higher vancomycin exposure as measured by AUC0–24. These results suggest that individualized patient dosing may be possible with dose modifications directed toward established pharmacodynamic targets while balancing AKI risks.
KEYWORDS: vancomycin, trough concentrations, pharmacokinetic, pharmacodynamics, AUC0–24, MRSA, nephrotoxicity, acute kidney injury, Bayesian estimation
INTRODUCTION
Vancomycin remains an important first line antimicrobial for the treatment of methicillin-resistant Staphylococcus aureus bacteremia (MRSA-B) (1–3). Current dosing strategies recommend a trough concentration of between 15 and 20 mg/liter to achieve the pharmacodynamic (PD) target (the ratio of the 24-h area under the concentration curve to the MIC [AUC0–24/MIC]) required for treatment efficacy of >400 mg · h/liter, provided the MIC of the MRSA isolate is ≤1 mg/liter (4, 5). This approach has been criticized, as trough concentrations underestimate the true AUC by about 25% on average, with such significant interpatient variability that a single patient may end up being either over- or underexposed (6). The consequence of underexposure is treatment failure, while overexposure may lead to increased toxicity, especially acute kidney injury (AKI) (7, 8).
The incidence of vancomycin associated AKI ranges from 12 to 43% according to current literature (5, 9). Although associated with longer lengths of hospital stay and possibly increased mortality, vancomycin-associated AKI is usually reversible and rarely requires dialysis (10, 11). Several risk factors, including higher vancomycin doses (4 g/day), higher initial trough concentrations (≥15 mg/liter), prolonged duration of therapy, and high vancomycin exposure (i.e., AUC0–24), have been associated with an increased risk for AKI (11, 12). Despite these risk factors, a direct correlation between vancomycin exposure (i.e., the AUC0–24) and AKI is lacking. In a population pharmacokinetic (PK) modeling and simulation study, an AUC0–24 value of 700 mg · h/liter at steady state was proposed as the threshold for nephrotoxicity. However, this has not been extensively studied or validated in a clinical setting (6). We therefore undertook this study to objectively determine a threshold for vancomycin AUC0–24 and increased risk of nephrotoxicity or AKI.
RESULTS
Demographics and outcomes.
Over the 6-year study period, 127 patients were identified that met the inclusion criteria. Of note, all patients were treated for MRSA-B for 2 to 6 weeks with vancomycin monotherapy based on source of bacteremia.
AKI (within the first 2 weeks of therapy) was observed in 15.7% of patients (20/127), with over half of episodes occurring in male patients over 70 years of age. Of note, no cases of AKI were observed after 2 weeks of therapy in patients prescribed longer durations of treatment. The patient characteristics of those who developed AKI and those who did not are shown in Table 1. The median weight of patients with AKI was 73.5 kg (interquartile range [IQR], 54 to 110 kg), and the patients had similar medical comorbidities. There was no difference in the median APACHE II scores, location at bacteremia (intensive care unit [ICU] or ward), or source of bacteremia compared to those in patients without AKI. In contrast, concomitant nephrotoxic agents (see footnote c to Table 1 for agents considered nephrotoxins) were significantly associated with an increased risk of AKI (18/20 [90%] versus 53/107 [49.5%]; P = 0.001). The three most frequent concomitantly prescribed nephrotoxins were diuretics (n = 18), angiotensin-converting enzyme inhibitors (n = 15), and aminoglycosides (n = 39). In all patients receiving concomitant aminoglycosides, these were prescribed only empirically, prior to the definitive bacteremic diagnosis, before being discontinued in all cases (maximum aminoglycoside doses received, 2).
TABLE 1.
