ABSTRACT
WCK 5222 consists of cefepime combined with zidebactam, a bicyclo-acyl hydrazide β-lactam enhancer antibiotic with a dual action involving binding to Gram-negative bacterial PBP2 and β-lactamase inhibition. We evaluated the in vitro activity of cefepime-zidebactam against 7,876 contemporary (2015) clinical isolates of Enterobacteriaceae (n = 5,946), Pseudomonas aeruginosa (n = 1,291), and Acinetobacter spp. (n = 639) from the United States (n = 2,919), Europe (n = 3,004), the Asia-Pacific (n = 1,370), and Latin America (n = 583). The isolates were tested by a reference broth microdilution method for susceptibility against cefepime-zidebactam (1:1 and 2:1 ratios) and comparator agents. Cefepime-zidebactam was the most active compound tested against Enterobacteriaceae (MIC50/90, ≤0.03/0.12 μg/ml [1:1] and 0.06/0.25 μg/ml [2:1]; 99.9% of isolates were inhibited at ≤4 [1:1] and ≤8 μg/ml [2:1]). Cefepime-zidebactam was active against individual Enterobacteriaceae species (MIC50/90, ≤0.03 to 0.06/≤0.03 to 0.5 μg/ml [1:1]) and retained potent activity against carbapenem-resistant isolates (MIC50/90, 1/4 μg/ml; 99.3% of isolates were inhibited at ≤8 μg/ml [1:1]). Cefepime-zidebactam activity was consistent among geographic regions, and only one isolate showed MIC values of >8 μg/ml (1:1). Cefepime-zidebactam was also very active against P. aeruginosa with MIC50/90 values of 1/4 μg/ml, and 99.5% of isolates were inhibited at ≤8 μg/ml (1:1). The MIC values for cefepime-zidebactam at the 1:1 ratio were generally 2-fold lower than those for cefepime-zidebactam at the 2:1 ratio (MIC50/90, 2/8 μg/ml) and zidebactam alone (MIC50/90, 4/8 μg/ml). Against Acinetobacter spp., cefepime-zidebactam at 1:1 and 2:1 ratios (MIC50/90, 16/32 μg/ml for both) was 4-fold more active than cefepime or ceftazidime. Zidebactam exhibited potent in vitro antimicrobial activity against some organisms. These results support the clinical development of WCK 5222 for the treatment of Gram-negative bacterial infections, including those caused by multidrug-resistant isolates.
KEYWORDS: carbapenem-resistant Enterobacteriaceae, CRE, MDR, XDR, KPC, metallo-β-lactamases
INTRODUCTION
The trend of increasing antimicrobial resistance is most troublesome for Gram-negative bacteria because few antimicrobial agents targeting this group of organisms have been developed successfully (1). The occurrence of carbapenemase-producing Enterobacteriaceae has increased rapidly in the last few years in some geographic regions (2–4). In particular, clonal Klebsiella pneumoniae strains with K. pneumoniae carbapenemases (KPC; class A carbapenemases) have disseminated widely in the United States, Israel, and some European countries (3–7). We are now facing infections caused by extensively drug-resistant (XDR) and pandrug-resistant (PDR) organisms that are resistant to all (PDR) or almost all (XDR) antimicrobial agents currently available for clinical use (8). Thus, the use of second-line and more toxic compounds, such as the polymyxins, has increased substantially in some geographic regions, and new antimicrobial agents for the treatment of infections caused by resistant Gram-negative organisms are greatly needed (1).
Zidebactam (C13H21N5O7S; Fig. 1) is a non-β-lactam antibiotic with a dual mode of action involving selective and high-affinity Gram-negative bacterial PBP2 binding and β-lactamase inhibition. Due to PBP2 binding, zidebactam demonstrates antibacterial activity against various Enterobacteriaceae and Pseudomonas aeruginosa isolates (9). Cefepime is a well-established parenteral fourth-generation cephalosporin with activity against both Gram-positive and Gram-negative aerobic bacteria. Cefepime and other β-lactam agents exert their antibacterial effects by binding to penicillin-binding proteins (PBP) (10).
FIG 1.
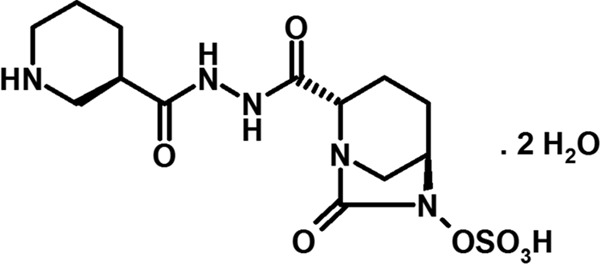
Chemical structure of zidebactam dihydrate.
Cefepime displays potent in vitro activity against all common pathogens from the family Enterobacteriaceae, including those that commonly produce chromosomally mediated β-lactamases. Type I (AmpC) β-lactamases have a low affinity for cefepime; therefore, cefepime retains its inhibitory activity against derepressed bacteria. In addition, cefepime is not susceptible to hydrolysis by plasmid-mediated AmpC β-lactamases expressed by Enterobacteriaceae species. However, similar to other β-lactams, cefepime can be hydrolyzed by some class A β-lactamases, including extended-spectrum β-lactamases (ESBLs) and KPCs, class B enzymes (metallo-β-lactamases [MBLs]), and some class D enzymes (OXA) (11, 12). Cefepime also has excellent activity against P. aeruginosa, and unlike imipenem and some second-generation cephalosporins, cefepime is a poor inducer of type I β-lactamases. Another important characteristic of cefepime pertains to its superior in vitro activity against some key Gram-positive bacterial pathogens, such as Streptococcus pneumoniae and methicillin-susceptible staphylococci, compared to the activities of other broad-spectrum cephalosporins. In summary, cefepime exhibits activity superior to the activities of ceftazidime and ceftriaxone against most clinically important Enterobacteriaceae and similar to the activity of ceftazidime against P. aeruginosa (10, 13).
