ABSTRACT
The Indian Revised National Tuberculosis (TB) Control Programme uses thrice-weekly treatment with standard drug dosages. The role of plasma drug levels and other factors in determining TB treatment outcomes is not well understood. We aimed to determine the factors influencing the concentrations of rifampin (RMP), isoniazid (INH), and pyrazinamide (PZA) at 2 h postdosing in adult TB patients and to study the factors impacting TB treatment outcome. We recruited 1,912 adult TB patients (newly treated and retreated patients) with pulmonary/extrapulmonary TB receiving antitubercular treatment (ATT) in the RNTCP in Chennai, India. At steady state, the concentrations of RMP, INH, and PZA were determined at 2 h postdosing after supervised drug administration. A total of 1,648 patients had a favorable outcome, while 264 (14%) had an unfavorable outcome. A total of 91%, 16%, and 17% of the patients had suboptimal concentrations of RMP (<8 μg/ml), INH (<3 μg/ml), and PZA (<20 μg/ml), respectively. Factors associated with treatment outcome were low RMP concentrations (adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.89 to 0.99; P = 0.036), category II ATT (aOR, 2.39; 95% CI, 1.56 to 3.65; P < 0.001), low body weight (aOR, 0.96; 95% CI, 0.94 to 0.98; P < 0.001), alcohol use (aOR, 2.17; 95% CI, 1.42 to 3.31; P < 0.001), male gender (aOR, 1.92; 95% CI, 1.02 to 3.62; P = 0.043), and baseline INH resistance (aOR, 5.74; 95% CI, 3.12 to 10.59; P < 0.001), which significantly increased the likelihood of an unfavorable outcome in multivariate logistic regression analysis. Further studies are needed to optimize anti-TB drug dosages and improve cure rates. Drug susceptibility testing at the baseline and attention to undernutrition and alcohol dependence are other important interventions.
KEYWORDS: tuberculosis, treatment outcome, rifampin, isoniazid, pharmacokinetics, pyrazinamide
INTRODUCTION
Tuberculosis (TB) is curable when adequate antitubercular treatment (ATT) is properly administered. Favorable treatment outcomes are achieved in about 85% of sputum smear-positive patients receiving standard short-course chemotherapy with isoniazid (INH), rifampin (RMP), pyrazinamide (PZA), and ethambutol (EMB) (1). Administration of medications through directly observed therapy (DOT) ensures patient adherence and is currently recommended in the Revised National TB Control Programme (RNTCP) in India. Despite these established standards, there is a paucity of information on the possible mechanisms to explain treatment failures, relapses, and acquired drug resistance in programmatic settings (2). While failure rates are generally low, several reports have documented recurrence rates of 12 to 15%, while multidrug resistance rates among previously treated patients vary from 12 to 17%, indicating that treatment results in the incomplete sterilization of lesions with the potential for the acquisition of drug resistance. Currently used dosages of anti-TB drugs are not based on careful pharmacokinetic studies, and a hot topic in TB research is the optimization of treatment with available drugs.
Low serum concentrations of anti-TB drugs have been associated with treatment failure, relapse, and acquired drug resistance (3–9). Recent modeling studies based on the ratio of plasma exposure to the MIC suggest that higher levels of RMP are associated with faster bacteriologic clearance and that this relationship is almost linear (10). Not many studies and none from India have examined the relationship between drug concentrations and TB treatment outcome prospectively. We undertook a study to determine the factors that influence the concentrations of RMP, INH, and PZA at 2 h postdosing in adult TB patients treated with thrice-weekly regimens in the RNTCP in Chennai, south India. We also explored the role of various factors, including plasma drug levels, on TB treatment outcomes.
RESULTS
A total of 1,928 patients were recruited; 16 patients were excluded from analysis (because the patients migrated for 9 patients, ATT was stopped due to toxicity for 1 patient, and multidrug resistance for 6 patients). Hence, 1,912 patients were included in the analysis (Fig. 1). The baseline details for the patients are given in Table 1. The median (interquartile range [IQR]) body weight was significantly lower in TB patients without diabetes mellitus (DM) (P < 0.001). The median blood glucose concentrations were 264 mg/dl (IQR, 176 to 365 mg/dl) and 91 mg/dl (IQR, 82 to 103 mg/dl) in TB patients with and without DM, respectively; the difference was statistically significant (P < 0.001). Table 1 shows the drug doses (in milligrams per kilogram) received by the different groups of patients. Significantly lower drug doses were received by patients with TB and DM, those with subtherapeutic drug concentrations, and patients with extrapulmonary TB (P < 0.001 for all drugs in all groups).
FIG 1.
Recruitment details.
TABLE 1.