Patient characteristics grouped by development of AKIa
| Variable | Value for patients with: |
P value | |
|---|---|---|---|
| AKI (n = 20) | No AKI (n = 107) | ||
| Age ≥ 70 yrs | 10 (50.0) | 44 (41.4) | 0.441 |
| Male sex | 13 (65.0) | 75 (69.2) | 0.731 |
| Wt, kg (range) | 73.5 (54–110) | 74.7 (39–184) | 0.791 |
| Comorbidities | |||
| Heart disease | 3 (15.0) | 25 (23.1) | 0.576 |
| Diabetes | 7 (35.0) | 36 (33.3) | 0.885 |
| Malignancy | 6 (30.0) | 30 (27.8) | 0.839 |
| Liver disease | 2 (10.0) | 7 (6.5) | 0.779 |
| Lung disease | 2 (10.0) | 14 (12.1) | 0.713 |
| Immunosuppression | 1 (5.0) | 21 (18.7) | 0.193 |
| Charlson weighted index ≥ 3 | 7 (35.0) | 47 (43.5) | 0.479 |
| Origin of bacteremia | 0.011 | ||
| Community onset | 8 (40.0) | 10 (9.3) | |
| Hospital onset | 12 (60.0) | 97 (90.6) | |
| Location at bacteremia | 0.447 | ||
| ICU | 6 (30.0) | 27 (25.2) | |
| Ward | 14 (70.0) | 80 (74.8) | |
| Source of infectionb | |||
| Low risk | 5 (25) | 33 (30.8) | 0.79 |
| Intermediate risk | 6 (30) | 44 (41.4) | 0.45 |
| High risk | 9 (45) | 30 (27.8) | 0.18 |
| APACHE II score (range) | 13.8 (0–37) | 11.2 (1–30) | 0.224 |
| Daily vancomycin dose, mg (median)* | 1,789 (750–4,000) | 1,880 (500–4,000) | 0.59 |
| Vancomycin AUC0–24 (median) | 536.36 | 440.33 | 0.02 |
| Vancomycin trough (median)* | 17.2 (9–36) | 13.1 (4–31) | 0.003 |
| Trough ≥ 15 mg/liter | 12 (60) | 38 (35.5) | 0.036 |
| Cmax (median) | 27.4 (24.7–32.2) | 24.0 (23.4–25.4) | 0.005 |
| Cmin (median) | 15.6 (13.9–19.0) | 13.1 (12.1–13.9) | 0.004 |
| AUC0–24 ≥ 563 | 8 (40) | 12 (11.2) | 0.002 |
| Concomitant use of nephrotoxinsc | 18 (90.0) | 53 (49.5) | 0.001 |
| Outcomes | |||
| Overall 30-day mortality | 6 (30.0) | 16 (15) | 0.098 |
Data are presented as numbers (percentages) of cases unless otherwise stated. Asterisks indicate vancomycin parameters determined at steady state. Cmin, minimum concentration of drug.
Source of bacteremia grouped into one of three groups based on overall mortality risk: low risk, line-related bacteremia (n = 35) and other sources (n = 3); intermediate risk, bone and joint (n = 14), skin and soft tissue infections (n = 20), deep abscess (n = 4), and no identified focus (n = 12); high risk, infective endocarditis (n = 12), pneumonia (n = 19), abdominal sources (n = 6), and nonendocarditis vascular sources (n = 2).
Any of the following agents were regarded as nephrotoxins: aminoglycosides, amphotericin B, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, colistin, contrast dye, cyclosporine, cisplatin, diuretics, nonsteroidal anti-inflammatory drugs, tacrolimus, and vasopressor medications.
Vancomycin pharmacokinetics and AKI.
The mean intravenous vancomycin dose at steady state was 2,000 mg/day (range, 500 to 4,000 mg/day) administered over 2 to 4 h, which resulted in a median trough concentration of 13.1 mg/liter (range 3.5 to 35.7 mg/liter) at steady state. Vancomycin dosing frequencies varied between 12 (93% of patients) and 24 h; no patients received 6- or 8-h dosing.
A positive correlation between vancomycin trough concentration and AUC0–24 was detected. However, a substantial proportion of patients (26.7%) achieved an AUC0–24 of >400 while demonstrating trough levels below current recommended trough targets (depicted by the open squares in Fig. 1). The median trough concentrations were significantly higher (trough, 17.2 mg/liter; IQR, 9 to 36 mg/liter) in patients who developed AKI than in those that did not (trough, 13.1 mg/liter; IQR, 4 to 31 mg/liter; P = 0.003). When examining trough strata, we determined that patients with trough concentrations greater >15 mg/liter were significantly more likely to develop AKI than patients with levels below 15 mg/liter (60% versus 35.5%; P = 0.036). The median AUC0–24 was also significantly higher (510 mg · h/liter) in patients with AKI than in those that did not develop AKI (436 mg · h/liter; P < 0.001) (Fig. 2).
FIG 1.
