ABSTRACT
The main goal of our study was to evaluate the in vitro bedaquiline susceptibility of six prevalent species of pathogenic nontuberculous mycobacteria (NTM) in China. In addition, we investigated the potential molecular mechanisms contributing to bedaquiline resistance in the different NTM species. Among slowly growing mycobacteria (SGM), bedaquiline exhibited the highest activity against Mycobacterium avium; the MIC50 and MIC90 values were 0.03 and 16 mg/liter, respectively. Among rapidly growing mycobacteria (RGM), Mycobacterium abscessus subsp. abscessus (M. abscessus) and Mycobacterium abscessus subsp. massiliense (M. massiliense) seemed more susceptible to bedaquiline than Mycobacterium fortuitum, with MIC50 and MIC90 values of 0.13 and >16 mg/liter, respectively, for both species. On the basis of bimodal distributions of bedaquiline MICs, we proposed the following epidemiological cutoff (ECOFF) values: 1.0 mg/liter for SGM and 2.0 mg/liter for RGM. Among M. avium, Mycobacterium intracellulare, Mycobacterium kansasii, M. abscessus, M. massiliense, and M. fortuitum isolates, 14 (29.8%), 41 (27.2%), 33 (39.3%), 44 (20.2%), 42 (25.8%), and 7 (31.8%), respectively, were resistant to bedaquiline. No significant differences in the proportions of bedaquiline resistance among these species were observed (P > 0.05). Genetic mutations were observed in 74 isolates (10.8%), with all nucleotide substitutions being synonymous. In conclusion, our data demonstrate that bedaquiline shows moderate in vitro activity against NTM species. Using the proposed ECOFF values, we could distinguish between bedaquiline-resistant and -susceptible strains with the broth dilution method. In addition, no nonsynonymous mutations in the atpE gene that conferred bedaquiline resistance in all six NTM species were identified.
KEYWORDS: nontuberculous mycobacteria, bedaquiline, ECOFF
INTRODUCTION
Nontuberculous mycobacteria (NTM) are a group of all Mycobacterium species with the exception of the obligate Mycobacterium tuberculosis complex and Mycobacterium leprae (1). Although NTM are considered typically environmental organisms, NTM infections have attracted more attention due to their increased prevalence worldwide in the past 2 decades (2, 3). In many high-income countries, NTM disease is a significant contributor to morbidity and death among immunocompromised individuals (4). The major obstacle to addressing NTM disease is associated with its natural resistance to antibacterial drugs, resulting in disappointing clinical outcomes with the currently available treatment regimens (5). Therefore, there is an urgent need to develop and to employ novel and more effective antibiotics for the treatment of NTM infections (6, 7).
Bedaquiline is a novel diarylquinoline antibiotic, which exhibits potent activity against mycobacteria by inhibiting ATP synthase (8). On the basis of favorable results in a number of preclinical and clinical trials, this drug was approved in 2012 by the U.S. FDA for use in the treatment of multidrug-resistant (MDR) tuberculosis (TB) (9). In addition, bedaquiline has been shown to have in vitro bacteriostatic activity against a wide range of NTM isolates (10). A recent preliminary report demonstrated potential clinical and microbiological activity of bedaquiline in patients with NTM disease. Taken together, the previous findings indicate promising prospects for the use of bedaquiline as part of combination therapy to treat NTM disease (4).
Prior to the application of bedaquiline, reliable results of in vitro antimicrobial susceptibility testing are urgently needed to guide the use of bedaquiline in the treatment of mycobacterial infections (6). Previous studies have recommended that the MIC breakpoint for the use of bedaquiline be 0.25 mg/liter for Mycobacterium tuberculosis (11), whereas very little attention has been paid to in vitro susceptibility profiles for bedaquiline against NTM. Therefore, data regarding the MIC distributions of different NTM species are essential for formulating practical recommendations regarding the use of bedaquiline for the treatment of infections due to different NTM species. The main goal of our study was to evaluate the in vitro susceptibility to bedaquiline of six prevalent mycobacterial species associated with NTM disease in China. Based on these distributions, the epidemiological cutoff (ECOFF) values for bedaquiline were proposed for these NTM species. In addition, we investigated the potential molecular mechanism contributing to the bedaquiline resistance in these different NTM species.
RESULTS
Bedaquiline MICs for NTM isolates.