It is also important to note that cefepime clinical breakpoints for Enterobacteriaceae have been revised by the Clinical and Laboratory Standards Institute (CLSI) on the basis of results from clinical and pharmacokinetic/pharmacodynamic (PK/PD) studies and contemporary MIC distributions (14, 15). According to the current CLSI breakpoint criteria for Enterobacteriaceae, susceptible and resistant breakpoints for cefepime are ≤2 and ≥16 μg/ml, respectively, and Enterobacteriaceae isolates with cefepime MIC values of 4 and 8 μg/ml should be reported as “susceptible-dose dependent” (SDD). The SDD interpretative criterion essentially provides three susceptible breakpoints for cefepime according to the dosage utilized, i.e., ≤2 μg/ml for a dosage of 1 g every 12 h (q12h) (low dose), ≤4 μg/ml for a dosage of 1 g every 8 h (q8h) or 2 g q12h, and ≤8 μg/ml for a dosage of 2 g q8h (high dose) (15).
Cefepime was initially approved by the U.S. Food and Drug Administration (U.S. FDA) in 1997. Current clinical indications include moderate to severe pneumonia, complicated and uncomplicated urinary tract infections, complicated intra-abdominal infections, and uncomplicated skin and skin structure infections, and it is also used as empirical therapy for febrile neutropenic patients (16). Zidebactam combined with cefepime (WCK 5222) is under clinical development with higher-dose regimens of 2 g of cefepime and 1 g of zidebactam every 8 h for the treatment of Gram-negative bacterial infections (registration no. NCT02707107 and NCT02674347 at www.ClinicalTrials.gov). We evaluated the in vitro activity of cefepime combined with zidebactam against a large worldwide collection of contemporary clinical isolates of Gram-negative organisms.