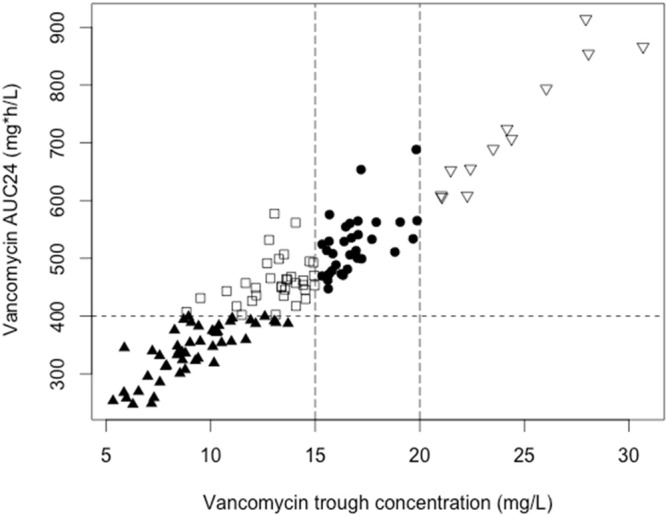
Scatterplot of vancomycin measured trough concentrations and AUC0–24. Vertical and horizontal lines represent current recommended vancomycin trough and AUC24 targets (Spearman rho, 0.923) for clinical efficacy. Shapes represent whether patients achieved AUC0–24 and/or appropriate trough thresholds (i.e., open squares represent patient measurements which achieved AUC0–24 targets but not trough targets).
FIG 2.
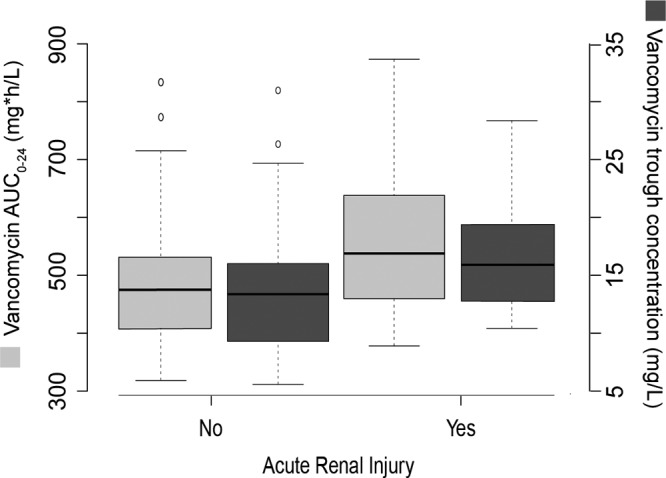
Box plots of vancomycin AUC0–24 and trough concentrations. Vancomycin AUC0–24 (light gray boxes) on y axis and trough concentrations (dark gray boxes) on the secondary axis are stratified by risk of acute kidney injury. Median and interquartile ranges are represented by horizontal lines within boxes and whiskers (representing minimum and maximum values), respectively. Circles represent outliers.
A vancomycin pharmacokinetic (PK) threshold at steady state for AKI of AUC0–24 of >563 mg · h/liter was detected by classification and regression tree (CART); this AUC0–24 cutoff was significantly associated with nephrotoxicity on univariate analysis (P = 0.002). An exposure-toxicity curve was observed, with the odds of nephrotoxicity increasing by 0.2% for every unit increase of AUC0–24 (odds ratio [OR], 1.002; 95% confidence interval [CI], 1.001 to 1.004; P = 0.021) when AUC0–24 was examined as a continuous variable (Fig. 3).
FIG 3.
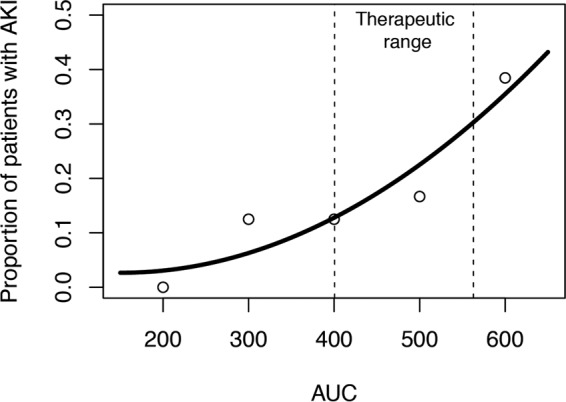
Vancomycin exposure-toxicity curve. Relationship between risk of acute kidney injury (AKI) and vancomycin AUC0–24 is represented by best-fit curve (open circles represented raw data at various AUC0–24 strata). The CART threshold is reflected by the vertical dashed lines and translates into a therapeutic vancomycin AUC0–24 range of 400 to 563.