A total of 685 NTM isolates were included in this study, including 47 Mycobacterium avium (6.9%), 151 Mycobacterium intracellulare (22.0%), 84 Mycobacterium kansasii (12.3%), 218 Mycobacterium abscessus subsp. abscessus (M. abscessus) (31.8%), 163 Mycobacterium abscessus subsp. massiliense (M. massiliense) (23.8%), and 22 Mycobacterium fortuitum (3.2%) isolates. The bedaquiline MICs for the NTM isolates are summarized in Table 1. Among slowly growing mycobacteria (SGM), bedaquiline exhibited the highest activity against M. avium; the MIC50 and MIC90 were 0.03 and 16 mg/liter, respectively. Among rapidly growing mycobacteria (RGM), M. abscessus and M. massiliense seemed more susceptible to bedaquiline than M. fortuitum, with MIC50 and MIC90 values of 0.13 and >16 mg/liter, respectively, for both species.
TABLE 1.
Distribution of bedaquiline MIC values for NTM isolates enrolled in this study
| Classification and speciesa | No. of strains with MIC of: |
Total no. of strains | MIC50 (mg/liter) | MIC90 (mg/liter) | Proportion of resistant strains (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 mg/liter | 0.031 mg/liter | 0.062 mg/liter | 0.12 mg/liter | 0.25 mg/liter | 0.5 mg/liter | 1 mg/liter | 2 mg/liter | 4 mg/liter | 8 mg/liter | 16 mg/liter | >16 mg/liter | |||||
| SGM | ||||||||||||||||
| M. avium | 23 | 5 | 2 | 1 | 1 | 1 | 0 | 1 | 2 | 5 | 3 | 3 | 47 | 0.03 | 16 | 29.8 |
| M. intracellulare | 68 | 16 | 10 | 8 | 5 | 2 | 1 | 1 | 3 | 4 | 12 | 21 | 151 | 0.03 | >16 | 27.2 |
| M. kansasii | 27 | 8 | 8 | 4 | 2 | 1 | 1 | 1 | 3 | 5 | 8 | 16 | 84 | 0.06 | >16 | 39.3 |
| RGM | ||||||||||||||||
| M. abscessus | 6 | 19 | 51 | 53 | 24 | 15 | 4 | 2 | 3 | 5 | 7 | 29 | 218 | 0.13 | >16 | 20.2 |
| M. massiliense | 5 | 18 | 37 | 29 | 14 | 10 | 5 | 3 | 2 | 2 | 6 | 32 | 163 | 0.13 | >16 | 25.8 |
| M. fortuitum | 1 | 2 | 4 | 3 | 2 | 2 | 1 | 0 | 1 | 1 | 2 | 3 | 22 | 0.25 | >16 | 31.8 |
SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria; MIC50, concentration required to inhibit the growth of 50% of the strains; MIC90, concentration required to inhibit the growth of 90% of the strains.
ECOFF values for NTM isolates.
As shown in Fig. 1 and 2, the bedaquiline MIC values of six NTM species followed bimodal distributions. Most of the tested isolates showed MIC values of less than 0.016 mg/liter and greater than 8 mg/liter. Based on the guidelines for determining ECOFF values, we propose the following ECOFF values: 1.0 mg/liter for SGM and 2.0 mg/liter for RGM. When 1.0 mg/liter was used as the cutoff value, 14/47 M. avium isolates (29.8%), 41/151 M. intracellulare isolates (27.2%), and 33/84 M. kansasii isolates (39.3%) were resistant to bedaquiline. Statistical analysis revealed that the proportions of bedaquiline-resistant isolates showed no significant differences among these species (P > 0.05). For RGM, resistance to bedaquiline was noted for 20.2% of M. abscessus isolates (44/218 isolates), 25.8% of M. massiliense isolates (42/163 isolates), and 31.8% of M. fortuitum isolates (7/22 isolates). Similar to SGM, no significant differences in the percentages of bedaquiline-resistant isolates were observed among RGM.
FIG 1.
