ABSTRACT
Preclinical characterization of velpatasvir (VEL; GS-5816), an inhibitor of the hepatitis C virus (HCV) NS5A protein, demonstrated that it has favorable in vitro and in vivo properties, including potent antiviral activity against hepatitis C virus genotype 1 to 6 replicons, good metabolic stability, low systemic clearance, and adequate bioavailability and physicochemical properties, to warrant clinical evaluation. The phase 1 (first-in-human) study evaluated the safety, tolerability, and pharmacokinetics of VEL in healthy human subjects following administration of single and multiple (n = 7) once-daily ascending doses and of VEL in the presence and absence of food. Following administration of single and multiple doses, VEL was safe and well tolerated when administered at up to 450 mg and when administered with food. The pharmacokinetic behavior of VEL observed in humans was generally in agreement with that seen during preclinical characterization. Following administration of multiple doses, VEL trough concentrations were significantly greater than the protein-adjusted half-maximal (50%) effective concentration of VEL against HCV genotype 1 to 6 replicons at all evaluated doses greater than 5 mg. The pharmacokinetics of VEL were not significantly affected by administration with food. Collectively, the results of this study support the further clinical investigation of VEL administered once daily as part of a regimen with other pangenotypic direct-acting antivirals for the treatment of HCV infection.
KEYWORDS: velpatasvir, HCV NS5A inhibitor, pharmacokinetics, safety, clinical trials
INTRODUCTION
Hepatitis C virus (HCV) infection is a global health challenge, with an estimated 180 million individuals worldwide being infected (1). Of the patients with chronic HCV infection, as many as 20% are estimated to develop complications, including cirrhosis, end-stage liver disease, and hepatocellular carcinoma (2). Genotype 1 HCV infection predominates in Western countries; genotype 3 HCV infection accounts for 35% to 80% of chronic HCV infections in regions such as the Indian subcontinent, southeast Asia, and Australia (3–5). In the United States, almost three-quarters of the reported HCV-related deaths occurred in the baby boomer generation, with the median age of death being 57 years, which is approximately 20 years less than the average life span (6). Successful treatment of chronic HCV infection reduces the need for liver transplantation, the incidence of hepatocellular carcinoma, and overall mortality (7).
The nonstructural 5A (NS5A) protein of HCV has been identified to be a target to inhibit the replication of HCV. The NS5A protein does not possess enzymatic activity but does have a role in HCV replication, assembly of HCV virions, and modulation of the host immune response (8–10). No human homolog of HCV NS5A exists, which engenders specificity for drugs against this target. Several NS5A inhibitors have demonstrated potent antiviral potency and are used as part of multidrug regimens to treat HCV infection (11–14). However, the antiviral potencies of these agents vary considerably across HCV genotypes (15, 16). Velpatasvir (VEL; GS-5816) is an HCV NS5A inhibitor with potent activity against HCV genotypes 1 to 6 (Table 1) (17). A short-duration, well-tolerated, and highly effective pangenotypic treatment for HCV could have a major impact on the global prevalence and burden of HCV infection, particularly for individuals with HCV genotype 3 infection, and, more broadly, would eliminate the diagnostic burden of genotyping that is currently necessary for proper regimen selection and, thus, a barrier to the implementation of treatment in resource-limited regions. To support the clinical development of VEL, the preclinical pharmacokinetic (PK) properties and the findings of an initial clinical characterization of VEL were evaluated and are described herein.
TABLE 1.
VEL in vitro preclinical profile
| Parameter | In vitro result |
|---|---|
| In vitro potency as EC50 (nMa) for replicons of genotype: | |
| 1a | 0.014 |
| 1b | 0.016 |
| 2a | 0.005–0.016 |
| 2b | 0.002–0.006 |
| 3a | 0.004 |
| 4a | 0.009 |
| 4d | 0.004 |
| 5a | 0.021–0.054 |
| 6a | 0.006–0.009 |
| 6e | 0.130 |
| ADME parametersb | |
| Caco-2 cell permeability (at 1 μM) | |
| Papp A → B (cm/s [106]) | 2.1 |
| Papp B → A (cm/s [106]) | 11.7 |
| Efflux ratio (B → A/A → B) | 5.6 |
| Predicted hepatic clearance (liter/h/kg) | |
| Rat hepatic microsomes | 0.74 |
| Dog hepatic microsomes | 0.37 |
| Monkey hepatic microsomes | <0.17 |
| Human hepatic microsomes | <0.17 |
| Human cryopreserved hepatocytes | <0.07 |
| Plasma protein binding by equilibrium dialysis (% free drug) | |
| Rat | 0.22 |
| Dog | 0.19 |
| Monkey | 0.41 |
| Human | 0.30 |
| Physicochemical properties (solubility [mg/ml]) | |
| Water, pH 7 | 0.003 |
| Water, pH 2 | >1 |
| FaSSIF,c pH 6.5 | 0.010 |
| FeSSIF,d pH 5.0 | 0.18 |
Mean values from multiple experiments with the same laboratory replicon.
ADME, absorption, distribution, metabolism, and excretion; Papp, apparent permeability; A → B, forward; B → A, reverse.
Contains 3 mM sodium taurocholate and 0.75 mM lecithin in water (pH adjusted to 6.5) with 50 mM phosphate buffer.
Contains 15 mM sodium taurocholate and 3.75 mM lecithin in water (pH adjusted to 5.0) with 50 mM acetate buffer.
RESULTS
Preclinical characterization.