Patient detailsa
| Characteristic | Value(s) |
|---|---|
| Median (IQR) age (yr) | 38 (27–50) |
| No. (%) of male patients | 1,291 (68) |
| Median (IQR) BMI (kg/m2) | 18.7 (16.4–21.6) |
| Median (IQR) body wt (kg) | 48 (42–55) |
| Nondiabetes patients | 46 (40–54)b |
| Diabetes patients | 53 (47–60)b |
| No. (%) of patients who were: | |
| Smokers | 612 (32) |
| Alcohol users | 742 (39) |
| Smear positive | 887 (46) |
| HIV seropositive | 19 (1.0) |
| No. (%) of patients who had: | |
| Diabetes mellitus | 452 (24) |
| Extrapulmonary TB | 671 (35) |
| Baseline INH resistance | 53 (8)c |
| Category I ATT | 1,659 (87) |
| Median (IQR) concn in clinical investigations | |
| Hemoglobin (g/dl) | 12.0 (10.9–13.3) |
| Glucose (mg/dl) | 96 (84–127) |
| Creatine (mg/dl) | 0.7 (0.6–0.9) |
| Urea (mg/dl) | 16 (13–20) |
| AST (U/liter) | 19.0 (16.0–25.0) |
| ALT (U/liter) | 13 (10–19) |
| Median (IQR) dose (mg/kg of body wt) | |
| RMP | 9.6 (8.7–10.7) |
| Nondiabetes patients | 10.0 (8.8–11.3)b |
| Diabetes patients | 9.0 (8.3–10.0)b |
| PTB patients | 10.0 (9.0–11.3)b |
| EPTB patients | 9.0 (8.3–10.0)b |
| Patients with RMP concn of <8 μg/ml | 9.6 (8.7–10.7)b |
| Patients with RMP concn of ≥8 μg/ml | 10.0 (9.0–11.3)b |
| INH | 12.5 (10.9–14.3) |
| Nondiabetes patients | 13.0 (11.1–15.0)b |
| Diabetes patients | 11.3 (10.0–12.8)b |
| PTB patients | 13.3 (11.3–15.0)b |
| EPTB patients | 11.3 (9.7–13.3)b |
| Patients with INH concn of <3 μg/ml | 12.0 (10.3–14.3)b |
| Patients with INH concn of ≥3 μg/ml | 12.5 (10.9–14.3)b |
| PZA | 31.3 (27.3–35.7) |
| Nondiabetes patients | 32.6 (27.8–37.5)b |
| Diabetes patients | 28.3 (25.0–31.9)b |
| PTB patients | 33.3 (28.3–37.5)b |
| EPTB patients | 28.3 (24.2–33.3)b |
| Patients with PZA concn of <20 μg/ml | 30.0 (26.3–35.7)b |
| Patients with PZA concn of ≥20 μg/ml | 31.3 (27.3–35.7)b |
| Median (IQR) drug concn (μg/ml) at 2 h postdosing | |
| RMP | 2.3 (0.6–5.0) |
| INH | 7.5 (4.5–11.0) |
| PZA | 33.5 (24.0–41.7) |
| No. (%) of patients with subtherapeutic level | |
| RMP (<8 μg/ml) | 1,740 (91) |
| INH (<3 μg/ml) | 309 (16) |
| PZA (<20 μg/ml) | 333 (17) |
| No. (%) of patients with the following treatment outcome: | |
| Favorable | 1,648 (86) |
| Cured | 687 (36) |
| Treatment completed | 961 (50) |
| Unfavorable | 264 (14) |
| Death | 31 (2) |
| Treatment failure | 20 (1) |
| Default | 213 (11) |
Data are for 1,912 patients unless indicated otherwise. BMI, body mass index; ATT, antitubercular treatment; AST, aspartate transaminase; ALT, alanine transaminase; PTB, pulmonary tuberculosis; EPTB, extrapulmonary tuberculosis; RMP, rifampin; INH, isoniazid; PZA, pyrazinamide.
P < 0.05 between the subgroups.
Data were available only for culture-positive cases (n = 651 patients).
Factors influencing drug concentrations.
Factors that were included in the univariate and multivariate analyses were gender, age, smoking, alcohol use, smear status, DM, baseline INH susceptibility, body weight, type of TB, and category of ATT (Table 2). In the univariate analysis, it was observed that females had significantly higher PZA concentrations than males (P < 0.001). Significantly higher RMP (P = 0.032), INH (P < 0.001), and PZA (P = 0.038) concentrations were observed in patients above 60 years than those <60 years of age. Plasma INH and PZA concentrations were significantly lower in TB patients with DM than those without DM, those with body weights of >48 kg than those with body weights of ≤48 kg, and those with extrapulmonary TB than those with pulmonary TB. In multiple linear regression analysis by the stepwise method, after adjusting for all factors, it was observed that males had lower concentrations of RMP (0.44 μg/ml) and PZA (3.16 μg/ml) than females. An increase in age by a year was accompanied by a marginal increase in RMP (0.02 μg/ml), INH (0.05 μg/ml), and PZA (0.12 μg/ml) levels. The coexistence of DM and TB reduced INH concentrations by 1.0 μg/ml and PZA concentrations by 2.7 μg/ml. There was a linear direct relationship between drug dose per unit of body weight and drug concentrations (Table 3).
TABLE 2.