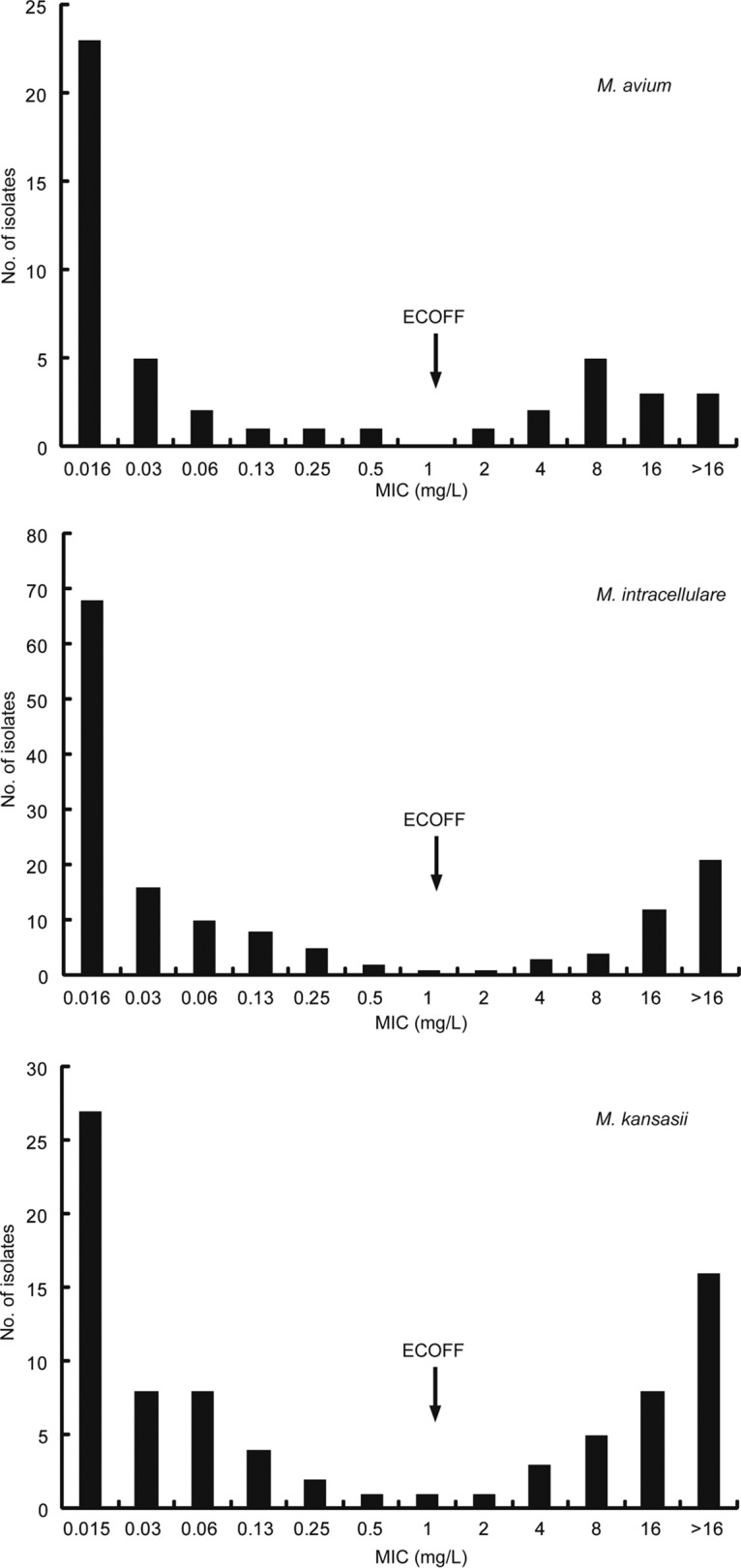
Distribution of MIC values for slowly growing mycobacterial strains. The arrows represent the proposed ECOFF value for slowly growing mycobacteria.
FIG 2.