The in vitro characteristics of VEL, including PK and physicochemical properties, are summarized in Table 1. The in vitro profiling of VEL in Caco-2 cell monolayers indicated that it has a good absorption potential with a low propensity for efflux (Table 1). Velpatasvir was stable in human hepatic microsomes and cryopreserved hepatocytes, with low predicted human hepatic clearances of <0.17 and <0.07 liter/h/kg of body weight, respectively (Table 1), though slow metabolic turnover by CYP3A4, CYP2B6, and CYP2C8 was detected in phenotyping experiments. Velpatasvir was highly bound to plasma proteins across species (fraction unbound [fu] range, ∼0.2% to 0.4%) by equilibrium dialysis. Velpatasvir is a weak base with pH-dependent solubility, ranging from being practically insoluble to slightly soluble in a range of physiologically relevant media (0.003 mg/ml at pH 7, >1 mg/ml at pH 2 [Table 1]); the presence of intestinal bile salts and surfactants improved VEL solubility (solubility in fasted-state simulated intestinal fluid [FaSSIF], 0.010 mg/ml; solubility in fed-state simulated intestinal fluid [FeSSIF], 0.18 mg/ml; Table 1) (18, 19).
The in vivo disposition of VEL was assessed preclinically in Sprague-Dawley rats, beagle dogs, and cynomolgus monkeys. Table 2 presents the values of the PK parameters obtained following the intravenous and oral administration of VEL to each species. Consistent with the nonclinical data, in vivo results demonstrate that VEL has low systemic clearance (<30% of hepatic blood flow) in the species in which the PK of VEL were evaluated preclinically (20). The volume of distribution and bioavailability (F) were similar across species at approximately 1.4 to 1.6 liters/kg and approximately 25% to 30%, respectively (Table 2).
TABLE 2.
Preclinical in vivo PK
| Route of administration and species | Dose (mg/kg) | Mean ± SD value of the following PK parametersa: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AUCinf (nM · h) | CL (liter/h/kg) | Vd (liters/kg) | Cmax (nM) | Tmax (h) | t1/2 (h) | MRT (h) | % F | ||
| Intravenous | |||||||||
| Rat | 1 | 1,323 ± 239 | 0.94 ± 0.19 | 1.61 ± 0.32 | 2.25 ± 0.34 | 1.71 ± 0.18 | |||
| Dog | 0.2 | 1,001 ± 190 | 0.25 ± 0.05 | 1.43 ± 0.42 | 5.20 ± 0.40 | 5.60 ± 0.70 | |||
| Cynomolgus monkey | 0.5 | 2,114 ± 769 | 0.30 ± 0.09 | 1.60 ± 0.45 | 5.03 ± 2.49 | 5.39 ± 0.76 | |||
| Oral | |||||||||
| Rat | 2 | 708 ± 478 | 116 ± 53 | 1.0 ± 0.0 | 2.23 ± 0.43 | 27.7 ± 18.7 | |||
| Dog | 0.5 | 580 ± 369 | 71 ± 27 | 1.3 ± 0.6 | 5.47 ± 1.85 | 25.0 ± 12.9 | |||
| Cynomolgus monkey | 1 | 1,279 ± 121 | 157 ± 25 | 3.3 ± 1.2 | 5.22 ± 0.25 | 29.7 ± 2.8 | |||
Vd, volume of distribution; MRT, mean residence time. The other abbreviations are defined in the text.
The predicted PK behavior of VEL in humans was estimated on the basis of data from preclinical in vitro and in vivo studies and a multicompartment model. The observed systemic VEL clearance (CL) values of 0.94, 0.25, and 0.30 liter/h/kg in rats, dogs, and monkeys, respectively (Table 2), were consistent with the metabolic clearance in hepatic microsomes predicted for each species (Table 1). The systemic clearance of VEL was scaled across species as a function of body weight by utilizing the allometric equation Y = a · Wb, where Y is the pharmacokinetic parameter, W is body weight, a is the allometric coefficient, and b is the allometric exponent. On the basis of this allometric scaling and in vitro metabolic stability, the systemic clearance of VEL in humans was estimated to be approximately 0.12 liter/h/kg (21). As the volume of distribution and plasma protein binding were similar across species (Tables 1 and 2), the estimated volume of distribution in humans was projected to be similar to that in other species (approximately 1.5 liters/kg). At a dose of 50 mg, the steady-state trough concentration was estimated to be approximately 23 ng/ml, which, on the basis of in vitro potency data (17), would be sufficient to provide significant antiviral activity in human subjects.
Collectively, data from preclinical in vitro and in vivo studies and simulated data supported the evaluation of VEL in human subjects. Accordingly, a first-in-human study was conducted to evaluate the safety, tolerability, and pharmacokinetics of VEL in healthy, non-HCV-infected humans.
Clinical study. (i) Study population disposition and demographics.
A total of 84 subjects were enrolled into 6 cohorts across the study, and all subjects completed the study (Table 3). Of the 84 subjects enrolled in the study, 72 subjects received VEL and 12 subjects received a placebo. For those that received VEL in the single- and multiple-ascending-dose portion of the study, the majority of subjects were male (85%), white (53%), and non-Hispanic/Latino (58%), and the subjects had a mean age of 33 years (range, 20 to 44 years) and a mean body mass index (BMI) of 26 kg/m2 (range, 24 to 28 kg/m2). In the food-effect portion of the study, the majority of the subjects were male (58% and 92% for the cohorts fed a light meal and a high-fat, high-calorie meal, respectively), white (50% and 92% for the cohorts fed a light meal and a high-fat, high-calorie meal, respectively), and Hispanic/Latino (50% and 92% for the cohorts fed a light meal and a high-fat, high-calorie meal, respectively), the mean ages of the subjects in the cohorts fed a light meal and a high-fat, high-calorie meal were 34 and 33 years, respectively (range, 20 to 45 years), and the mean BMIs of the subjects in the cohorts fed a light meal and a high-fat, high-calorie meal were 26 and 25 kg/m2, respectively.
TABLE 3.