Drug concentrations at 2 h postdosing in different groups of patientsa
| Characteristic | No. (%) of patients | Median (IQR) concn (μg/ml) |
||
|---|---|---|---|---|
| RMP | INH | PZA | ||
| Sex | ||||
| Male | 1,291 (68) | 2.3 (0.7–4.8) | 7.4 (4.5–11.0) | 32.3 (23.7–40.5) |
| Female | 621 (32) | 2.3 (0.6–5.4) | 7.7 (4.4–11.4) | 35.1 (24.9–45.4) |
| P value | 0.470 | 0.235 | <0.001 | |
| Age (yr) | ||||
| 18–59 | 1,754 (92) | 2.3 (0.6–4.9) | 7.4 (4.4–10.9) | 33.3 (23.9–41.4) |
| >60 | 158 (8) | 2.8 (1.0–6.0) | 9.0 (5.5–12.0) | 36.9 (24.5–44.9) |
| P value | 0.033 | <0.001 | 0.038 | |
| Smoker | ||||
| Yes | 612 (32) | 2.3 (0.7–4.8) | 7.8 (5.0–11.0) | 33.3 (25.3–40.8) |
| No | 1,300 (68) | 2.3 (0.6–5.0) | 7.4 (4.3–11.0) | 33.5 (23.5–42.0) |
| P value | 0.844 | 0.072 | 0.733 | |
| Alcohol user | ||||
| Yes | 742 (39) | 2.3 (0.7–4.7) | 7.7 (4.9–11.1) | 33.5 (25.0–41.5) |
| No | 1,170 (61) | 2.3 (0.6–5.1) | 7.4 (4.2–11.0) | 33.5 (23.5–42.0) |
| P value | 0.590 | 0.232 | 0.953 | |
| Smear statusb | ||||
| Positive | 887 (71) | 2.3 (0.6–4.9) | 7.9 (4.7–11.3) | 34.5 (24.1–42.3) |
| Negative | 354 (29) | 2.4 (0.8–5.3) | 7.7 (4.9–11.4) | 33.4 (24.9–43.0) |
| P value | 0.340 | 0.938 | 0.913 | |
| Diabetes | ||||
| Yes | 452 (24) | 2.3 (0.7–4.9) | 6.6 (3.9–9.8) | 31.0 (22.3–38.0) |
| No | 1,460 (76) | 2.3 (0.6–5.0) | 7.8 (4.7–11.3) | 34.1 (24.6–42.7) |
| P value | 0.858 | <0.001 | <0.001 | |
| Baseline INH susceptibilityc | ||||
| Sensitive | 598 (92) | 2.2 (0.6–4.8) | 8.0 (4.6–11.3) | 34.5 (24.1–42.8) |
| Resistant | 53 (8) | 2.6 (0.5–5.8) | 9.0 (5.7–11.4) | 37.9 (29.1–44.0) |
| P value | 0.376 | 0.442 | 0.127 | |
| Body wt (kg) | ||||
| ≤48 | 961 (50) | 2.3 (0.7–5.2) | 8.4 (5.0–12.0) | 37.6 (25.3–46.0) |
| >48 | 951 (50) | 2.3 (0.6–4.8) | 6.8 (4.0–9.7) | 30.6 (23.0–37.1) |
| P value | 0.1681 | <0.001 | <0.001 | |
| Disease | ||||
| PTB | 1,241 (65) | 2.3 (0.6–5.0) | 7.9 (4.7–11.3) | 34.1 (24.4–42.6) |
| EPTB | 671 (35) | 2.3 (0.6–5.0) | 7.0 (4.0–10.2) | 32.2 (23.5–39.8) |
| P value | 0.981 | 0.001 | 0.003 | |
| Category of ATT | ||||
| I | 1,659 (87) | 2.3 (0.7–5.0) | 7.4 (4.6–11.0) | 33.4 (24.3–41.6) |
| II | 253 (13) | 2.2 (0.6–4.9) | 7.8 (4.0–11.4) | 33.7 (20.5–42.3) |
| P value | 0.960 | 0.780 | 0.487 | |
Data are for 1,912 patients unless indicated otherwise.
Data are only for pulmonary TB patients (n = 1,241).
Data are only for culture-positive cases (n = 651).
TABLE 3.
Factors influencing drug concentrationsa
| Type of analysis and factor | RMP |
INH |
PZA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| Univariate analysis | |||||||||
| Gender | −0.334 | −0.664 to −0.005 | 0.047 | −0.311 | −0.746 to 0.123 | 0.160 | −2.779 | −4.115 to −1.442 | <0.001 |
| Age | 0.015 | 0.004 to 0.027 | 0.007 | 0.036 | 0.022 to 0.051 | <0.001 | 0.052 | 0.006 to 0.098 | 0.026 |
| Smoker | −0.083 | −0.414 to 0.248 | 0.621 | 0.294 | −0.142 to 0.730 | 0.186 | −0.298 | −1.646 to 1.050 | 0.664 |
| Alcohol user | −0.175 | −0.492 to 0.142 | 0.279 | 0.175 | −0.242 to 0.593 | 0.411 | −0.071 | −1.362 to 1.219 | 0.914 |
| Smear status | −0.150 | −0.514 to 0.214 | 0.420 | 0.141 | −0.349 to 0.630 | 0.573 | −0.426 | −1.925 to 1.074 | 0.578 |
| Diabetes mellitus | −0.092 | −0.456 to 0.271 | 0.619 | −0.960 | −1.437 to −0.483 | <0.001 | −3.255 | −4.727 to −1.783 | <0.001 |
| Body wt | −0.016 | −0.029 to −0.002 | 0.025 | −0.074 | −0.102 to −0.067 | <0.001 | −0.281 | −0.335 to −0.227 | <0.001 |
| Baseline INH susceptibilityb | NA | NA | NA | 0.699 | −0.584 to 1.982 | 0.285 | NA | NA | NA |
| Type of TB | 0.002 | −0.322 to 0.325 | 0.992 | 0.789 | 0.364 to 1.215 | <0.001 | 1.668 | 0.353 to 2.984 | 0.013 |
| Category of ATT | 0.080 | −0.375 to 0.536 | 0.730 | 0.024 | −0.577 to 0.625 | 0.938 | −1.150 | −3.004 to 0.705 | 0.224 |
| Drug dose | 0.175 | 0.078 to 0.271 | <0.001 | 0.398 | 0.322 to 0.473 | <0.001 | 0.520 | 0.428 to 0.613 | <0.001 |
| Multivariate analysis | |||||||||
| Gender | −0.442 | −0.782 to −0.102 | 0.011 | NA | NA | NA | −3.161 | −4.499 to −1.823 | <0.001 |
| Age | 0.020 | 0.009 to 0.032 | <0.001 | 0.051 | 0.036 to 0.066 | <0.001 | 0.122 | 0.073 to 0.170 | <0.001 |
| Diabetes mellitus | NA | NA | NA | −1.006 | −1.511 to −0.501 | <0.001 | −2.676 | −4.233 to −1.119 | 0.001 |
| Body wt | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Type of TB | NA | NA | NA | NS | NS | NS | NS | NS | NS |
| Drug dose | 0.171 | 0.075 to 0.268 | <0.001 | 0.375 | 0.298 to 0.451 | <0.001 | 0.482 | 0.387 to 0.576 | <0.001 |
NS, not significant; NA, not applicable; β, regression coefficient, which indicates the change per 1 unit.