RESULTS
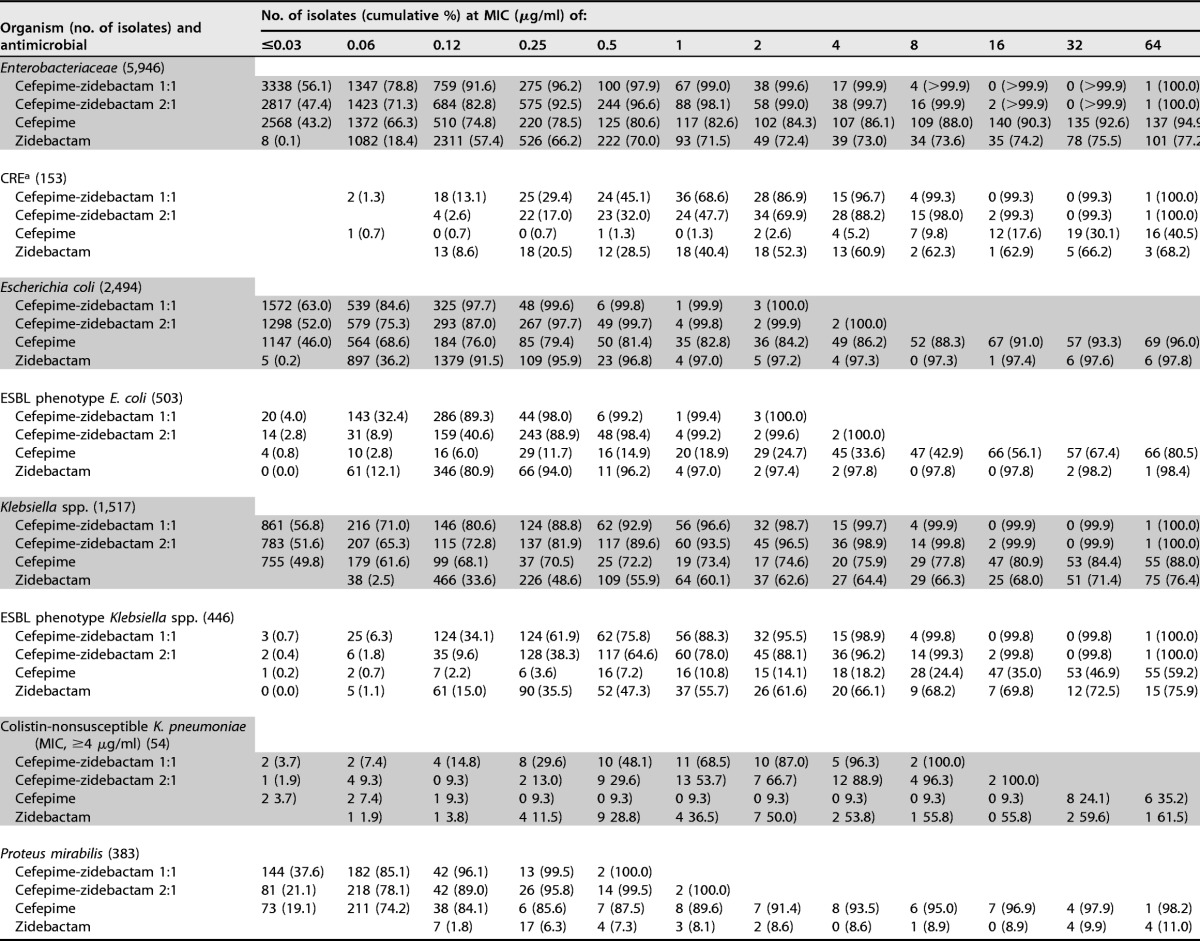
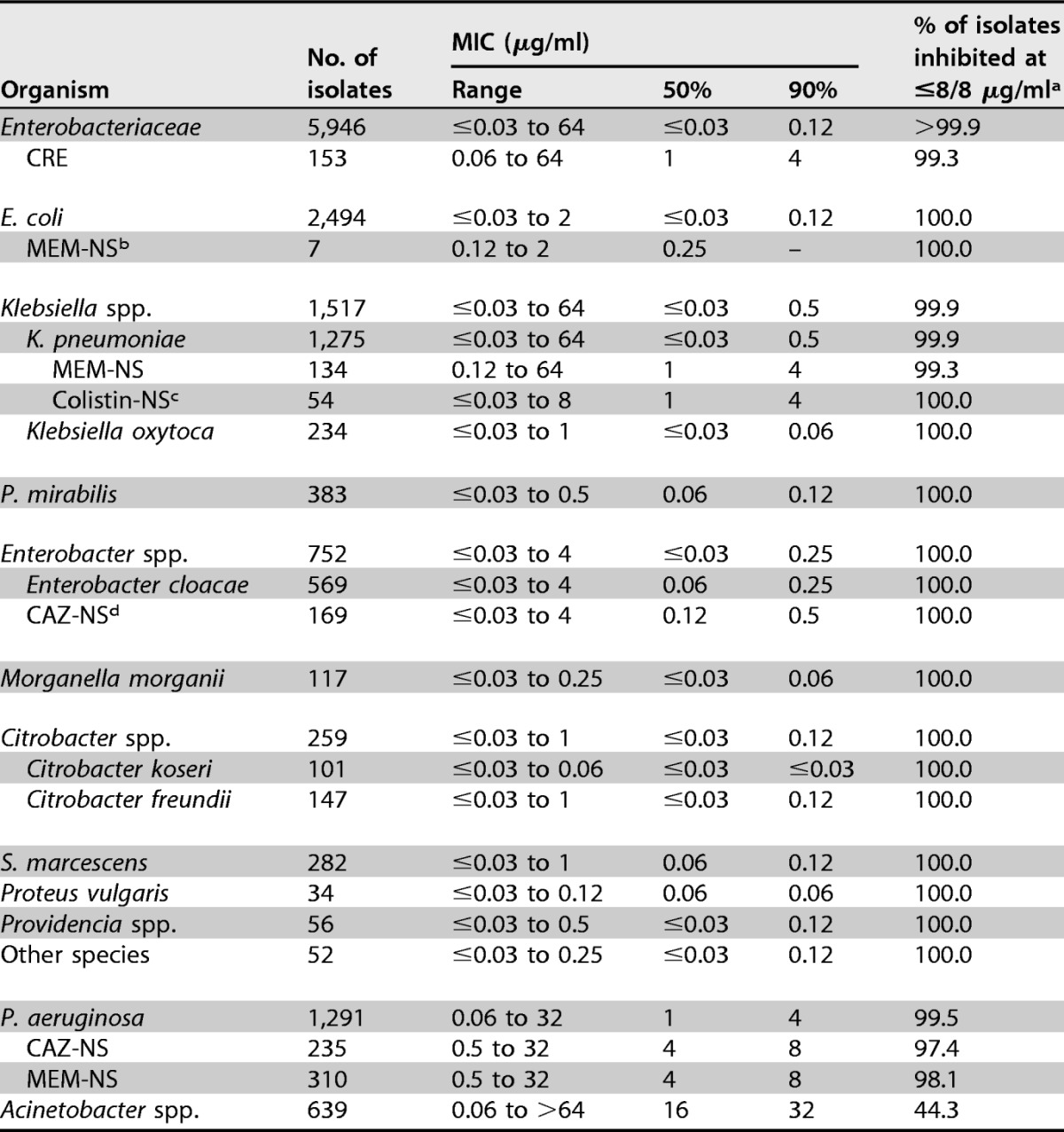
Cefepime-zidebactam was the most active compound tested against Enterobacteriaceae, with MIC50/90 values of ≤0.03/0.12 (1:1 ratio) and 0.06/0.25 μg/ml (2:1 ratio) (Table 1). Moreover, 99.6 and >99.9% of isolates were inhibited at ≤2 and ≤8 μg/ml (1:1 ratio), respectively (Table 2). Only one isolate (a K. pneumoniae isolate) showed a cefepime-zidebactam (1:1) MIC value of >8 μg/ml.
TABLE 1.
Summary of cefepime-zidebactam (1:1) activity against Enterobacteriaceae isolates included in this study
a For comparison purposes only.
b MEM-NS, meropenem nonsusceptible (MICs, ≥2 μg/ml for Enterobacteriaceae and ≥4 μg/ml for P. aeruginosa) (15).
c Colistin-NS, colistin nonsusceptible (MIC, ≥4 μg/ml) (23).
d CAZ-NS, ceftazidime nonsusceptible (MIC, ≥8 μg/ml for Enterobacter spp. and ≥16 μg/ml for P. aeruginosa) (15).
TABLE 2.
Antimicrobial activity of cefepime-zidebactam (1:1 and 2:1), cefepime, and zidebactam tested against the main organisms and organism groups of isolates included in this study
a The organisms included Enterobacter aerogenes (n = 2), Enterobacter cloacae species complex (n = 12), Enterobacter gergoviae (n = 1), Escherichia coli (n = 7), Klebsiella oxytoca (n = 1), K. pneumoniae (n = 122), Klebsiella variicola (n = 1), Proteus mirabilis (n = 1), and Serratia marcescens (n = 6).