On multivariable logistic regression analysis, independent predictors of AKI included concomitant use of nephrotoxins (OR, 7.71; 95% CI, 1.62 to 35.76; P = 0.009) and vancomycin overexposure (AUC0–24) greater than 563 mg · h/liter (OR, 5.07; 95% CI, 1.57 to 16.29; P = 0.006) (Table 2). Of note, despite the correlation observed between trough and AUC0–24, only the latter remained significant on multivariate analysis (adjusted OR [aOR], 1.004; 95% CI, 1.001 to 1.009; P = 0.004).
TABLE 2.
Predictors of acute kidney injury on multivariate analysis
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Concomitant nephrotoxins | 7.71 (1.62–35.76) | 0.009 |
| Vancomycin AUC0–24 > 563 mg · h/liter | 5.07 (1.57–16.29) | 0.006 |
The majority (85% [17/20]) of patients had reversible AKI, with the remaining 3 patients progressing to long-term dialysis. Despite the reversible nature of the associated AKI, patients who developed AKI had an increased chance of death at 30 days compared to that of patients who did not develop AKI (30% [6/20] versus 15% [16/107]; P = 0.098) (Table 1).
DISCUSSION
Despite the availability of other antimicrobials for treatment of MRSA infections, vancomycin still remains the agent of choice (3). However, current dosing regimens and targets (vancomycin trough concentrations) are an area of great controversy with respect to efficacy. This is partly because of evidence showing a poor correlation between established pharmacodynamic (AUC0–24) target attainment and measured trough concentrations (6, 8). Similar to others, a substantial number of our patients achieved an AUC0–24 of >400 despite having vancomycin trough concentrations below guideline recommendations (≤15 mg/liter) (6). Of note, we observed that clinicians increased the total vancomycin dose prescribed in these patients, resulting in higher exposures and increasing the risk of AKI. Therefore, ideally vancomycin dosing should be directly tailored toward established AUC0–24 targets rather than trough concentration ranges (6, 13).
One advantage of this approach is that dosing is aimed at the pharmacodynamic parameter linked with treatment efficacy and improved clinical outcomes rather than a correlate (i.e., trough concentration) (13). Consequently, levels below this threshold could lead to treatment failures in moderate- and high-risk MRSA infections. Several obstacles remain, however, to individualized dosing becoming the standard of care. These include the availability of appropriate software tools, the level of expertise required to calculate and verify Bayesian estimations, and the lack of a well-established upper limit of an AUC0–24 “therapeutic range” which is clearly linked with toxicity.
In the current study, we have determined a vancomycin AUC0–24 (albeit refined for overweight patients), which is independently associated with nephrotoxicity (>563 mg · h/liter) and represents the upper limit of a possible vancomycin therapeutic range. This cutoff is lower than previous predicted thresholds of between 648 and 1,300 mg · h/liter (6, 7, 14). Possible explanations for these differences include the patient subsets studied (empirical therapy versus definitive MRSA infections) and models used for AUC0–24 estimations based on subtle differences between models (1 versus 2 compartment). Although we utilized a validated pharmacokinetic software tool for such calculations, the intermodel variability is unknown but is likely to differ, especially when covariates such as creatinine clearance (CLCR) (a measure that can fluctuate greatly depending on the equation used) are included in the model. Nevertheless, we observed a definitive dose-toxicity curve with AUC0–24 (with a 0.2% increase risk of nephrotoxicity for every unit rise in AUC0–24) but acknowledge that the optimal upper limit for any therapeutic range requires further study (15).
Controversy remains regarding whether vancomycin has a direct toxic effect or whether the higher vancomycin exposure is secondary to renal impairment in the first place. Nephrotoxicity in the setting of vancomycin use is likely to occur in the context of several other factors, including concomitant nephrotoxins and severity of illness which is consistently associated with AKI (15, 16). Even so, like others, we found that when adjusted for concomitant nephrotoxin use, vancomycin AUC0–24 remains a strong independent predictor of nephrotoxicity (aOR, 1.004; 95% CI, 1.001 to 1.009; P = 0.004) (12, 17).
It is well known that the AUC0–24/MIC ratio is a PD parameter associated with treatment success (13, 17). This PD parameter is dependent on MIC value, which is higher with Etest MIC-based results than with broth microdilution MIC results (18–20). Overall, our data indicate that individualized dosing is feasible, with clinicians able to optimize vancomycin doses to attain appropriate PD targets when MIC data become available from the microbiological laboratory. An additional benefit of such an approach would be better refinement of local empirical usage guidelines for MRSA infections, combining individual PK with local antimicrobial MIC surveillance data.