This factor was tested against INH concentrations only.
TB treatment outcome.
Of the 1,912 patients for whom outcomes were available, 1,648 (86%) of all patients, 1,011 (81%) of the pulmonary TB patients, and 700 (79%) of the smear-positive patients had favorable outcomes. The corresponding numbers with unfavorable outcomes were 264 (14%), 230 (19%), and 187 (21%) (Table 1).
The influence of such factors as gender, age, smoking, alcohol use, smear status, DM, baseline INH susceptibility, body weight, type of TB, category of ATT, and plasma concentrations of RMP, INH, and PZA at 2 h postdosing on treatment outcome was tested. Among these variables, RMP concentrations (adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.89 to 0.99; P = 0.036), category II ATT (aOR, 2.39; 95% CI, 1.56 to 3.65; P < 0.001), body weight (aOR, 0.96; 95% CI, 0.94 to 0.98; P < 0.001), alcohol use (aOR, 2.17; 95% CI, 1.42 to 3.31; P < 0.001), male gender (aOR, 1.92; 95% CI, 1.02 to 3.62; P = 0.043), and baseline INH resistance (aOR, 5.74; 95% CI, 3.12 to 10.59; P < 0.001) significantly influenced treatment outcomes (Table 4). An increase in the plasma RMP level by 1 μg/ml and an increase in body weight by 1 kg decreased the chances of an unfavorable outcome by 6% and 4%, respectively.
TABLE 4.
Factors associated with treatment outcome
| Factor | Outcomea: |
ORb (95% CI) | P value for OR | aOR (95% CI) | P value for aOR | |
|---|---|---|---|---|---|---|
| Favorable | Unfavorable | |||||
| Sex | ||||||
| Male | 1,056 (64.1) | 235 (89) | 4.54 (3.05–6.76) | <0.001 | 1.92 (1.02–3.62) | 0.043 |
| Female | 592 (35.9) | 29 (11) | Reference | Reference | ||
| Age (yr) | ||||||
| 18–59 | 1,519 (92.2) | 235 (89) | Reference | 0.085 | NSc | NS |
| ≥60 | 129 (7.8) | 29 (11) | 1.45 (0.95–2.22) | NS | NS | |
| Smoker | ||||||
| No | 1,172 (71.1) | 128 (48.5) | Reference | <0.001 | NS | NS |
| Yes | 476 (28.9) | 136 (51.5) | 2.62 (2.01–3.41) | NS | NS | |
| Alcohol user | ||||||
| No | 1,077 (65.4) | 93 (35.2) | Reference | <0.001 | Reference | <0.001 |
| Yes | 571 (34.6) | 171 (64.8) | 3.47 (2.64–4.55) | 2.17 (1.42–3.31) | ||
| Smear statusd | ||||||
| Negative | 515 (42.4) | 59 (24) | Reference | <0.001 | NS | NS |
| Positive | 700 (57.6) | 187 (76) | 2.33 (1.7–3.19) | NS | NS | |
| Diabetes | ||||||
| No | 1,251 (75.9) | 209 (79.2) | Reference | 0.248 | NS | NS |
| Yes | 397 (24.1) | 55 (20.8) | 0.83 (0.6–1.14) | NS | NS | |
| Baseline INH susceptibilitye | ||||||
| Sensitive | 687 (96.5) | 147 (84) | Reference | <0.001 | Reference | <0.001 |
| Resistant | 25 (3.5) | 28 (16) | 5.23 (2.97–9.24) | 5.74 (3.12–10.59) | ||
| Body wt (kg) | 49 (42–56) | 45 (40–50) | 0.96 (0.95–0.97) | <0.001 | 0.96 (0.94–0.98) | <0.001 |
| Disease | ||||||
| EPTB | 637 (38.7) | 34 (12.9) | Reference | <0.001 | NS | NS |
| PTB | 1,011 (61.3) | 230 (87.1) | 4.26 (2.93–6.19) | NS | NS | |
| Category | ||||||
| I | 1,464 (88.8) | 195 (73.9) | Reference | <0.001 | Reference | <0.001 |
| II | 184 (11.2) | 69 (26.1) | 2.82 (2.06–3.86) | 2.39 (1.56–3.65) | ||
| Concn (μg/ml) at 2 h postdosing | ||||||
| RMP | 2.3 (0.7–5) | 2.1 (0.5–4.4) | 0.97 (0.93–1.01) | 0.140 | 0.94 (0.89–0.99) | 0.036 |
| INH | 7.4 (4.5–10.9) | 8.4 (4.7–11.8) | 1.02 (0.99–1.05) | 0.116 | NS | NS |
| PZA | 33.3 (24–41.5) | 35.8 (23.7–42.5) | 0.99 (0.98–1.01) | 0.726 | NS | NS |
Data represent the number (percent) of patients for all factors except body weight and the concentration at 2 h postdosing, for which the data represent the median (IQR).
OR, odds ratio.
NS, not significant.
Data are only for pulmonary TB patients (n = 1,241).
Data are only for culture-positive cases (n = 651).
After defaulted patients were excluded from the unfavorable outcome group and when only failures and deaths were considered to be unfavorable outcomes, RMP concentrations (aOR, 0.86; 95% CI, 0.75 to 0.98; P = 0.029), category II ATT (aOR, 2.91; 95% CI, 1.33 to 6.35; P = 0.007), body weight (aOR, 0.95; 95% CI, 0.91 to 0.99; P = 0.014), and baseline INH resistance (aOR, 13.16; 95% CI, 5.68 to 30.49; P < 0.001) significantly influenced treatment outcomes. A higher proportion of previously treated patients (category II) than newly treated patients had unfavorable outcomes (27% versus 12%; P < 0.001).