Demographic and baseline characteristics
| Characteristic | Value for subjects receiving the following VEL dosea: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 50 mg | 100 mg | 150 mg | 450 mg | 100 mg, fed (light meal) | 100 mg, fed (HFMb) | |
| % of male subjects | 83 | 75 | 92 | 75 | 83 | 92 | 58 | 92 |
| Mean (range) age (yr) | 30 (19–45) | 33 (20–43) | 32 (21–44) | 34 (20–45) | 33 (21–44) | 30 (22–40) | 34 (22–45) | 33 (22–43) |
| Mean (range) BMI (kg/m2) | 25 (20–30) | 28 (20–30) | 24 (20–27) | 25 (20–30) | 27 (20–30) | 26 (20–29) | 26 (21–30) | 25 (20–30) |
| % of subjects by race | ||||||||
| White | 33 | 67 | 50 | 71 | 50 | 67 | 50 | 92 |
| Black/African heritage | 67 | 33 | 50 | 25 | 50 | 33 | 42 | 8 |
| % of subjects by ethnicity | ||||||||
| Hispanic/Latino | 25 | 50 | 17 | 71 | 50 | 67 | 50 | 92 |
| Not Hispanic/Latino | 75 | 50 | 83 | 29 | 50 | 33 | 50 | 8 |
Twelve subjects were enrolled in each dose cohort, and all subjects in all dose cohorts completed the study.
HFM, high-fat, high-calorie meal.
(ii) Safety.
Single and multiple doses of VEL were generally well tolerated when it was administered in the fasted or fed state. A total of 21 of 84 subjects had an adverse event (AE) in the study. All AEs reported were grade 1 (mild) in severity, except for the AEs in 2 subjects receiving placebo, who experienced grade 2 (moderate) AEs of presyncope, orthostatic hypotension, viral infection, and pityriasis rosea. The most frequently reported AEs across all subjects were upper respiratory tract infection (5 subjects) and nausea (3 subjects). In the single- and multiple-ascending-dose portion of the study, there was no increase in the incidence of AEs with increasing doses of VEL; the treatment-emergent AEs related to study drug are presented in Table 4. There was no difference in the overall incidence of AEs when VEL was administered in the fasted or fed state.
TABLE 4.
Treatment-emergent AEs related to study drug by preferred term
| Preferred term | No. (%) of subjects receiving the following treatment with a treatment-emergent AE: |
|||||
|---|---|---|---|---|---|---|
| 5 mg (n = 12) | 50 mg (n = 12) | 100 mg (n = 24) | 150 mg (n = 12) | 450 mg (n = 12) | Placebo (n = 12) | |
| Any AE | 0 | 0 | 0 | 2 (16.7) | 0 | 2 (16.7) |
| Constipation | 0 | 0 | 0 | 1 (8.3) | 0 | 0 |
| Nausea | 0 | 0 | 0 | 1 (8.3) | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 1 (8.3) | 0 | 0 |
| Presyncope | 0 | 0 | 0 | 0 | 0 | 1 (8.3) |
| Orthostatic hypotension | 0 | 0 | 0 | 0 | 0 | 1 (8.3) |
| Dermatitis | 0 | 0 | 0 | 0 | 0 | 1 (8.3) |
The majority of AEs were considered by the investigators to be not related to the study drug. A total of 4 subjects experienced AEs considered to be related to the study drug (2 subjects receiving placebo; 2 subjects receiving VEL at 150 mg). No deaths or pregnancies were reported, and no serious AEs (grade 3 or 4) or AEs leading to discontinuation of the study drug were reported in any cohort.
The majority of laboratory abnormalities were grade 1 (low) or grade 2 (moderate) in severity. Grade 3 and 4 (severe) abnormalities included hematuria in 6 subjects, all of which were in female subjects with ongoing menses. Grade 3 elevated low-density lipoprotein (LDL) levels were observed in 3 subjects, all of whom had elevated LDL levels prior to dosing. One grade 4 abnormality of elevated lipase levels was observed in a subject receiving placebo, but this resolved during the conduct of the study. No notable changes in vital signs (temperature, heart rate, systolic/diastolic blood pressure, or respiration rate) were reported during the study. No clinically significant treatment-emergent electrocardiographic (ECG) abnormalities were reported during the study. No subject had an absolute QT interval adjusted for heart rate using Fridericia's formula (QTcF) value of >450 ms or an increase in the QTcF value of >60 ms from the baseline value following initiation of treatment with the study drug.
(iii) Pharmacokinetics. (a) Single and multiple ascending doses.
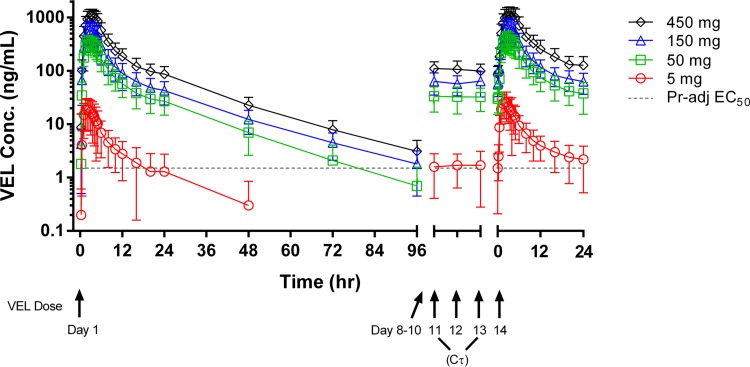
Figure 1 presents the mean (standard deviation [SD]) VEL plasma concentration-time profiles following the administration of single and multiple once-daily doses of VEL at 5 mg, 50 mg, 150 mg, and 450 mg. Following the administration of single or multiple doses of VEL, mean concentrations in plasma increased with increasing doses, maximal concentrations in plasma (Cmaxs) were reached within approximately 3 h postdosing, and the concentrations in plasma declined in a biexponential manner. Velpatasvir concentrations were quantifiable at 24 h postdosing for the majority of subjects who received VEL at 5 mg and in all subjects who received VEL doses of 50 mg or higher.
FIG 1.