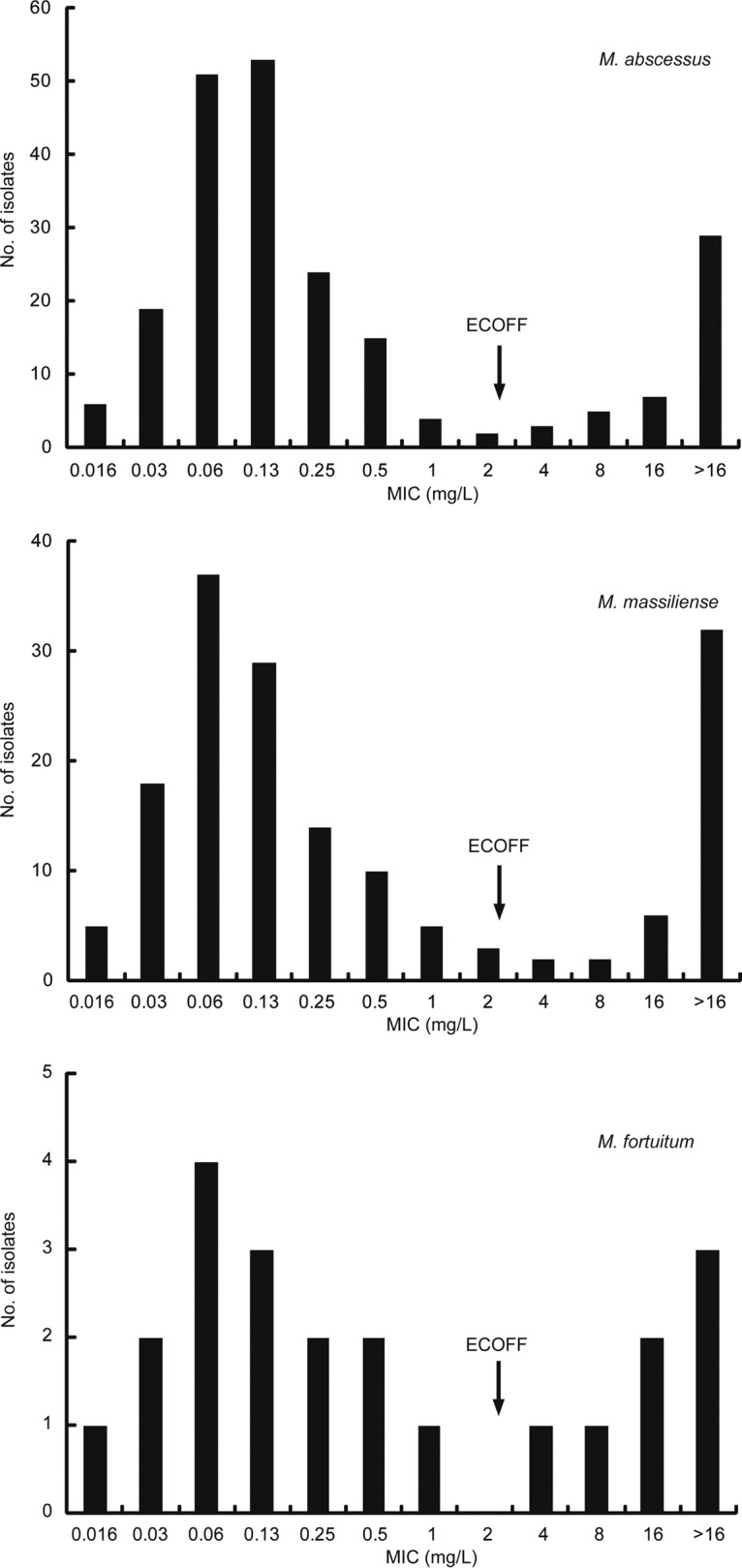
Distribution of MIC values for rapidly growing mycobacterial strains. The arrows represent the proposed ECOFF value for rapidly growing mycobacteria.
Mutations in atpE genes.
The entire atpE genes of 685 NTM isolates were sequenced. The DNA sequence chromatogram found that genetic mutations were observed in 74 (10.8%) of those 685 isolates; all of those nucleotide substitutions were synonymous mutations, resulting in no amino acid change. As shown in Table 2, M. fortuitum had the highest frequency of genetic mutations (22.7% [5/22 isolates]), followed by M. massiliense (12.8% [21/163 isolates]), M. avium (10.6% [5/47 isolates]), M. intracellulare (10.6% [16/151 isolates]), M. abscessus (9.6% [21/218 isolates]), and M. kansasii (7.1% [6/84 isolates]). We further analyzed the relationship between nucleotide substitutions and bedaquiline MIC values. The majority of isolates harboring mutations (91.9% [68/74 isolates]) were susceptible to bedaquiline, whereas only six isolates (8.1% [6/74 isolates]) showed resistance to bedaquiline, indicating that these synonymous nucleotide polymorphisms may not be associated with bedaquiline resistance.