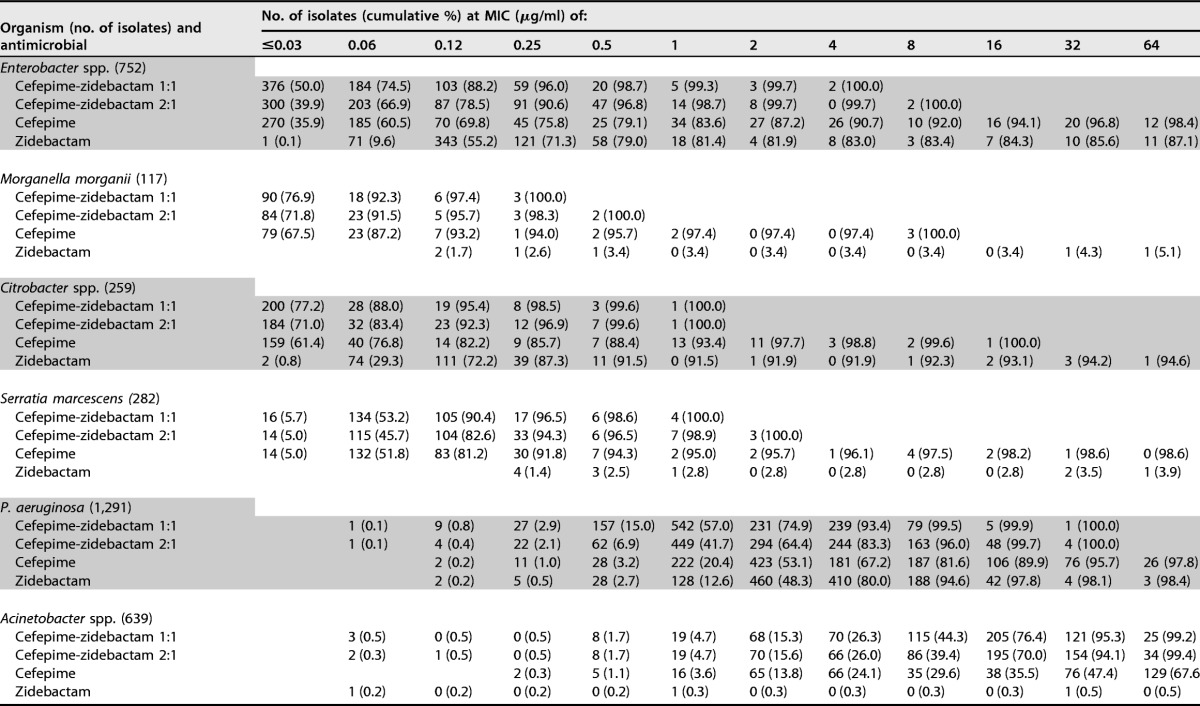
Cefepime-zidebactam at a 1:1 ratio was generally 2-fold more active than cefepime-zidebactam at a 2:1 ratio, and zidebactam alone exhibited variable antibacterial activity (MIC50/90, 0.12/>64 μg/ml) when tested against Enterobacteriaceae (Table 2). Overall, Escherichia coli (MIC50/90, 0.12/0.12 μg/ml) and Citrobacter species (MIC50/90, 0.12/0.5 μg/ml) isolates exhibited low zidebactam MIC values, whereas Proteus mirabilis, indole-positive Proteeae, and Serratia marcescens showed much higher zidebactam MIC results (MIC50, >64 μg/ml). Among the Klebsiella species (MIC50/90, 0.5/>64 μg/ml) and Enterobacter species (MIC50/90, 0.12/>64 μg/ml) isolates, zidebactam MIC values ranged from ≤0.03 to >64 μg/ml, and 66.3 and 83.4% of isolates were inhibited at ≤8 μg/ml of zidebactam, respectively (Table 2).
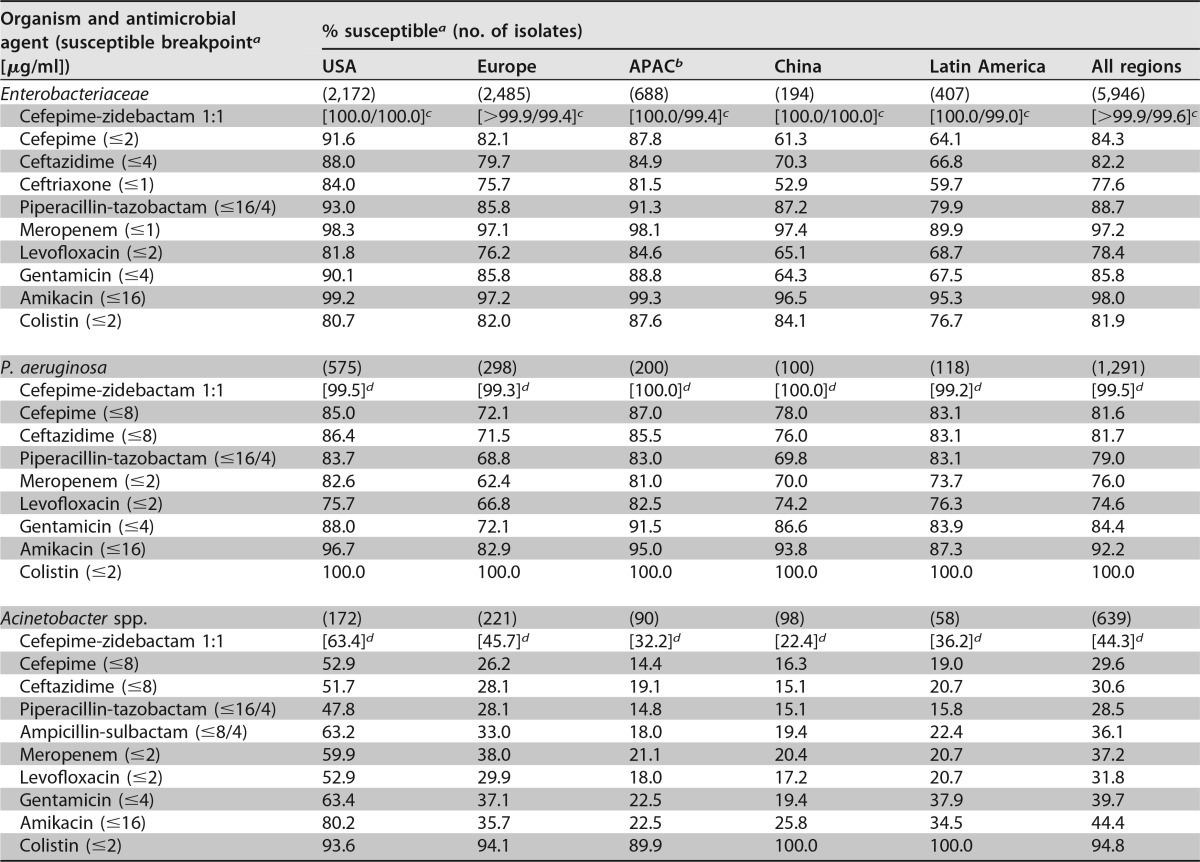
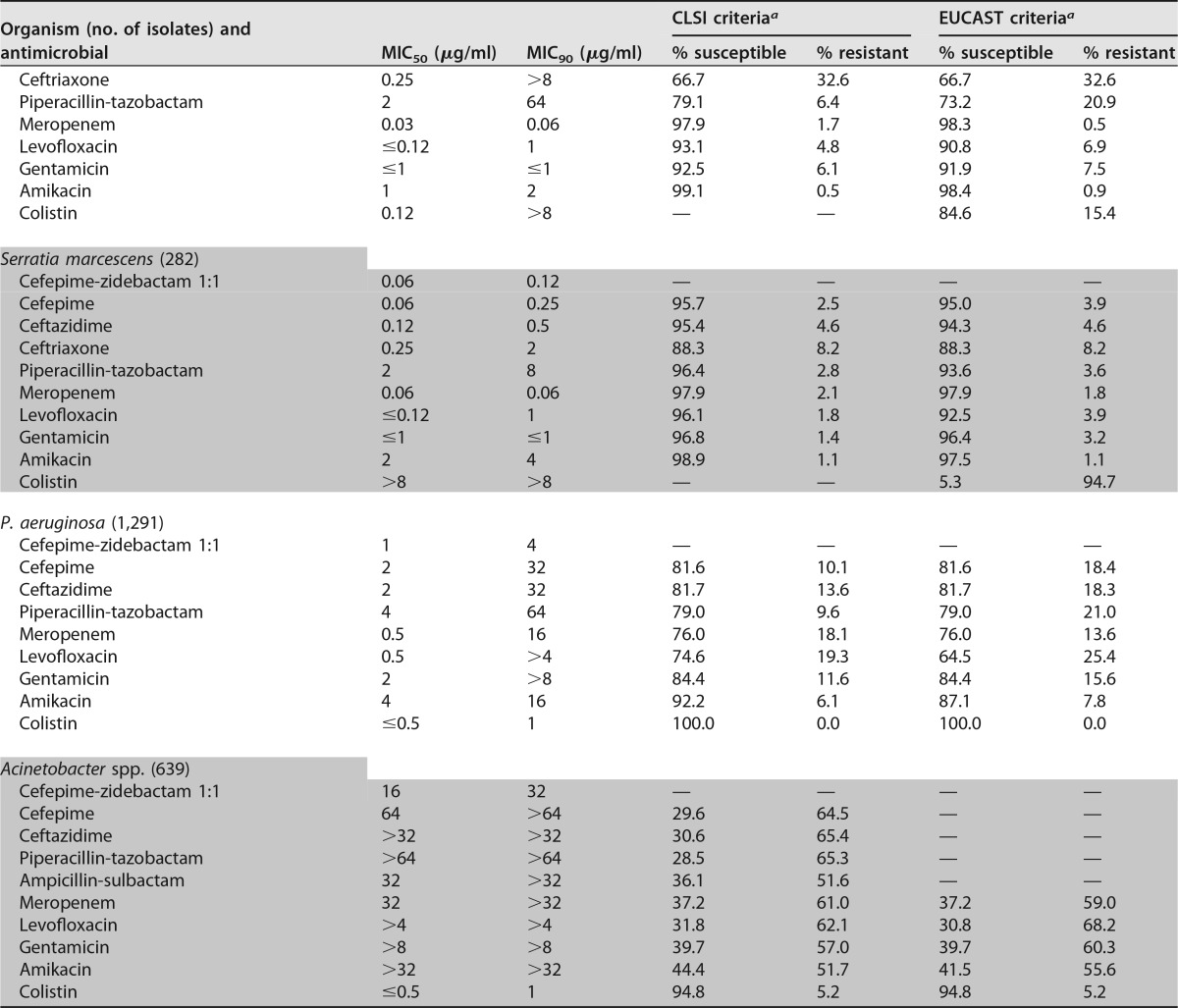