VEL concentration-time profiles following administration of single and multiple doses. Dashed line, the protein-adjusted (Pr-adj) EC50 for the genotype 6e HCV replicon (1.5 ng/ml); this is the highest EC50 of VEL established for an HCV replicon.
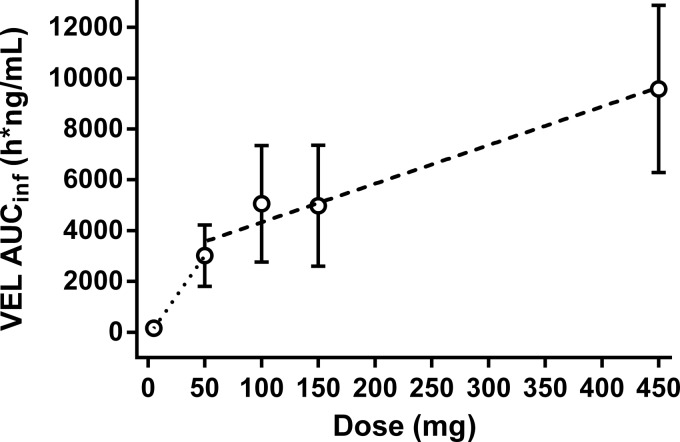
Table 5 presents the values of the PK parameters for VEL following the administration of a single or multiple once-daily doses of 5, 50, 150, and 450 mg VEL under fasting conditions; Table 5 also includes the values of the VEL plasma PK parameters following administration of a single 100-mg dose of VEL under fasting conditions, conducted as part of the food-effect portion of the study (pooled data for 24 subjects). Velpatasvir exposure, as measured by determination of the area under the concentration-time curve (AUC) and Cmax, increased in a greater than dose-proportional manner from 5 mg to 50 mg and in a less than dose proportional manner from 50 mg to 450 mg (Table 5 and Fig. 2). The median times to the maximal concentrations in plasma (Tmaxs) ranged from 1.50 to 3.25 h postdosing following the administration of a single dose and 2 to 3 h postdosing following the administration of multiple doses. The median half-life (t1/2) ranged from 11.2 to 16.2 h.
TABLE 5.
Values of PK parameters for VEL after single and multiple dosesa
| VEL dose (mg) | Single dose |
Multiple doses |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUClast (ng · h/ml) | AUCinf (ng · h/ml) | Cmax (ng/ml) | C24 (ng/ml) | Tmax (h) | t1/2 (h) | CL/F (ml/h) | AUCtau (ng · h/ml) | Cmax (ng/ml) | Ctau (ng/ml) | Tmax (h) | t1/2 (h) | CLss/F (ml/h) | |
| 5 (n = 12) | 134 (69.6) | 159 (64.0) | 22.4 (55.4) | 1.3 (112)b | 1.50 (1.50, 2.00) | 11.2 (5.40, 16.9) | 58,400 (124) | 172 (51.7) | 28.3 (49.3) | 2.2 (76.0)c | 2.00 (1.25, 2.50) | 13.7 (13.2, 15.9) | 36,100 (46.4) |
| 50 (n = 12) | 2,970 (40.1) | 3,020 (40.1) | 371 (32.7) | 27.0 (45.1) | 2.50 (2.00, 3.00) | 13.6 (10.6, 16.5) | 19,200 (39.2) | 3,030 (40.4) | 411 (40.7) | 37.9 (59.5) | 2.50 (2.25, 3.00) | 13.0 (11.4, 16.2) | 19,600 (50.5) |
| 100 (n = 24) | 4,990 (44.8) | 5,060 (45.3) | 575 (37.2) | 47.2 (51.6) | 2.50 (2.50, 3.00) | 15.7 (12.6, 17.1) | 24,600 (50.8) | ||||||
| 150 (n = 12) | 4,930 (48.0) | 4,980 (47.8) | 608 (46.7) | 42.8 (46.5) | 2.75 (2.50, 3.50) | 16.2 (14.6, 17.6) | 72,200 (196) | 4,890 (45.4) | 669 (48.1) | 63.4 (42.8) | 2.50 (2.50, 3.50) | 15.2 (12.0, 15.6) | 45,100 (88.3) |
| 450 (n = 12) | 9,500 (34.5) | 9,580 (34.3) | 1,120 (31.7) | 87.5 (37.9) | 3.25 (25.0, 3.75) | 15.0 (12.9, 16.7) | 53,700 (42.5) | 9,510 (40.9) | 1,200 (38.0) | 128 (44.3) | 3.00 (2.50, 4.25) | 11.7 (10.6, 13.1) | 58,800 (57.3) |
All PK parameters are reported as mean (range of the percent coefficient of variation), except for Tmax and t1/2, which are reported as median (quartile 1, quartile 3). Data are for the number of subjects (n) indicated in the first column, unless indicated otherwise.
n = 7.
n = 10.
FIG 2.
VEL dose linearity.
Accumulation indices were calculated for AUCs (the AUC from 0 to 24 h [AUC0–24; following a single dose] and the AUC over a dosing interval [AUCtau; at steady state]), Cmax, and trough concentrations (the trough concentration at 24 h following the administration of a single dose [C24] and the concentration at the end of a dosing interval [Ctau]) and are presented in Table 6. Consistent with the moderate t1/2 of VEL, modest accumulation was observed in all cohorts and ranged from approximately 17% to 51%, as measured by determination of the AUC.
TABLE 6.
VEL accumulation ratio
| PK parameter | % GLSM (90% CI) for multiple doses/single dose ratioe |
|||
|---|---|---|---|---|
| 5 mg (n = 12)c | 50 mg (n = 12)d | 150 mg (n = 12) | 450 mg (n = 12) | |
| AUCtau (ng · h/ml)a | 151 (117, 195) | 120 (100, 142) | 129 (88.4, 189) | 117 (96.6, 141) |
| Cmax (ng/ml) | 137 (108, 174) | 107 (86.6, 133) | 116 (73.3, 184) | 102 (82.4, 126) |
| Ctau (ng/ml)b | 123 (92.3, 163) | 144 (120, 172) | 164 (120, 225) | 142 (122, 165) |
Ratio of AUCtau (day 14; multiple dose)/AUC0–24 (day 1; single dose).