Two-month smear status.
Of the 887 patients who were initially smear positive, smear results at 2 months posttreatment were available in the RNTCP records for 795 patients. Among them, 722 were smear negative. The median drug concentrations between those who converted to smear negativity and those who did not were not significantly different.
NAT2 gene polymorphism and INH acetylator status.
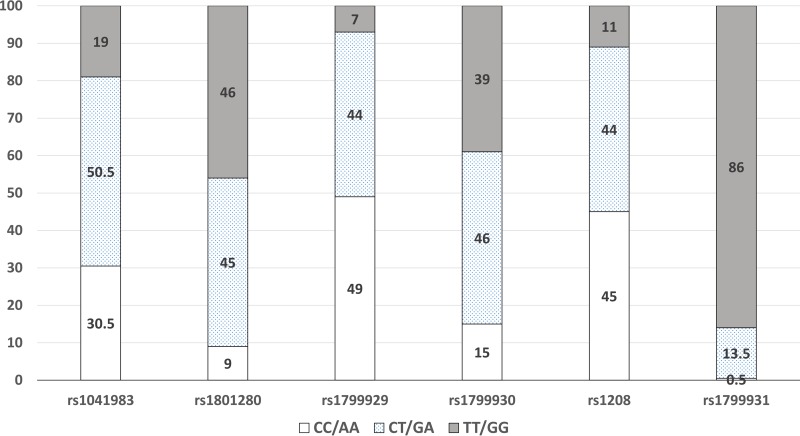
INH acetylator status, based on the NAT2 gene polymorphism, was available for 632 patients. The allele frequencies for the six genotypes are shown in Fig. 2. The numbers of slow, intermediate, and rapid acetylators were 361, 228, and 43, respectively. The median INH concentrations in these groups were 9.5 μg/ml (IQR, 5.0 to 12.6 μg/ml), 7.5 μg/ml (IQR, 4.3 to 10.8 μg/ml), and 4.3 μg/ml (IQR, 2.2 to 7.3 μg/ml), respectively; the differences were statistically significant (P < 0.001). There were no significant differences in the distribution of NAT2 genotypes among patients infected with INH-resistant and -sensitive Mycobacterium tuberculosis strains or among those with favorable and unfavorable outcomes. However, there was a significant difference in the proportion of patients with suboptimal INH concentrations among the patients with the three genotypes, with the highest being among rapid acetylators (29.5%), followed by intermediate acetylators (16.7%) and slow acetylators (13.3%) (P = 0.025). The trend in the proportion of patients with subtherapeutic INH concentrations among the patients with the three genotypes was statistically significant (P = 0.011).
FIG 2.
Distribution of allele frequencies for the six genotypes of the NAT2 gene.
DISCUSSION
According to the WHO Global TB Report, in 2015, 2.2 million cases of the estimated global annual incidence of 9.6 million TB cases occurred in India (11). India continues to use thrice-weekly anti-TB regimens, with a recent policy change to the daily regimens being made. Even though large numbers of patients are treated through the RNTCP, not much is known about the factors influencing key anti-TB drug concentrations or the risk factors associated with poor treatment outcomes. The present study identified factors that impact key first-line anti-TB drug concentrations and, importantly, examined the relationship between drug concentrations and TB treatment outcome in adult TB patients treated through the RNTCP. Since this study was undertaken in programmatic settings, we chose to estimate the drug concentrations at 2 h postdosing, which is predictive of the pharmacokinetic parameters obtained from intensive sampling (12). We have shown that the RMP, INH, and PZA concentrations at 2 h postdosing correlate with the peak concentrations and are often used as a surrogate for peak concentrations, though this sample does not account for slow drug absorption (13).
Several factors were associated with lower drug levels in the univariate analysis. However, in the multivariate analysis, only a few factors were significant. For example, age and diabetes significantly influenced INH and PZA concentrations. Patients above 60 years of age had significantly higher drug concentrations than those below 60 years of age. Physiological changes during the aging process can have a significant impact on drug pharmacokinetics, such as the volume of distribution, the protein binding capacity, and hepatic and renal clearance. Elderly patients exhibit frequent side effects, probably due to higher drug concentrations (14). Older patients tend to accumulate drugs for longer periods of time, and some might require dosage adjustments. Studies of the pharmacokinetics of RMP and rifapentine in the elderly population, however, do not suggest the need for any dose modifications (15, 16).
We have reported for the first time that INH and PZA concentrations are lower in TB patients with diabetes than those without diabetes. Babalik et al. reported that the plasma INH concentration at 2 h postdosing was lower in patients with TB and DM than those with TB (17). These decreases are most likely due to higher body weights of TB patients with DM and, hence, the lower drug doses (in milligrams per kilogram of body weight) received by TB patients with DM than those without DM. Similarly, patients with extrapulmonary TB received lower drug doses (in milligrams per kilogram) than those with pulmonary TB, leading to lower drug concentrations. Females had higher INH and PZA levels than males, with these findings being consistent with those of other studies (5, 8), though the mechanism of sex-related differences in drug pharmacokinetics is poorly understood.
We undertook substudies in the same population to examine the influence of polymorphisms in the NAT2 and SLCO genes on plasma INH and RMP concentrations, respectively. While the NAT2 genotypes significantly impacted INH levels, the SLCO genotypes (rs11045819, rs4149032, and rs4149033) did not influence RMP concentrations (18, 19). In this study, we undertook NAT2 genotyping in only about a third of the patients. Although no significant associations between the NAT2 genotypes and INH susceptibility or treatment outcome were observed, among the rapid acetylators, the proportion of patients with suboptimal INH concentrations was higher.