Cefepime-zidebactam was active against individual Enterobacteriaceae species (MIC50/90, ≤0.03 to 0.06/≤0.03 to 0.5 μg/ml [1:1 ratio]) and retained potent activity against carbapenem-resistant Enterobacteriaceae (CRE; MIC50/90, 1/4 μg/ml; 99.3% of isolates were inhibited at ≤8 μg/ml [1:1]), ESBL screening-positive E. coli (MIC50/90, 0.12/0.25 μg/ml; 100.0% were inhibited at ≤8 μg/ml [1:1]), ESBL screening-positive Klebsiella spp. (MIC50/90, 0.25/2 μg/ml; 99.8% were inhibited at ≤8 μg/ml [1:1]), meropenem-nonsusceptible K. pneumoniae (MIC50/90, 1/4 μg/ml; 99.3% were inhibited at ≤8 μg/ml [1:1]), colistin-nonsusceptible K. pneumoniae (MIC50/90, 1/4 μg/ml; 100.0% were inhibited at ≤8 μg/ml [1:1]), and ceftazidime-nonsusceptible Enterobacter spp. (MIC50/90, 0.12/0.5 μg/ml; 100.0% were inhibited at ≤8 μg/ml [1:1]) (Table 1). Moreover, cefepime-zidebactam (1:1 and 2:1 ratios) activity was consistent among geographic regions, with >99.9 to 100.0% of isolates being inhibited at ≤8 μg/ml and 99.0 to 100.0% being inhibited at ≤2 μg/ml (1:1 ratio) (see Table 4).
TABLE 4.