TABLE 2.
Distribution of MIC values for NTM isolates harboring mutations in the atpE gene
| Species | Mutation in atpE gene |
No. of strains with MIC of: |
Total no. of strains | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change | Amino acid change | 0.016 mg/liter | 0.031 mg/liter | 0.062 mg/liter | 0.12 mg/liter | 0.25 mg/liter | 0.50 mg/liter | 1 mg/liter | 2 mg/liter | 4 mg/liter | 8 mg/liter | 16 mg/liter | >16 mg/liter | ||
| M. avium | C186T | Val62Val | 1 | 1 | 1 | 3 | |||||||||
| C186A | Val62Val | 1 | 1 | 2 | |||||||||||
| M. intracellulare | C147T | Gly49Gly | 6 | 2 | 3 | 2 | 1 | 1 | 1 | 16 | |||||
| M. kansasii | T186C | Gly62Gly | 2 | 3 | 1 | 6 | |||||||||
| M. abscessus | T72C | Gly24Gly | 3 | 1 | 3 | 7 | |||||||||
| T105C | Ala35Ala | 2 | 5 | 4 | 2 | 1 | 14 | ||||||||
| M. massiliense | T72C | Gly24Gly | 3 | 7 | 4 | 3 | 1 | 18 | |||||||
| T105C | Ala35Ala | 1 | 1 | 1 | 3 | ||||||||||
| M. fortuitum | A195G | Glu65Glu | 2 | 1 | 1 | 1 | 5 | ||||||||
DISCUSSION
Due to the intrinsic resistance to most available antibiotics, the nontuberculous mycobacteria pose a unique challenge for clinical treatment (12). There is interest in evaluating new anti-TB compounds against NTM, which will provide new options for treatment of NTM infections (12). In this study, we first evaluated the in vitro efficacy of bedaquiline with a large number of clinical NTM isolates. Although a bedaquiline breakpoint was established for M. tuberculosis (11), there were no clear criteria regarding whether a given NTM isolate is susceptible to bedaquiline. Hence, the most important finding of this study is the proposal of ECOFF values, i.e., 1.0 mg/liter for SGM and 2.0 mg/liter for RGM. On the basis of the ECOFF values, our data demonstrate that bedaquiline exhibits moderate activity against various NTM species. For M. avium, the proportion of bedaquiline-resistant isolates was 29.8%, which is greater than the values for clarithromycin (3.0%), amikacin (9.2%), and moxifloxacin (10.8%) but lower than those for rifampin (RIF) (38.5%), linezolid (40.0%), and ethambutol (EMB) (40.0%) (13). Similar to the findings for M. avium, the proportion of bedaquiline-resistant strains was smaller than the values for RIF (66.0%) and EMB (49.5%) for M. intracellulare in China (13). Clarithromycin in combination with RIF and EMB is the cornerstone of the treatment of M. avium complex (MAC) lung disease (6). In view of the high-level resistance to RIF and EMB of MAC strains in China, bedaquiline may provide an alternative to generate an effective regimen for MAC infections. In addition, a recent report on in vitro bedaquiline susceptibility testing of MAC strains by Brown-Elliott and colleagues revealed that 50% of the MAC isolates had MIC50 values of ≤0.008 mg/liter (14), which is significantly lower than the MIC50 of 0.03 mg/liter from the current study. Although the exact reasons remain unknown, several potential reasons may be responsible for the discrepancy with respect to other studies. In China, due to the lack of the capability to identify mycobacterial species, a larger proportion of NTM cases may be misdiagnosed as MDR-TB, resulting in potential exposure to second-line anti-TB drugs, including clofazimine. In view of the cross-resistance between clofazimine and bedaquiline (15, 16), preliminary exposure to clofazimine may be an important contributor to the significant difference in MICs. Alternatively, the high MIC values for bedaquiline against MAC strains may be due to the abuse of antibiotics in the animal and food industries, which is associated with high concentrations of antibiotics in the environment (17). Because they are opportunistic pathogens, overexposure to broad-spectrum antibiotics in the natural habitat may accelerate the emergence of intrinsically drug-resistant MAC strains by decreasing cell permeability, which also may be a potential reason for the different bimodal MIC distribution profiles of MAC strains.