Several groups from across the world that have studied anti-TB drug pharmacokinetics have reported suboptimal drug concentrations, particularly suboptimal RMP and INH concentrations (20–28). However, not many studies have related drug concentrations with outcomes. Although our finding that the majority of patients in this study had RMP concentrations of less than 8 μg/ml at 2 h postdosing is concerning, not all patients had an unfavorable outcome. We explored possible reasons for low RMP levels. Random samples of RMP tablets from the same batch consumed by patients were checked for the amount of the active ingredient, and we found that the drug contents in the tablets were within 5% of the stated content. The drugs were administered under DOT on the study day. Genetic polymorphism (at least for the SLCO1 gene) did not have a role. We were aware that most patients took their anti-TB medications after the consumption of food. Although we have shown that food significantly reduces the plasma concentrations of RMP, INH, and PZA (29), the reason why RMP levels alone were severely affected is unclear. It would be interesting to identify genetic or other factors that are unique to the Indian population, though the most obvious reason is that the patients are underdosed, with 10 mg/kg thrice-weekly being an insufficient dose.
The relationship between plasma drug concentrations and TB treatment outcome remains a complex one, with various studies being divided in their findings. Apart from drug concentrations, treatment outcome is driven by multiple factors, such as the bacillary load, type of strain, virulence, MIC in relation to drug concentrations, drug concentrations at the site of lesion, duration of infection, extent of disease, and the immune status and nutritional status of the subject. Mota et al. performed a systematic review and meta-analysis relating the concentrations of first-line anti-TB drugs at 2 h postdosing and clinical outcomes (26). They observed that of the 12 studies taken up for analysis, only 3 studies demonstrated an association between drug levels and unsuccessful treatment outcomes, thus failing to draw a firm association between serum drug concentrations and treatment outcome (26). This review failed to draw a firm association between serum drug concentrations and treatment outcome. A study from Indonesia showed that though low RMP, INH, and PZA concentrations occurred in many patients even with the DOT strategy, most patients had a good treatment outcome (30). Narita and colleagues did not find an association between TB recurrence and the serum levels of anti-TB drugs (31). In another study, it was observed that low RMP and INH peak concentrations preceded acquired drug resistance and that low drug exposures were predictive of clinical outcomes in TB patients (32). Low peak concentrations of RMP were found to influence the treatment outcome and/or the acquisition of RMP resistance (5, 9). A retrospective cohort study from Virginia reported that most patients who responded slowly to treatment had RMP and INH concentrations below the expected range at 2 h postdosing (33). It has been reported that a longer time to culture conversion and treatment failures were more frequent in those having drug concentrations below the expected range (20, 27). We explored whether baseline drug levels could predict a slow response, but we did not observe any difference in drug levels between patients who were sputum smear positive and those who were sputum smear negative at 2 months. A lack of an association between the RMP peak concentration and slow culture conversion has been reported previously (34).
Numerous reports on the factors influencing TB treatment outcomes are available, but not many of these studies have included drug concentrations in their analysis. Our study, done with a large, adequately powered, and representative patient population, has shown that low RMP levels had a significant impact on treatment outcome, with the other factors being male gender, low body weight, baseline INH resistance, alcohol use, and previous treatment for TB. The same factors emerged to be significant when defaults were excluded from the unfavorable outcome group and also in the subgroups of patients analyzed separately (patients with pulmonary TB, smear-positive patients, patients treated with category I ATT). Our study is the first and only one from India that observed low RMP concentrations to be a risk factor for a poor outcome.
Even though several studies reported drug concentrations below the expected range in a high proportion of patients, it is often observed that not all patients have unsuccessful outcomes. A recent report by Maze et al. from New Zealand observed that even though patients had drug concentrations below the international therapeutic ranges, they were successfully treated (35). This raises a pertinent question: is there a need to define cutoff drug concentrations in various settings that would be a predictive marker of an unfavorable outcome?
This study had some limitations. Since the study was performed under programmatic settings, we could not control the timing of food intake, but we made a note of the time at which the patients took their breakfast and the drugs. We observed that most patients took drugs 15 to 30 min after breakfast. We also observed that drug concentrations did not significantly differ when the drugs were taken 30 or 60 min after the consumption of food (unpublished findings). In a substudy, we did observe that food significantly reduced RMP, INH, and PZA concentrations (29). Further, while ATT was taken under DOT according to programmatic guidelines, drug administration was supervised on the study day. Although the concentrations at 2 h postdosing have consistently been used by other researchers, this alone may not be informative, in view of the high degree of variability of RMP absorption observed; the use of the trough concentrations of drugs would have been ideal. Also, we did not follow the patients after the end of ATT for recurrences, which could have underestimated the number of unfavorable outcomes.
In summary, this study, undertaken in adult TB patients treated in the RNTCP in Chennai in south India under programmatic settings, has shown that age, gender, drug doses, and DM are important factors that impact RMP, INH, and PZA concentrations. We identified male gender, low RMP, low body weight, baseline INH resistance, alcohol use, and previous treatment to be risk factors for an unfavorable outcome. It is important to ensure that patients take their treatment regularly to prevent being retreated. It is important to identify patients with low RMP concentrations early during ATT so that the dose can be appropriately increased. Although it would be difficult to have RMP levels routinely estimated in the RNTCP, this facility can be established in select places. Drug susceptibility testing at the baseline and attention to undernutrition and alcohol dependence are other important interventions that can be used to improve TB treatment outcomes. There is a need to undertake this study with long term follow-up in different parts of India, in order to generalize the findings.
MATERIALS AND METHODS
Patients.