Activity of cefepime-zidebactam (1:1) and comparator antimicrobial agents when tested against Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp. stratified by geographic region
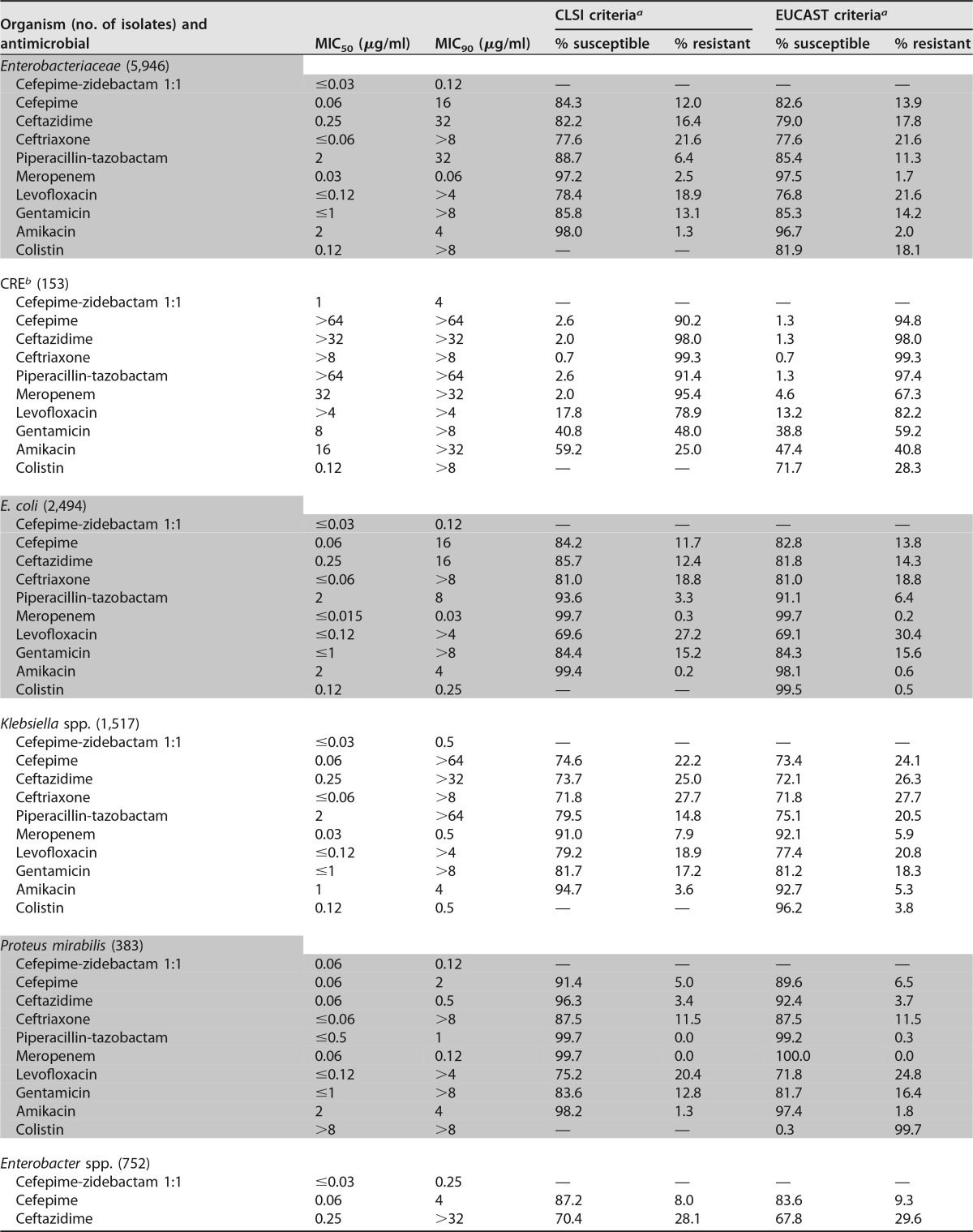
Amikacin (MIC50/90, 2/4 μg/ml; 98.0/96.7% of isolates were susceptible at the respective CLSI/EUCAST susceptible breakpoints) and meropenem (MIC50/90, 0.03/0.06 μg/ml; 97.2/97.5% were susceptible at the respective CLSI/EUCAST susceptible breakpoints) were also very active overall, whereas cefepime (MIC50/90, 0.06/16 μg/ml) and gentamicin (MIC50/90, ≤1/>8 μg/ml) were active against 84.3/82.6% and 85.8/85.3% of Enterobacteriaceae isolates at the respective CLSI/EUCAST susceptible breakpoints (Table 3).
TABLE 3.
Activity of cefepime-zidebactam (1:1) and comparator antimicrobial agents when tested against 5,946 Enterobacteriaceae isolates
b The organisms included Enterobacter aerogenes (n = 2), Enterobacter cloacae species complex (n = 12), Enterobacter gergoviae (n = 1), Escherichia coli (n = 7), Klebsiella oxytoca (n = 1), K. pneumoniae (n = 122), Klebsiella variicola (n = 1), Proteus mirabilis (n = 1), and Serratia marcescens (n = 6).
The Enterobacteriaceae susceptibility rate for meropenem was the lowest in Latin America (89.9%) when the rate was compared to that in the other geographic regions (97.1 to 98.3%), and rates of susceptibility to ceftriaxone ranged from 52.9% in China, 59.7% in Latin America, 75.7% in Europe, and 81.5% in the Asia-West Pacific (APAC) region, excluding China, to 84.0% in the United States (Table 4).
Susceptibility rates for meropenem among K. pneumoniae isolates were lower in Latin America (70.9%; data not shown) than the other regions (87.6 to 94.7%; data not shown), and the most active compounds tested against meropenem-nonsusceptible K. pneumoniae isolates were cefepime-zidebactam (MIC50/90, 1/4 μg/ml; 99.3% of isolates were inhibited at ≤8 μg/ml), colistin (MIC50/90, 0.25/>8 μg/ml; 71.4% were susceptible), and amikacin (MIC50/90, 16/>32 μg/ml; 54.1% were susceptible) (Table 3).
Cefepime-zidebactam was also very active against P. aeruginosa isolates, with MIC50/90 values being 1/4 μg/ml and 99.5% of isolates being inhibited at ≤8 μg/ml (1:1 ratio) (Tables 1 and 2), and retained potent in vitro activity against isolates nonsusceptible to ceftazidime (MIC50/90, 4/8 μg/ml; 97.4% were inhibited at ≤8 μg/ml [1:1 ratio]) and/or meropenem (MIC50/90, 4/8 μg/ml; 98.1% were inhibited at ≤8 μg/ml [1:1 ratio]) (Table 1). Furthermore, cefepime-zidebactam exhibited consistent activity against P. aeruginosa isolates from all continents, whereas the susceptibility rates for the comparator agents were generally lower in Europe than the other geographic regions (Table 4).
The in vitro activity of cefepime-zidebactam tested at the 2:1 ratio was slightly lower (2-fold) than that of cefepime-zidebactam tested at the 1:1 ratio, with MIC50/90 values being 2/8 μg/ml and 96.0% of isolates being inhibited at ≤8 μg/ml, and zidebactam alone also exhibited potent in vitro activity against P. aeruginosa (MIC50/90, 4/8 μg/ml), inhibiting 94.6% of isolates at ≤8 μg/ml (Table 2).