M. abscessus infections are associated with the lowest cure rate among various NTM species, which is largely due to the emergence of inducible macrolide resistance in M. abscessus (12). As a consequence, the treatment of clarithromycin-resistant M. abscessus relies on the use of amikacin and cefoxitin (12, 18), although a recent study from China reported that 32% and 55% of M. abscessus isolates were resistant and intermediate to cefoxitin, respectively (19). Considering the large proportion of cefoxitin-resistant isolates, the use of bedaquiline is more likely to be a promising choice for M. abscessus infections in China. Consistent with our findings, a small preliminary report by Philley and colleagues revealed that bedaquiline produced potential clinical and microbiological activity in patients with advanced MAC or M. abscessus disease (4). In contrast, nude mouse model experiments demonstrated that bedaquiline did not prevent death when used alone, which might be associated with high minimal bactericidal concentrations (18). Despite the conflicting observations from different studies, our in vitro susceptibility data indicate that the addition of bedaquiline to a preferred drug combination may serve as a starting point for the optimized use of this novel anti-TB compound against M. abscessus. Large clinical trials are urgently needed to confirm the efficacy of bedaquiline in the management of M. abscessus and other NTM lung diseases.
Resistance to bedaquiline is associated with genetic mutations in the atpE gene in M. tuberculosis, which encodes subunit c of the F0 subunit of ATP synthase (the target of bedaquiline). Numerous reports have found nucleotide substitutions in selected bedaquiline-resistant mutants, such as A63P and I66M in M. tuberculosis (20–22). Similar to those findings in M. tuberculosis, a recent study by Alexander et al. indicated that one nonsynonymous mutation in the atpE gene was associated with a 50-fold increase in the bedaquiline MIC in M. intracellulare (23). However, no nonsynonymous mutations in the atpE gene that conferred bedaquiline resistance in all six NTM species were identified in our study. In a study by Huitric et al., only 15 (28.3%) of 53 bedaquiline-resistant M. tuberculosis isolates harbored mutations in atpE (24). The unsatisfactory correlation between in vitro susceptibility and genotypes in mycobacteria indicates that alternative resistance mechanisms must be involved in bedaquiline resistance. Several potential mechanisms conferring bedaquiline resistance in M. tuberculosis have been reported (15, 25). Milano and colleagues found that mutations in Rv0678, which encodes a regulatory protein of the MmpS5-MmpL5 efflux system, were associated with bedaquiline and clofazimine cross-resistance in MDR-TB patients receiving bedaquiline treatment (25). Another gene, i.e., pepQ, encoding a putative Xaa-Pro aminopeptidase, has also been determined to confer low-level resistance to bedaquiline in M. tuberculosis (15). The efflux pump and other natural mechanisms doubtless result in low-level resistance, while high-level drug resistance is attributed to mutations in the target genes (26). Hence, we hypothesize that bedaquiline must engage targets other than atpE to achieve its bacteriostatic activity.
This report has several limitations. First, all of the experiments in this study were carried out in vitro with clinical NTM isolates. Future studies are needed to determine the correlation between in vitro susceptibility and treatment outcomes in clinical trials. Second, some NTM isolates in this study had MICs of <0.016 mg/liter, which were not covered by our experimental system; this may hinder us in determining their true MICs. Third, sequencing of the atpE gene alone, and not Rv0678 and pepQ, was included in our study. Further analysis of the latter two genes will extend our knowledge of the molecular mechanisms conferring bedaquiline resistance in NTM species. Fourth, there is strong evidence that bedaquiline exhibits cross-resistance with clofazimine in M. tuberculosis (15, 16), although the cross-resistance profiles of these two compounds were not evaluated in the final analysis. Nevertheless, our observations provide important insights into the clinical application of bedaquiline for the treatment of NTM infections.
In conclusion, our data demonstrate that bedaquiline shows moderate in vitro activity against NTM species. Using ECOFF values of 1.0 mg/liter for SGM and 2.0 mg/liter for RGM, we could distinguish between bedaquiline-resistant and -susceptible strains by using the broth dilution method. In addition, no nonsynonymous mutations in the atpE gene that conferred bedaquiline resistance in all six NTM species were identified. Further studies are urgently needed to investigate the molecular mechanisms conferring bedaquiline resistance in NTM species.
MATERIALS AND METHODS
Ethics statement.