There are several limitations to our retrospective cohort study. Of note, our results are not applicable to pediatric patients or patients with preexisting renal impairment requiring dialysis, as these patients were excluded secondary to the lack of standardized methodology in calculating renal clearance. A more accurate AUC0–24 could have been obtained when using the RIFLE criteria to define acute kidney injury. These criteria were unable to be applied, as urine output was not documented in the medical records for the majority of patients (21). A recent meta-analysis demonstrated a significantly lower risk of nephrotoxicity (relative risk, 0.6; 95% CI, 0.4 to 0.9; P = 0.02) in patients treated with continuous infusion than for those treated standard intermittent administration (22). A possible explanation for this observation is that patients on continuous infusion are more likely to achieve reliable exposures when doses are adjusted to concentrations of 20 mg/liter (i.e., 20 mg/liter over 24 h results in an AUC0–24 of 480 mg · h/liter, which is below the nephrotoxicity threshold). Alternatively, renal toxicity may be secondary to vancomycin pharmacokinetics (e.g., maximum concentration of drug [Cmax]), and therefore, we would suggest confirming our results in patients on continuous infusion prior to wide-scale adoption.
Estimates were made using single trough concentration taken within a dosing interval. Based on previous reports, it is likely that greater accuracy would have been obtained if AUC0–24 estimations were based on increased sampling within the same dosing interval (23). However, whether this would have resulted in a substantial difference in our findings is unknown. Similarly, optimal timing of blood samples for effective Bayesian estimation is unclear but is unlikely to be the same for all patients and requires further review (6, 24).
In conclusion, AKI is associated with higher vancomycin exposure as measured by AUC0–24. We have been able to define the upper limit of a vancomycin therapeutic range, with a vancomycin AUC0–24 of >563 mg · h/liter associated with significantly increased risks of toxicity. The establishment of a possible therapeutic exposure range allows for individualized patient dosing and dose modifications directed toward pharmacodynamic targets to achieve treatment success in conjunction with balancing AKI risks.
MATERIALS AND METHODS
Study population and vancomycin pharmacokinetics.
All adult patients (>18 years old) admitted to Liverpool Hospital, Sydney, Australia, over a 6-year period (2006 to 2012) with MRSA-B who met the inclusion criteria were enrolled into this retrospective observational cohort study. At our hospital, most patients with sepsis receive empirical therapy with a β-lactam in combination with an aminoglycoside or vancomycin based on presumptive diagnosis. Once a diagnosis is established (generally within 48 h), antibiotics are rationalized to cover the causative pathogen. To be included in this study, patients were required to have received ≥7 days of vancomycin monotherapy with assessable drug charts (documenting timing of doses) and a minimum of 2 timed drug concentrations following MRSA-B diagnosis. The treating clinician in conjunction with infectious diseases advice ultimately determined the duration of vancomycin therapy; no patients received less than 14 days of therapy, all of which occurred in the setting of uncomplicated bacteremia. Only the first episode was included in the study for patients with multiple MRSA-B episodes over the study period. The outcome of interest was AKI (within in the first 2 weeks of therapy), defined as serum creatinine of ≥0.5 mg/liter and a 50% increase from baseline on 2 or more consecutive measurements (4, 8, 9). Local institutional ethics approval was obtained.
Vancomycin concentrations were determined using a commercially available competitive enzyme immunoassay, which has a quantification range between 1.7 mg/liter and 80 mg/liter. No plasma sample included in the study returned a concentration outside this range.
A maximum a posteriori (MAP) Bayesian estimation, using a priori pharmacokinetic parameters of a previous population pharmacokinetic model, was used to predict vancomycin AUC0–24 at 72 h (i.e., which predominantly corresponds with steady state; henceforth AUC0–24) using dose individualization software (DoseMe; DoseMe Pty Ltd., Brisbane, Australia) (5, 25). This one-compartment model was used to determine the AUC0–24 from a vancomycin trough concentration obtained at steady state (i.e., 72 h). Vancomycin clearance (CL) and volume of distribution (V) were determined by the software based on the equations CL (liters/hour) = 1.08 × CLCR (Cockcroft-Gault) and V (liters) = 0.98 × TBW, where CLCR is creatinine clearance and TBW is total body weight, with coefficients of variance of 28.16% and 37.15% for CL and V, respectively. The mean values obtained for vancomycin clearance and volume of distribution in this study were 1.19 ml/min/kg of body weight and 1.05 liters/kg.
Patients were excluded from the study if no weight was recorded in the medical records or patients required dialysis for chronic renal impairment prior to admission. Creatinine clearance was derived using the Cockcroft-Gault equation with adjusted body weight (ideal body weight + 0.4 × [actual − ideal body weight]) used for overweight patients (weight > 100 kg).