Colistin (MIC50/90, ≤0.5/1 μg/ml; 100.0% of isolates were susceptible) and amikacin (MIC50/90, 4/16 μg/ml; 92.2% were susceptible) were also very active against P. aeruginosa. In contrast, meropenem (MIC50/90, 0.5/16 μg/ml), piperacillin-tazobactam (MIC50/90, 4/64 μg/ml), and ceftazidime (MIC50/90, 2/32 μg/ml) were active against only 76.0%, 79.0%, and 81.7% of isolates at the current CLSI susceptible breakpoint, respectively (Table 3).
Cefepime-zidebactam at 1:1 and 2:1 ratios (MIC50/90, 16/32 μg/ml at both ratios) was at least 4-fold more active than cefepime (MIC50/90, 64/>64 μg/ml) against Acinetobacter spp. (Table 2). The most active compounds tested against Acinetobacter spp. were colistin (MIC50/90, ≤0.5/1 μg/ml; 94.8% of isolates were susceptible) and amikacin (MIC50/90, >32/>32 μg/ml; 44.4% were susceptible) (Table 3). The rates of susceptibility to most antimicrobial agents tested of Acinetobacter isolates collected from U.S. medical centers were substantially higher than those of Acinetobacter isolates collected from other geographic regions (Table 3).
DISCUSSION
The results of the present study clearly demonstrate that the cefepime-zidebactam combination possesses potent in vitro activity against Enterobacteriaceae, including isolates producing the β-lactamases most commonly found in hospitals worldwide, such as ESBLs, KPCs, and MBLs (3, 4, 17–20). Cefepime-zidebactam inhibited >99.9% of Enterobacteriaceae strains at MIC values of ≤8 μg/ml, including 99.3% (152/153) of CRE strains. Only one isolate, a K. pneumoniae isolate from Turkey harboring a blaNDM-1 gene, showed a cefepime-zidebactam MIC value of >8 μg/ml (1:1 ratio). Moreover, a cefepime-zidebactam MIC of 8 μg/ml (1:1 ratio) was exhibited by only four isolates (0.07%), all of which were K. pneumoniae isolates (one each from Poland, Russia, Singapore, and Turkey). Screening of these four isolates for β-lactamase genes revealed that two isolates harbored a blaNDM-1 gene and the other two harbored a blaKPC-2 gene. Further characterization of these four K. pneumoniae isolates is warranted since cefepime-zidebactam has demonstrated in vitro activity against other Enterobacteriaceae isolates, including K. pneumoniae isolates, producing KPC-like enzymes, NDM-1, and other MBLs (20).
Cefepime-zidebactam was also highly active against P. aeruginosa and inhibited 99.5% of the isolates tested at ≤8 μg/ml. Cefepime-zidebactam (MIC50/90, 1/4 μg/ml) exhibited greater anti-P. aeruginosa activity than any other β-lactam and inhibited 98.1% of meropenem-nonsusceptible strains and 97.4% of ceftazidime-nonsusceptible strains at ≤8 μg/ml. Moreover, a previous investigation from our group has indicated that cefepime-zidebactam exhibits good in vitro activity against MBL-producing P. aeruginosa strains, with MIC50 and MIC90 values of 4 and 8 μg/ml, respectively, when testing 12 isolates, including strains producing IMP-13 (n = 1 isolate), IMP-15 (n = 1), VIM-1 (n = 1), VIM-2 (n = 6), VIM-4 (n = 2), and VIM-7 (n = 1) (20).
Similar to other β-lactams and to most antimicrobial agents tested, cefepime-zidebactam showed higher MIC values against Acinetobacter spp. than against other Gram-negative organisms; however, cefepime-zidebactam at 1:1 and 2:1 ratios (MIC50/90, 16/32 μg/ml at both ratios) was at least 4-fold more active than cefepime (MIC50/90, 64/>64 μg/ml) and ceftazidime (MIC50/90, >32/>32 μg/ml) against these organisms.
In summary, WCK 5222 (cefepime-zidebactam) demonstrated potent in vitro activity against a large worldwide collection of contemporary (2015) clinical isolates of Enterobacteriaceae and P. aeruginosa. The results of this investigation also show that zidebactam possesses robust in vitro antimicrobial activity against some organisms. Studies on the mechanism of action and pharmacodynamics of zidebactam in combination with cefepime are warranted to establish the potential of this combination in providing therapeutic coverage against infections caused by multidrug-resistant (MDR) and XDR pathogens (9, 21). These in vitro results clearly support the further clinical development of cefepime-zidebactam for the treatment of serious Gram-negative bacterial infections, especially those caused by MDR and XDR organisms.
MATERIALS AND METHODS
Susceptibility testing.
MIC values were determined using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology, as described in CLSI document M07-A10 (22). The combination of cefepime-zidebactam (WCK 5222; ratio concentrations of 1:1 and 2:1), both compounds alone, and various comparator agents were tested in 96-well, frozen-form panels produced by JMI Laboratories (North Liberty, IA, USA). The cefepime-zidebactam combination was tested at fixed ratio instead of at a fixed concentration of zidebactam due to the potent intrinsic antimicrobial activity of zidebactam against some organisms. Quality control (QC) isolates were tested daily, and the inoculum density was monitored by colony counts. QC ranges and interpretive criteria for the comparator compounds were those published in CLSI document M100-S26 (15) and by EUCAST (23). The sponsor provided available MIC information for cefepime-zidebactam and zidebactam alone tested against the listed QC organisms. The tested QC strains for Escherichia coli were ATCC 25922, ATCC 35218, and NCTC 13353; those for Klebsiella pneumoniae were ATCC 700603 and ATCC BAA-1705; and the QC strain for P. aeruginosa was ATCC 27853.
Organism collection.