The protocols used in this study were approved by the Ethics Committee of the Chinese Center for Disease Control and Prevention.
Bacterial strains.
The strains used in this study, representing different geographical origins, were collected between 2011 and 2015 from Guangdong Chest Hospital, Shanghai Pulmonary Hospital, Lianyungang Fourth Hospital, Chongqing Yongchuan Hospital (affiliated with Chongqing Medical University), Inner Mongolia Fourth Hospital, and Kaifeng Pulmonary Hospital. All of the strains were identified as NTM species using multilocus sequence analysis, including 16S rRNA, hsp65, rpoB, and a 16S-23S rRNA internal transcribed spacer (ITS) sequence (13). The most prevalent NTM isolates associated with NTM diseases, including M. avium, M. intracellulare, M. abscessus, M. massiliense, M. kansasii, and M. fortuitum, were included, whereas the other rare subspecies belonging to the M. avium complex, M. abscessus complex, and M. fortuitum complex were excluded from the current study.
MIC assays.
Pure bedaquiline powder was a gift from Johnson & Johnson (Beerse, Belgium). To determine the bedaquiline susceptibility of NTM strains, broth microdilution assays were performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (27). Cation-adjusted Mueller-Hinton broth (CAMHB) enriched with oleic acid-albumin-dextrose-catalase (OADC) was used for SGM, while CAMHB without OADC was used for RGM. The bacterial suspensions were prepared from subcultures collected from 4-week-old cultures in Löwenstein-Jensen medium. The broth microdulution format was set up with 2-fold dilutions, and the concentrations of bedaquiline ranged from 0.016 to 16 mg/liter. Briefly, a suspension was prepared at a 0.5 McFarland standard, diluted, and inoculated into 96-well microtiter plates to achieve final organism concentrations of 105 cells/ml for both SGM and RGM. All plates were incubated at 37°C for 7 days for SGM and 3 days for RGM. All experiments were performed in triplicate. The MIC was defined as the lowest concentration that inhibited visible growth. The ECOFFs were determined according to the distribution profiles of MIC values. For unimodal MIC distributions, ECOFFs were defined as concentrations representing ≥99.9% of the bacterial population; for bimodal MIC distributions, ECOFFs were set between the two populations (28).
DNA amplification and sequencing.
The atpE gene encodes subunit c of the F0 subunit of ATP synthase, which is the target of bedaquiline (8). In this study, the atpE genes from different NTM species were analyzed by Sanger sequencing. DNA fragments were amplified with the primers listed in Table S1 in the supplemental material. PCR was performed in a final volume of 50 μl, containing 5 μl 10× PCR buffer, 200 μM each deoxynucleoside triphosphate (dNTP), 0.2 μM each primer set, and 1 U HotStar Taq polymerase (Qiagen). PCR was performed as follows: initial denaturation of 5 min at 94°C; 35 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C; and final extension of 10 min at 72°C. The amplification products were sent to Tsingke Co. (Beijing, China) for DNA sequencing. DNA sequences were aligned with the homologous sequences of the reference mycobacterial strains by using BioEdit Sequence Alignment Editor 7.1.3 (http://www.mbio.ncsu.edu/bioedit/bioedit.html).
Statistical analysis.
The chi-square test was performed to compare the proportions of bedaquiline-resistant isolates between different NTM species, using SPSS 14.0 (SPSS Inc., Chicago, IL). Differences were considered significant if the P values were <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key Research Program of China (grant 2014ZX10003-002).
We thank the staff members of the National Tuberculosis Reference Laboratory, affiliated with the Chinese Center for Disease Control and Prevention, for technical help.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02627-16.