Ratio of Ctau (day 14; multiple dose)/C24 (day 1; single dose).
For Ctau, n = 10 for multiple doses and n = 7 for a single dose.
For Ctau, n = 11 for multiple doses and n = 12 for a single dose.
Data are for the indicated number of subjects unless indicated otherwise. GLSM, geometric least-squares mean.
(b) Food effect.
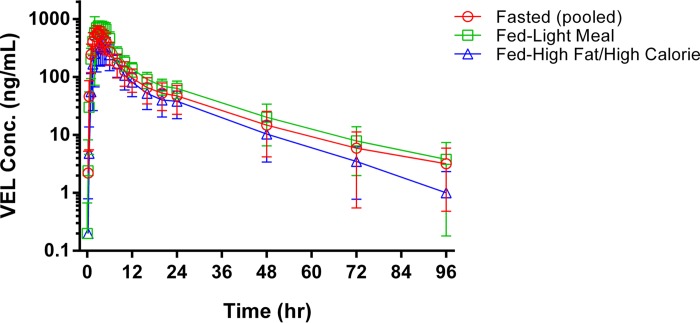
Figure 3 presents the mean (SD) plasma VEL concentration-time profiles following administration of a single dose of VEL at 100 mg under fasting conditions, with a light meal, or with a high-fat, high-calorie meal. Table 7 presents the values of the PK parameters for VEL following administration under fasting conditions or with food. The administration of VEL with food modestly extended the VEL Tmax; the median Tmaxs were 3.25 h following a light meal, 3.50 h following a high-fat, high-calorie meal, and 2.50 h following administration under fasting conditions. Mean VEL plasma concentrations were modestly higher following administration of VEL with a light meal (the AUC from time zero to infinity following a single dose [AUCinf] increased by 25% and Cmax increased by 35% compared with the values under fasting conditions) and were modestly lower following administration with a high-fat, high-calorie meal (the AUCinf decreased by 14% and Cmax decreased by 25% compared with the values obtained under fasting conditions).
FIG 3.
VEL concentration-time profiles following administration with and without food.
TABLE 7.
Values of VEL plasma PK parameters following administration with or without food
| Meal type and PK parameter | Value for the following treatmenta: |
% GLSMb ratio (90% CI) for test value/reference value | |
|---|---|---|---|
| 100 mg, fed (n = 12) | 100 mg, fasted (n = 12) | ||
| Light meal | |||
| AUCinf (ng · h/ml) | 7,120 (30.2) | 5,980 (40.8) | 125 (110, 141) |
| Cmax (ng/ml) | 800 (19.3) | 624 (34.3) | 135 (117, 156) |
| Tmax (h) | 3.25 (2.75, 4.25) | 2.50 (2.50, 3.00) | |
| t1/2 (h) | 16.7 (15.3, 18.2) | 17.0 (14.5, 18.3) | |
| High-fat, high-calorie meal | |||
| AUCinf (ng · h/ml) | 3,620 (48.0) | 4,130 (42.6) | 86.3 (73.4, 101) |
| Cmax (ng/ml) | 398 (42.2) | 526 (40.2) | 75.0 (62.6, 90.0) |
| Tmax (h) | 3.50 (2.75, 5.00) | 2.50 (2.50, 3.25) | |
| t1/2 (h) | 14.2 (11.8, 15.9) | 13.9 (11.6, 16.1) | |
All PK parameter values are reported as mean (percent coefficient of variation), except for Tmax and t1/2, which are reported as the median (quartile 1, quartile 3).
GLSM, geometric least-squares mean.
DISCUSSION
The preclinical characterization of VEL, an inhibitor of the HCV NS5A protein, demonstrated favorable in vitro and in vivo properties, including potent antiviral activity, good metabolic stability, low systemic clearance, and adequate bioavailability and physicochemical properties, that collectively warranted clinical evaluation. This phase 1 study evaluated the safety, tolerability, and pharmacokinetics of VEL in healthy human subjects following administration of single and multiple once-daily ascending doses and in the presence and absence of food.
Eighty-four subjects were enrolled into 6 cohorts, with 72 subjects receiving VEL and 12 subjects receiving placebo. In general, VEL was safe and well tolerated throughout the study. No serious AEs were reported; 4 subjects experienced AEs considered to be related to study drug, with 2 of these subjects receiving VEL and 2 receiving placebo. Laboratory abnormalities were generally of low to moderate severity. No notable changes to vital signs or ECG results were observed following the administration of VEL.
Following the administration of single and multiple doses of VEL, maximal concentrations in plasma were achieved at 1.5 to 3.25 h postdosing (median range). The median time required to reach the maximal concentration increased with dose, suggesting a solubility/dissolution-limited absorption at higher doses, which may explain the less than dose proportional increase in VEL exposure at doses above 50 mg (Table 5; Fig. 2). In vitro solubility data suggest that gastrointestinal (GI) concentrations could reach solubility saturation at VEL doses of >50 mg (50-mg dose in a 250-ml GI fluid volume = ∼0.2 mg/ml), assuming the solubility determined in FeSSIF (0.18 mg/ml); however, this assumption is complicated by the potential for extensive solubilization in the stomach under fasting conditions followed by precipitation in the small intestine (22) and the nature of the drug product, an amorphous solid dispersion of VEL, which could enhance the physiological behavior of VEL (23). Following multiple once-daily doses of VEL, modest accumulation was observed (≤64% by any exposure metric) across the dose range of 5 mg to 450 mg, consistent with the observed elimination half-life of approximately 16 h. After multiple once-daily doses of VEL at doses greater than 5 mg, mean plasma VEL concentrations at 24 h postdosing were well above the protein-adjusted half-maximal (50%) effective concentration (EC50) for genotype 1 to 6 HCV replicons (17).