A total of 7,876 Gram-negative isolates collected as part of a global surveillance program were included in this investigation. Only clinically significant isolates were included in the investigation (one per infection episode). All isolates were collected in 2015, except for those from China (392 isolates), which were collected in 2013. Isolates were consecutively collected from 134 medical institutions worldwide, including the United States (2,919 isolates from 64 medical centers), Europe (3,004 isolates from 38 medical centers), Latin America (583 isolates from eight medical centers), the Asia-West Pacific (APAC) region (excluding China; 978 isolates from 14 medical centers), and China (392 isolates from 10 medical centers). Most medical centers providing the isolates included in this investigation were large/teaching hospitals. Escherichia coli and Klebsiella species isolates were grouped as the extended-spectrum β-lactamase (ESBL) screening-positive phenotype on the basis of the CLSI screening criteria for ESBL production, i.e., an MIC of ≥2 μg/ml for ceftazidime, ceftriaxone, and/or aztreonam (15), for the purpose of susceptibility testing result analysis. Although other β-lactamases, such as AmpC and KPC, may also produce an ESBL screening-positive phenotype, these strains were grouped together because they usually demonstrate resistance to various broad-spectrum β-lactam compounds. A carbapenem-resistant Enterobacteriaceae (CRE) isolate was defined to be resistant (MIC, ≥4 μg/ml [CLSI criteria]) (15) to imipenem (excluding Proteus mirabilis and indole-positive Proteeae), meropenem, or doripenem. Species identification was confirmed when necessary by matrix-assisted laser desorption ionization (MALDI)–time of flight mass spectrometry (TOF MS) using a Bruker Daltonics MALDI biotyper (Billerica, MA, USA) by following the manufacturer's instructions. Enterobacteriaceae isolates with elevated cefepime-zidebactam MIC values were tested for β-lactamase-encoding genes using a microarray-based Check-MDR CT101 assay kit (Check-Points, Wageningen, Netherlands). The assay was performed according to the manufacturer's instructions. This kit has the capabilities to detect CTX-M groups 1, 2, 8 plus 25, and 9; the TEM wild type (WT) and ESBL; the SHV WT and ESBL; and ACC, ACT/MIR, CMYII, DHA, FOX, KPC, and NDM-1, as previously reported (4).
ACKNOWLEDGMENTS
This study was supported by Wockhardt Bio AG (Zug, Switzerland).
JMI Laboratories was contracted to perform services from 2014 to 2016 for Achaogen, Actavis, Actelion, Allecra Therapeutics, Allergan, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Duke, Durata, Entasis Therapeutics, Inc., Exela, Forest Research Institute, Furiex, Genentech, Geom Therapeutics, Inc., GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Scynexis, Seachaid, Shionogi, Tetraphase, The Medicines Co., Theravance, Thermo Fisher, VenatoRX, Vertex, Zavante, and some other corporations. Some JMI Laboratories employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, and Cempra. In regard to speakers' bureaus and stock options, the authors have no conflicts to declare.
REFERENCES
- 1.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D, for the Infectious Diseases Society of America. 2013. 10 x '20 progress—development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush K. 2013. Proliferation and significance of clinically relevant beta-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 3.Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 4.Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. 2013. Prevalence of β-lactamase encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 USA hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010). Antimicrob Agents Chemother 57:3012–3020. doi: 10.1128/AAC.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanheira M, Farrell SE, Wanger A, Rolston KV, Jones RN, Mendes RE. 2013. Rapid expansion of KPC-2-producing Klebsiella pneumoniae isolates in two Texas hospitals due to clonal spread of ST258 and ST307 lineages. Microb Drug Resist 19:295–297. doi: 10.1089/mdr.2012.0238. [DOI] [PubMed] [Google Scholar]
- 6.Jacob JT, Klein E, Laxminarayan R, Beldavs Z, Lynfield R, Kallen AJ, Ricks P, Edwards J, Srinivasan A, Fridkin S, Rasheed KJ, Lonsway D, Bulens S, Herrera R, McDonald LC, Patel J, Limbago B, Bell M, Cardo D. 2013. Vital signs: carbapenem-resistant enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 7.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Palwe SR, Joshi PR, Khande HN, Biniwale SS, Bhagwat SS, Patel MV. WCK 5107 (zidebactam, ZID): a pan Gram-negative beta-lactam enhancer augmenting beta-lactam pharmacodynamics in wild type and carbapenemase producers (CP), abstr Sunday-438. ASM Microbe, 16 to 20 June 2016, Boston, MA, USA. [Google Scholar]
- 10.Barradell LB, Bryson HM. 1994. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 47:471–505. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HM, Shier KL, Graber CJ. 2014. Determining a clinical framework for use of cefepime and beta-lactam/beta-lactamase inhibitors in the treatment of infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 69:871–880. doi: 10.1093/jac/dkt450. [DOI] [PubMed] [Google Scholar]
- 12.Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther 6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sader HS, Fritsche TR, Jones RN. 2005. Potency and spectrum trends for cefepime tested against 65,746 clinical bacterial isolates collected in North American medical centers: results from the SENTRY Antimicrobial Surveillance Program (1998-2003). Diagn Microbiol Infect Dis 52:265–273. doi: 10.1016/j.diagmicrobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN. 2013. Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa. I. Cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2016. M100-S26. Performance standards for antimicrobial susceptibility testing: 26th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.B. Braun Medical, Inc. 2015. Maxipime package insert, November 2015. B. Braun Medical, Inc, Bethlehem, PA. [Google Scholar]
- 17.Bush K. 2015. A resurgence of beta-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int J Antimicrob Agents 46:483–493. doi: 10.1016/j.ijantimicag.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Jones RN, Flonta M, Gurler N, Cepparulo M, Mendes RE, Castanheira M. 2014. Resistance surveillance program report for selected European nations (2011). Diagn Microbiol Infect Dis 78:429–436. doi: 10.1016/j.diagmicrobio.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Mendes RE, Mendoza M, Singh KKB, Castanheira M, Bell JM, Turnidge JD, Lin SSF, Jones RN. 2013. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011). Antimicrob Agents Chemother 57:5721–5726. doi: 10.1128/AAC.01121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. WCK 5222 (cefepime-zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother, in press. [DOI] [PubMed] [Google Scholar]
- 21.Khande HN, Joshi PR, Palwe SR, Bhagwat SS, Patel MV. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: activity against ESBL, class C, and KPC-expressing enterics and Pseudomonas (PA) expressing AmpC (PA AmpC) or OXA beta-lactamases (PA OXA), abstr Sunday-442 ASM Microbe, 16 to 20 June 2016, Boston, MA, USA. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2015. M07-A10 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.EUCAST. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0, January 2016. European Committee on Antimicrobial Susceptibility Testing. [Google Scholar]