REFERENCES
- 1.van Ingen J. 2013. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med 34:103–109. doi: 10.1055/s-0033-1333569. [DOI] [PubMed] [Google Scholar]
- 2.van Ingen J. 2015. Microbiological diagnosis of nontuberculous mycobacterial pulmonary disease. Clin Chest Med 36:43–54. doi: 10.1016/j.ccm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Maugein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabo N, Thomson R, Tortola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 4.Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. 2015. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148:499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raju RM, Raju SM, Zhao Y, Rubin EJ. 2016. Leveraging advances in tuberculosis diagnosis and treatment to address nontuberculous mycobacterial disease. Emerg Infect Dis 22:365–369. doi: 10.3201/eid2203.151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliott BA, Nash KA, Wallace RJ Jr. 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 25:545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arend SM, van Soolingen D, Ottenhoff TH. 2009. Diagnosis and treatment of lung infection with nontuberculous mycobacteria. Curr Opin Pulm Med 15:201–208. doi: 10.1097/MCP.0b013e3283292679. [DOI] [PubMed] [Google Scholar]
- 8.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 9.Fox GJ, Menzies D. 2013. A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther 2:123–144. doi: 10.1007/s40121-013-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huitric E, Verhasselt P, Andries K, Hoffner SE. 2007. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 51:4202–4204. doi: 10.1128/AAC.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrea G, Coeck N, Desmaretz C, Van De Parre T, Van Poucke T, Lounis N, de Jong BC, Rigouts L. 2015. Bedaquiline susceptibility testing of Mycobacterium tuberculosis in an automated liquid culture system. J Antimicrob Chemother 70:2300–2305. doi: 10.1093/jac/dkv117. [DOI] [PubMed] [Google Scholar]
- 12.Kasperbauer SH, De Groote MA. 2015. The treatment of rapidly growing mycobacterial infections. Clin Chest Med 36:67–78. doi: 10.1016/j.ccm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Pang Y, Wang Y, Cohen C, Zhao Y, Liu C. 2015. Differences in risk factors and drug susceptibility between Mycobacterium avium and Mycobacterium intracellulare lung diseases in China. Int J Antimicrob Agents 45:491–495. doi: 10.1016/j.ijantimicag.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ Jr. 2017. In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 61:e01798-16. doi: 10.1128/AAC.01798-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida D, Ioerger T, Tyagi S, Li SY, Mdluli K, Andries K, Grosset J, Sacchettini J, Nuermberger E. 2016. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:4590–4599. doi: 10.1128/AAC.00753-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. 2015. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 18.Lerat I, Cambau E, Roth Dit Bettoni R, Gaillard JL, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. doi: 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- 19.Li YM, Tong XL, Xu HT, Ju Y, Cai M, Wang C. 2016. Prevalence and antimicrobial susceptibility of Mycobacterium abscessus in a general hospital, China. Biomed Environ Sci 29:85–90. doi: 10.3967/bes2016.009. [DOI] [PubMed] [Google Scholar]
- 20.Haagsma AC, Abdillahi-Ibrahim R, Wagner MJ, Krab K, Vergauwen K, Guillemont J, Andries K, Lill H, Koul A, Bald D. 2009. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob Agents Chemother 53:1290–1292. doi: 10.1128/AAC.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palomino JC, Martin A. 2013. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol 8:1071–1080. doi: 10.2217/fmb.13.85. [DOI] [PubMed] [Google Scholar]
- 22.Petrella S, Cambau E, Chauffour A, Andries K, Jarlier V, Sougakoff W. 2006. Genetic basis for natural and acquired resistance to the diarylquinoline R207910 in mycobacteria. Antimicrob Agents Chemother 50:2853–2856. doi: 10.1128/AAC.00244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander DC, Vasireddy R, Vasireddy S, Philley JV, Brown-Elliott BA, Perry BJ, Griffith DE, Benwill JL, Cameron AD, Wallace RJ Jr. 2017. Emergence of mmpT5 variants during bedaquiline treatment of Mycobacterium intracellulare lung disease. J Clin Microbiol 55:574–584. doi: 10.1128/JCM.02087-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. 2010. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 54:1022–1028. doi: 10.1128/AAC.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milano A, Pasca MR, Provvedi R, Lucarelli AP, Manina G, Ribeiro AL, Manganelli R, Riccardi G. 2009. Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5-MmpL5 efflux system. Tuberculosis (Edinb) 89:84–90. doi: 10.1016/j.tube.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Pang Y, Wang Y, Liu C, Zhao Y. 2014. Beijing genotype of Mycobacterium tuberculosis is significantly associated with linezolid resistance in multidrug-resistant and extensively drug-resistant tuberculosis in China. Int J Antimicrob Agents 43:231–235. doi: 10.1016/j.ijantimicag.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; approved standard—2nd ed. CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 28.Morrissey I, Oggioni MR, Knight D, Curiao T, Coque T, Kalkanci A, Martinez JL. 2014. Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 9:e86669. doi: 10.1371/journal.pone.0086669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