A prospective study was undertaken in adult patients with pulmonary/extrapulmonary TB receiving ATT in RNTCP treatment centers of the Chennai Corporation from October 2013 to September 2015. Patients were recruited from eight TB units across the city of Chennai. Diagnosis and treatment were according to RNTCP guidelines (36). Patients were treated with either the category I treatment (RMP, INH, PZA, and EMB for 2 months, followed by RMP and INH for 4 months) or the category II treatment (streptomycin, INH, RMP, PZA, and EMB for 2 months, followed by INH, RMP, PZA, and EMB for 1 month and INH, RMP, and EMB for the remaining 5 months) (36). The entire treatment was thrice weekly, with the drug doses being 450 mg for RMP (600 mg for those with body weights of ≥60 kg), 600 mg for INH, 1,200 mg for EMB, 1,500 mg for PZA, and 0.75 g for streptomycin. Rifampin, INH, PZA, and EMB were administered as separate tablets, and streptomycin was administered as an injection. Patients meeting the following study criteria were recruited: (i) they were age 18 years or above, (ii) they had a body weight of >30 kg, (iii) they had received ATT for a minimum of 2 weeks, (iv) they were not very sick or moribund, (v) they were willing to participate and give informed written consent, and (vi) they agreed to visit the same DOT center until study completion. A structured questionnaire was used to collect patient details. Those with a known history of type 2 diabetes mellitus (DM) with or without a random blood glucose level of ≥200 mg/dl on the study day were considered diabetic. The study was approved by the ethics committee of the National Institute for Research in Tuberculosis, Chennai, India.
Study procedures.
The study was conducted at the TB units of the Chennai Corporation, after patients had received a minimum of seven doses of ATT. On the study day, the anti-TB drugs were administered under direct supervision, and about 3 ml blood was collected at 2 h postdosing. Clinical biochemistry tests (random glucose and liver and renal function tests), routine hematological tests, and HIV testing were carried out, in addition to estimation of RMP, INH, and PZA concentrations. Five hundred microliters of blood was used for DNA extraction and NAT2 genotyping.
Drug measurements.
The blood was immediately centrifuged, and the plasma was separated and stored at −20°C until analysis. Measurement of plasma concentrations of RMP, INH, and PZA was undertaken within a week of sample collection by high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan) using validated methods as previously described (37, 38).
NAT2 genotyping.
Genomic DNA was used to analyze six single nucleotide polymorphisms (rs1041983, rs1801280, rs1799929, rs1799930, rs1208, and rs1799931) in the NAT2 gene by real-time PCR and with sequence detection software (SDS; version 1.3.1). The slow, intermediate, and rapid NAT2 acetylator genotypes were determined using the NAT2PRED web server (39).
Sputum samples.
Sputum samples were collected at the baseline (before the start of ATT) from pulmonary TB patients (wherever possible) to perform culture and drug susceptibility testing using standard methods (40). Sputum smear examination and other diagnostic tests were performed, and the results were interpreted by the treating physicians at the TB units.
Follow-up and TB treatment outcome.
All patients continued to receive ATT according to RNTCP guidelines. Standard default retrieval procedures were adopted. TB treatment outcomes (cured/treatment completed, failure, death, or default) at the end of ATT were noted from the TB register/treatment card. Cured and treatment completed were taken as favorable outcomes, and default, death, and failure were taken as unfavorable outcomes.
Statistical analysis and sample size calculation.
Data analysis was performed using SPSS software (version 20.0). The data were verified, and normality was checked by the Shapiro-Wilks test. Summary statistics are presented as proportions for categorical variables and as medians with interquartile ranges for continuous variables. The Mann-Whitney U test was used to compare drug concentrations between patient groups. Comparison of proportions was performed using the z proportion test. The subtherapeutic cutoff concentrations were taken as <8 μg/ml for RMP, <3 μg/ml for INH, and <20 μg/ml for PZA (12).
Univariate linear regression analysis followed by multiple linear regression analysis using the stepwise method was performed to determine the factors associated with drug concentrations.
Binary logistic regression was carried out to identify the factors that were independently associated with treatment outcome (with and without defaults in the unfavorable outcome group). Adjusted odds ratios (aOR) and their 95% confidence intervals were obtained using the stepwise method.
Sample size was calculated on the basis of a previous study (41), which reported the proportions of defaults, failures, and deaths to be 29% (194/676). With a precision of 10%, a power of 90%, an α value of 5%, a design effect of 2, and a 1% loss during follow-up being accounted for, the required sample size was 1,928.
ACKNOWLEDGMENTS
We thank the patients who took part in the study, V. Sudha for estimation of drug concentrations by high-performance liquid chromatography, the staff of the Clinical Biochemistry and Hematology laboratories for blood estimations, the data entry operators, the field investigators engaged with patient recruitment, the staff at the RNTCP treatment centers of the Chennai Corporation, and the financial support by the United States Agency for International Development through the World Health Organization, SEARO, New Delhi, India.
G.R. designed the study and wrote the study protocol, G.R. obtained regulatory approvals, H.K.A.K. and G.R. conducted the study, H.K.A.K. supervised estimation of the drug concentrations, A.D. carried out bacteriological investigations, R.K. performed the genotyping experiments, L.J. recruited the study patients, C.V. and K.T. performed the statistical analysis, V.R. carried out data cleaning and error checking, G.R. drafted the manuscript, and S.S. provided critical inputs and overall guidance.
This study was supported by the United States Agency for International Development through the World Health Organization, SEARO, New Delhi, India.
We have no conflicts of interest to declare.