Statistical analysis.
Categorical variables were compared using the χ2 test or Fisher's exact test, and continuous variables were compared by the Student t test or Mann-Whitney U test. Classification and regression tree (CART) analysis (which uses decision tree algorithms to determine the best if-then split conditions that accurately predict an outcome of interest) was used to identify vancomycin AUC0–24 values for AKI. Vancomycin overexposure was subsequently defined as the first dichotomous value obtained on CART analysis, which was associated with development of AKI. A P value of <0.05 was considered significant. Multivariable backward stepwise logistic regression analysis was performed to identify predictors of AKI. Adjusted odds ratio calculation was done to determine relation between vancomycin PK parameters and AKI (adjusted for concomitant nephrotoxins and onset of bacteremia). The nonparametric, 2-tailed Spearman rank correlation coefficient was calculated to determine the statistical dependence between AUC0–24 and trough concentration. All calculations were computed using SPSS (version 23.0) and CART software (Salford Predictive Modeler version 7; Salford Systems, San Diego, CA).
ACKNOWLEDGMENTS
We acknowledge Robert McLeay (DoseMe Pty Ltd., Brisbane, Australia) for assistance with data analysis.
No funding was received for this study.
All authors report no conflict of interest related to this study.
REFERENCES
- 1.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak JM, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 2.Gould FK, Brindle R, Chadwick PR, Fraise AP, Hill S, Nathwani D, Ridgway GL, Spry MJ, Warren RE. 2009. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother 63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 3.Therapeutic Guidelines Ltd. Antibiotic eTG version 15. Severe sepsis and septic shock: Staphylococcus aureus. Accessed 15 June 2015 https://tgldcdp.tg.org.au.acs.hcn.com.au/severe-sepsis. [Google Scholar]
- 4.Wong-Beringer A, Joo J, Tse E, Beringer P. 2011. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents 37:95–101. doi: 10.1016/j.ijantimicag.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 6.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodise TP, Patel N, Lomaestro BM, Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 8.Prybylski JP. 2015. Vancomycin trough concentration as a predictor of clinical outcomes in patients with Staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy 35:889–898. doi: 10.1002/phar.1638. [DOI] [PubMed] [Google Scholar]
- 9.van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations: a literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 11.Costa e Silva VT, Marçal LJ, Burdmann EA. 2014. Risk factors for vancomycin nephrotoxicity: still a matter of debate. Crit Care Med 42:2635–2636. doi: 10.1097/CCM.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 62:168–171. doi: 10.1093/jac/dkn080. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I, Itoh H, Hiramatsu K, Takeyama M, Kadota JI. 2012. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 58:308–312. [DOI] [PubMed] [Google Scholar]
- 16.Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, Romanowski G, Vo T, Bradley J. 2015. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Ped Infect Dis Soc 4:e109–. doi: 10.1093/jpids/piu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant staphylococcus aureus infections. Arch Intern Med 166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh N, Chavada R, Maley M, Hal SJ. 2014. Impact of source of infection and vancomycin AUC0–24/MICBMD targets on treatment failure in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect 20:O1098–O1105. doi: 10.1111/1469-0691.12695. [DOI] [PubMed] [Google Scholar]
- 19.Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, McNutt LA. 2014. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 59:666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 20.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MVN, Anderson TL, Roberts SA, Warren SJC, Gao W, Howden BP, Johnson PDR. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes JA, Jorge S. 2012. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. J Clin Kidney 6:8–14. doi: 10.1093/ckj/sfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24. doi: 10.1093/jac/dkr442. [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, Roberts JA. 2014. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med 42:2527–2536. doi: 10.1097/CCM.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 24.Hale CM, Seabury RW, Steele JM, Darko W, Miller CD. 12 April 2016. Are vancomycin trough concentrations of 15 to 20 mg/L associated with increased attainment of an AUC/MIC ≥ 400 in patients with presumed MRSA infection? J Pharm Pract. doi: 10.1177/0897190016642692. [DOI] [PubMed] [Google Scholar]
- 25.Buelga DS, del Mar Fernandez de Gatta M, Herrera EV, Dominguez-Gil A, Garcia MJ. 2005. Population pharmacokinetic analysis of vancomycin in patients with haematological malignancies. Antimicrob Agents Chemother 49:4934–4941. doi: 10.1128/AAC.49.12.4934-4941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