The effect of food on the PK of VEL was assessed following the administration of two different meals, a light meal or a high-fat, high-calorie meal, and the effect of the administration of VEL under fed conditions was compared to that under fasting conditions. Velpatasvir demonstrated the highest aqueous solubility under acidic conditions (Table 1) as well as increased solubility in the presence of intestinal bile salts (0.18 mg/ml in FeSSIF; Table 1); this solubility profile suggests the potential for different dissolution behaviors in the presence of different kinds of meals. In general, a light snack or small meal could increase the VEL dissolution rate (and, potentially, the extent of dissolution) via decreased gastric pH, while a large meal, typically resulting in a higher gastric pH due to the increased protein load (and, thus, an increased buffering capacity), could negatively affect the VEL dissolution rate (19, 24).
Administration of VEL with a light meal or a high-fat, high-calorie meal resulted in a change to the rate and, to a lesser degree, the extent of absorption. The time to reach maximal VEL concentrations following administration with either meal was delayed compared to that under fasting conditions, consistent with the increased gastric residence time associated with the presence of food (25). While the extent of the change in VEL exposure was small following the administration of a high-fat, high-calorie meal (the AUC decreased 14% and Cmax decreased 25% compared with the values under fasting conditions), the decrease in exposure is consistent with the increased gastric pH following a meal that has a high protein content and the corresponding decrease in VEL solubility.
The small increase in VEL exposure following the administration of VEL with a light meal (an increase in the AUC of 25% and an increase in Cmax of 35% compared with the values under fasting conditions) also aligns with VEL solubility behavior, as a meal with a limited calorie content has a reduced capacity to buffer gastric acid secretion following a food stimulus, resulting in a more uniformly acidic gastric environment across subjects. This effect is likely also responsible for the observed reduction in variability following the administration of VEL with a light meal compared to that after administration under fasting conditions. Collectively, the administration of food with VEL resulted in small changes to VEL PK that are not expected to be of clinical relevance and are generally consistent with the physicochemical properties of VEL.
Collective single- and multiple-dose PK data, including food-effect data, demonstrated that the doses used and once-daily dosing of VEL are expected to provide significant antiviral activity and thus formed the basis for the dosing schemes evaluated in a phase 1b proof-of-concept study and subsequent phase 2 and phase 3 clinical studies (26–31).
In conclusion, this study assessed the safety, tolerability, and pharmacokinetics of VEL in healthy human subjects following the administration of single and multiple once-daily ascending doses of VEL in the presence and absence of food. Velpatasvir was safe and well tolerated following the administration of single and multiple doses up to 450 mg or when it was administered with food. The pharmacokinetic behavior of VEL observed in humans was generally in agreement with the findings of the preclinical characterization and was not significantly affected by administration with food. Mean VEL trough concentrations were above the protein-adjusted EC50 of VEL against HCV genotypes 1 to 6 following the administration of VEL at all evaluated doses greater than 5 mg, supporting once-daily administration. Collectively, the results of this study support the clinically relevant dose range found in a proof-of-concept study in HCV-infected subjects and subsequent phase 2 and phase 3 clinical studies as part of a regimen with other pangenotypic direct-acting antivirals for the treatment of HCV infection.
MATERIALS AND METHODS
Preclinical characterization.
The bidirectional permeability of VEL was determined using monolayers of cells of the human colonic adenocarcinoma cell line Caco-2 cultured in transwell plates. Caco-2 cell monolayers were evaluated to assess membrane integrity, and controls were used to assess permeability (propranolol) and efflux (digoxin). Velpatasvir concentrations were determined using liquid chromatography-tandem mass spectrometry.
The rate of hepatic metabolism of VEL was assessed in pooled hepatic microsomes from rats, dogs, monkeys, and humans and in cryopreserved human hepatocytes. Cytochrome P450 (CYP) reaction phenotyping was determined by incubating VEL with cDNA-expressed human CYP enzyme preparations coexpressed with human NADPH CYP reductase. Compounds known to be metabolized by each CYP enzyme in the panel were used as controls (CYP1A2, ethoxycoumarin; CYP2B6, efavirenz; CYP2C8, amodiaquine; CYP2C9, diclofenac; CYP2C19, diazepam; CYP2D6, dextromethorphan; CYP3A4, testosterone).
The extent of in vitro VEL binding to rat, dog, monkey, and human plasma was assessed by equilibrium dialysis (37°C). The aqueous solubility of VEL was assessed at room temperature in pH 2 and 7 water, with the pH being adjusted using HCl, in fasted-state simulated intestinal fluid (FaSSIF) and in fed-state simulated intestinal fluid (FeSSIF) using high-performance liquid chromatography with UV detection (18).
Clinical characterization. (i) Study drug.
The investigational products, VEL 5- and 50-mg tablets and placebo to match, were manufactured at Patheon Inc. (Mississauga, Ontario, Canada) on behalf of Gilead Sciences, Inc., in accordance with good manufacturing practice.
(ii) Study population.
Eligible subjects were an approximately even distribution of healthy males and nonpregnant, nonlactating females ages 18 to 45 years with a body mass index (BMI) of 19 to 30 kg/m2, 12-lead electrocardiograms (ECGs) without clinically significant abnormalities, normal renal function (creatinine clearance [CLCR], ≥80 ml/min by the Cockcroft-Gault equation), and no significant medical history. Written informed consent was obtained from each subject included in the study. Institutional review boards (Seaview Research) provided approval for the study, which was conducted in accordance with good clinical practice and the ethical guidelines of the Declaration of Helsinki.
Study design. (i) Single and multiple ascending doses.
The dose escalation portion of this phase 1 study was a randomized, double-blind, placebo-controlled, single- and multiple-dose study with staggered and adaptive dose escalations. Following screening procedures and baseline assessments, eligible subjects were randomized to receive VEL (n = 12 subjects per cohort) or placebo (n = 3 subjects per cohort; n = 12 subjects total). The safety, tolerability, and PK of VEL were evaluated in 4 cohorts receiving VEL doses of 5 mg, 50 mg, 150 mg, and 450 mg. The subjects in each cohort were administered a single dose of VEL or placebo under fasting conditions, followed by a 6-day washout and then seven once-daily doses of VEL or placebo under fasting conditions. All treatments were administered orally.
(ii) Food effect.
The food-effect portion of this phase 1 study was a randomized and single-dose study. Following screening procedures and baseline assessments, eligible subjects were randomized to receive VEL in one of two cohorts. Subjects in the first cohort were administered a single oral dose of VEL at 100 mg under fasting conditions (n = 12), followed by a 6-day washout and administration of a single oral dose of VEL at 100 mg with a light meal (n = 12), defined as ∼400 kcal and 30% fat. Subjects in the second cohort were administered a single oral dose of VEL at 100 mg under fasting conditions (n = 12), followed by a 6-day washout and administration of a single oral dose of VEL at 100 mg with a high-fat, high-calorie meal (n = 12), defined as ∼800 kcal and 50% fat.
Safety assessments.
Safety was assessed during the study by clinical laboratory tests; physical examinations, including monitoring of vital signs and collection of ECGs at various time points during the study; and documentation of the adverse events (AEs) experienced and the concomitant medications taken throughout the study. Vital signs were monitored at pre- and postdose assessments. Electrocardiograms were collected predosing and 4 h after each dose following the administration of single and multiple doses. The administration of multiple doses within a cohort was commenced after a review of the safety data by the sponsor in a blind manner following single-dose administration. Similarly, dose escalation for single-dose administration was determined after a review in a blind manner of the safety data from all subjects administered multiple doses in the lower-dose cohort.
Sample collection for pharmacokinetic analysis.
Blood samples were drawn from the subjects in the dose escalation cohorts to determine plasma VEL concentration. Following administration of a single dose, samples were collected at 0 h (predosing) and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 10, 12, 16, 20, 24, 48, 72, and 96 h postdosing. During multiple-dose administration, predose (trough) samples were collected following administration of the 4th, 5th, and 6th once-daily dose of study drug. Intensive sampling was conducted at 0 h (predosing) and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 10, 12, 16, 20, and 24 h postdosing following administration of the 7th once-daily dose of study drug.
Blood samples were drawn from subjects in the cohorts used to determine the effect of food to determine plasma VEL concentrations. Following administration of a single VEL dose under fasting or fed conditions, samples were collected at selected time points up to 96 h postdosing.
Blood samples were centrifuged for the collection of plasma. Plasma velpatasvir concentrations were determined using 100 μl of human plasma, a supported liquid extraction (SLE) method, and a validated high-performance liquid chromatography-tandem mass spectroscopy method with an assay range of detection of 1 to 1,000 ng/ml. An API 4000 mass spectrometer was operated in the selected reaction monitoring (SRM) mode under optimized conditions for VEL positive ions formed by electrospray ionization. The interassay accuracy range was −1.5 to 4.1 (percent relative error). The interassay precision range was 1.7 to 4.9% (coefficient of variation). Analysis of VEL was performed at Tandem Laboratories (Salt Lake City, UT, USA).
The values of the plasma pharmacokinetic parameters were estimated using Phoenix WinNonlin software (version 6.3, Pharsight, St. Louis, MO, USA) by application of a nonlinear model using noncompartmental methods. The plasma pharmacokinetic parameters estimated included AUC (the AUC from time zero to infinity [following a single dose] [AUCinf], the AUC from time zero to the last measurable time point [AUClast], and the AUC over a dosing interval [at steady state; AUCtau]), Cmax, the time to Cmax (Tmax), the concentration at the end of a dosing interval (Ctau), the elimination half-life (t1/2), and the apparent oral clearance (clearance following administration of a single dose [CL/F] and clearance at steady state [CLss/F]).
Statistical analysis.
Data for all randomized subjects who received at least one dose of study medication were included in the safety analysis (n = 84). Adverse events were recorded and summarized by system organ class and preferred term. The frequency of subjects who experienced AEs was summarized by treatment.
The data for subjects who received the study medication and for whom the values of the pharmacokinetic parameters could be appropriately estimated were included in the pharmacokinetic analysis (n = 72). Dose proportionality was obtained by comparing the values of the VEL PK parameters across the evaluated doses. The accumulation ratio was assessed by constructing 90% confidence intervals (CIs) for the ratio of the geometric least-squares means for the area under the concentration-time curve over the dosing interval (AUCtau [at steady state], AUC0–24 [following administration of a single dose]), Cmax, and trough concentrations (Ctau [at steady state] and C24 [at 24 h following administration of a single dose]) of VEL following the administration of single and multiple doses of VEL, consistent with the two-1-sided-tests approach.
To evaluate the effect of food on VEL, the values of the primary PK parameters (AUCinf, AUClast, and Cmax) of VEL were compared following the administration of VEL under fasting conditions or following the administration of VEL with a light meal or a high-fat, high-calorie meal. The 90% CIs for the ratio of the geometric least-squares means of the treatments under fed and fasted conditions were calculated for each parameter, consistent with the two-1-sided-tests approach.
All statistical analyses were performed using SAS software (SAS Institute, Cary, NC, USA).
ACKNOWLEDGMENTS
We thank the volunteers and staff who participated in the study.
This study was funded by Gilead Sciences, Inc.
Erik Mogalian, Polina German, Brian P. Kearney, Cheng Yong Yang, Diana Brainard, John Link, John McNally, LingLing Han, John Ling, and Anita Mathias are employees of Gilead Sciences, Inc.
REFERENCES
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. 2009. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naggie S, Patel K, McHutchison J. 2010. Hepatitis C virus directly acting antivirals: current development with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother 65:2063–2069. doi: 10.1093/jac/dkq284. [DOI] [PubMed] [Google Scholar]
- 3.Negro F, Alberti A. 2011. The global health burden of hepatitis C virus infection. Liver Int 31(Suppl 2):1–3. doi: 10.1111/j.1478-3231.2011.02537.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, Dalgard O, Dillion JF, Flisiak R, Forns X, Frankova S, Goldis A, Goulis I, Halota W, Hunyady B, Lagging M, Largen A, Makara M, Manolakopoulos S, Marcellin P, Marinho RT, Pol S, Poynard T, Puoti M, Sagalova O, Sibbel S, Simon K, Wallace C, Young K, Yudaydin C, Zuckerman E, Negro F, Zeuzem S. 2011. A systematic review of hepatitis C virus epidemiology in Europe, Canada, and Israel. Liver Int 31(Suppl 2):30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 5.Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, Khayat H, Elshazly M, Esmat G, Guan R, Hand KH, Koike K, Largen A, McCaughan G, Mogawer S, Monis A, Nawaz A, Piratvisuth T, Sanai FM, Sharara AI, Sibbel S, Sood A, Suh DJ, Wallace C, Young K, Negro F. 2011. A systematic review of hepatitis C virus epidemiology in Asia, Australia, and Egypt. Liver Int 31(Suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Morgan ML, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, Alter M, Yartel A, Ward JW, Centers for Disease Control and Prevention. 2012. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 61(RR-4):1–32. [PubMed] [Google Scholar]
- 7.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. 2011. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 9:509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J Virol 75:1252–1264. doi: 10.1128/JVI.75.3.1252-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tellinghuisen TL, Foss KL, Treadaway J. 2008. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog 4:e1000032. doi: 10.1371/journal.ppat.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale MJ Jr, Korth MJ, Tang NM, Tan SL, Hopkins DA, Dever TE, Polyak SJ, Gretch DR, Katze MG. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 11.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Brau N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P. 2014. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, Reddy R, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P. 2014. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA. 2015. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. 2014. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Jia L, O'Boyle DR, Sun JH, Rigat K, Valera L, Nower P, Huang X, Kienzle B, Roberts S, Gao M, Fridell RA. 2014. Comparison of daclatasvir resistance barriers on NS5A from hepatitis C virus genotypes 1 to 6: implications for cross-genotype activity. Antimicrob Agents Chemother 58:5155–5163. doi: 10.1128/AAC.02788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stirnimann G. 2014. Ombitasvir (ABT-267), a novel NS5A inhibitor for the treatment of hepatitis C. Expert Opin Pharmacother 15:2609–2622. doi: 10.1517/14656566.2014.972364. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Yu M, Peng B, Lee Y, Trejo-Martin A, Gong R, Bush C, Worth A, Nash M, Chan K, Yang H, Beran R, Tian Y, Taylor J, Yang C, Paulson M, Delaney W, Link JO. 2013. GS-5816, a second generation HCV HS5A inhibitor with potent antiviral activity, broad genotypic coverage and a high resistance barrier. J Hepatol 58:S484–S485. [Google Scholar]
- 18.Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. 2009. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J Pharm Sci 98:1177–1192. doi: 10.1002/jps.21502. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Pharmacopeia. 2011. General notices and requirements: applying to standards, tests, assays, and other specifications of the USP. Version 1 May 2011 U.S. Pharmacopeia, Rockville, MD. [Google Scholar]
- 20.Davies B, Morris T. 1993. Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- 21.Mordenti J. 1986. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci 75:1028–1040. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- 22.Kostewicz ES, Wunderlich M, Brauns U, Becker R, Bock T, Dressman JB. 2004. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J Pharm Pharmacol 56:43–51. [DOI] [PubMed] [Google Scholar]
- 23.Craig DQM. 2002. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231:131–144. doi: 10.1016/S0378-5173(01)00891-2. [DOI] [PubMed] [Google Scholar]
- 24.Carver PL, Fleisher D, Zhou SY, Kaul D, Kazanjian P, Li C. 1999. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm Res 16:718–724. doi: 10.1023/A:1018880726035. [DOI] [PubMed] [Google Scholar]
- 25.Winstanley PA, Orme ML. 1989. The effects of food on drug bioavailability. Br J Clin Pharmacol 28:621–628. doi: 10.1111/j.1365-2125.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawitz E, Freilich B, Link J, German P, Mo H, Han L, Brainard DM, McNally J, Marbury T, Rodriguez-Torres M. 2015. A phase 1, randomized, dose-ranging study of GS-5816, a once-daily NS5A inhibitor, in patients with genotype 1-4 hepatitis C virus. J Viral Hepat 22:1011–1019. doi: 10.1111/jvh.12435. [DOI] [PubMed] [Google Scholar]
- 27.Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, McNally J, Brainard DM, Han L, Doehle B, Mogalian E, McHutchison JG, Rabinovitz M, Towner WJ, Gane EJ, Stedman CAM, Reddy KR, Roberts SK. 2015. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection. Ann Intern Med 163:809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 28.Everson GT, Towner WJ, Davis MN, Wyles DL, Nahass RG, Thuluvath PJ, Etzkorn K, Hinestrosa F, Tong M, Rabinovitz M, McNally J, Brainard DM, Han L, Doehle B, McHutchison JG, Morgan T, Chung RT, Tran TT. 2015. Sofosbuvir with velpatasvir in treatment-naïve noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection. Ann Intern Med 163:818–826. doi: 10.7326/M15-1000. [DOI] [PubMed] [Google Scholar]
- 29.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HLY, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S. 2015. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 30.Foster GR, Afdahl N, Roberts SK, Brau N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourliere M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CAM, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M. 2015. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 31.Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton RSM. 2015. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]