REFERENCES
- 1.Jindani A, Nunn AJ, Enarson DA. 2004. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 364:1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 2.Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. 1995. Eleven years of community-based directly observed therapy for tuberculosis. JAMA 274:945–951. [PubMed] [Google Scholar]
- 3.Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. 1998. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 113:1178–1183. doi: 10.1378/chest.113.5.1178. [DOI] [PubMed] [Google Scholar]
- 4.Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, Oyewo A, Talbot EA, Kenyon TA, Moeti TL, Moffat HJ, Peloquin CA. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–469. doi: 10.1086/431984. [DOI] [PubMed] [Google Scholar]
- 5.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner M, Burman W, Vernon A, Benator D, Peloquin CA, Khan A, Weis S, King B, Shah N, Hodge T, Tuberculosis Trials Consortium. 2003. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 167:1341–1347. doi: 10.1164/rccm.200208-951OC. [DOI] [PubMed] [Google Scholar]
- 7.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, Jones B, Silva-Trigo C, Zhao Z, Hodge T, Tuberculosis Trials Consortium. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 8.Um SW, Lee SW, Kwon SY, Yoon HI, Park KU, Song J, Lee CT, Lee JH. 2007. Low serum concentrations of anti-TB drugs and determinants of their serum levels. Int J Tuberc Lung Dis 11:972–978. [PubMed] [Google Scholar]
- 9.Mehta JB, Shantaveerapa H, Byrd RP Jr, Morton SE, Fountain F, Roy TM. 2001. Utility of rifampin blood levels in the treatment of active pulmonary tuberculosis patients who were slow to respond to routine directly observed therapy. Chest 120:1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 10.Savic RM, Ruslami R, Hibma JE, Hesseling A, Ramachandran G, Ganiem AR, Swaminathan S, McIlleron H, Gupta A, Thakur K, van Crevel R, Aarnoutse R, Dooley KE. 2015. Pediatric tuberculous meningitis: model-based approach to determining optimal doses of rifampin and levofloxacin for children. Clin Pharmacol Ther 98:622–629. doi: 10.1002/cpt.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. 2016. Global TB report. WHO, Geneva, Switzerland. [Google Scholar]
- 12.Alsultan A, Peloquin CA. 2014. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 13.Hemanth Kumar AK, Kannan T, Chandrasekaran V, Sudha V, Vijayakumar A, Ramesh K, Lavanya J, Swaminathan S, Ramachandran G. 2016. Pharmacokinetics of thrice weekly rifampicin, isoniazid and pyrazinamide in adult tuberculosis patients in India. Int J Tuberc Lung Dis 20:1236–1241. doi: 10.5588/ijtld.16.0048. [DOI] [PubMed] [Google Scholar]
- 14.Walubo A, Chan K, Woo J, Chan HS, Wong CL. 1991. The disposition of antituberculous drugs in plasma of elderly patients. II. Isoniazid, rifampicin and pyrazinamide. Methods Find Exp Clin Pharmacol 13:551–558. [PubMed] [Google Scholar]
- 15.Advenier C, Gobert C, Houin G, Bidet D, Richelet S, Tillement JP. 1983. Pharmacokinetic studies of rifampicin in the elderly. Ther Drug Monit 5:61–65. [DOI] [PubMed] [Google Scholar]
- 16.Keung AC, Eller MG, Weir SJ. 1998. Single-dose pharmacokinetics of rifapentine in elderly men. Pharm Res 15:1286–1291. doi: 10.1023/A:1011960428896. [DOI] [PubMed] [Google Scholar]
- 17.Babalik A, Ulus IH, Bakirci N, Kuyucu T, Arpag H, Dagyildizi L, Capaner E. 2013. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother 57:5740–5742. doi: 10.1128/AAC.01345-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemanth Kumar AK, Ramesh K, Kannan T, Sudha V, Hemalatha Haribabu, Lavanya J, Swaminathan S, Ramachandran G. 2016. NAT2 gene polymorphisms and plasma isoniazid concentrations in tuberculosis patients in South India. Indian J Med Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramesh K, Hemanth Kumar AK, Kannan T, Vijayalakshmi R, Sudha V, Manohar Nesakumar S, Bharathiraja T, Lavanya J, Swaminathan S, Ramachandran G. 2016. SLCO1B1 gene polymorphisms do not influence plasma rifampicin concentrations in a south Indian population. Int J Tuberc Lung Dis 20:1231–1235. doi: 10.5588/ijtld.15.1007. [DOI] [PubMed] [Google Scholar]
- 20.Babalik A, Mannix S, Francis D, Menzies D. 2011. Therapeutic drug monitoring in the treatment of active tuberculosis. Can Respir J 18:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahimi F, Tabarsi P, Kobarfard F, Bozorg BZ, Goodarzi A, Dastan F, Shahsavari N, Emami S, Habibi M, Salamzadeh J. 2013. Isoniazid, rifampicin and pyrazinamide plasma concentrations 2 and 6 h post dose in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 17:1602–1606. doi: 10.5588/ijtld.13.0019. [DOI] [PubMed] [Google Scholar]
- 22.Ray J, Gardiner I, Marriott D. 2003. Managing antituberculosis drug therapy by therapeutic drug monitoring of rifampicin and isoniazid. Intern Med J 33:229–234. doi: 10.1046/j.1445-5994.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 23.Tostmann A, Mtabho CM, Semvua HH, Boogaard JVD, Kibiki GS, Boeree MJ, Aarnoutse RE. 2013. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob Agents Chemother 57:3208–3213. doi: 10.1128/AAC.02599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Crevel R, Alisjahbana B, de Lange WC, Borst F, Danusantoso H, van der Meer JW, Burger D, Nelwan RH. 2002. Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int J Tuberc Lung Dis 6:497–502. [DOI] [PubMed] [Google Scholar]
- 25.Van Tongeren L, Nolan S, Cook VJ, FitzGerald JM, Johnston JC. 2013. Therapeutic drug monitoring in the treatment of tuberculosis: a retrospective analysis. Int J Tuberc Lung Dis 17:221–224. doi: 10.5588/ijtld.12.0279. [DOI] [PubMed] [Google Scholar]
- 26.Mota L, Al-Efraij K, Campbell JR, Cook VJ, Marra F, Johnson J. 2016. Therapeutic drug monitoring in anti-tuberculosis treatment: a systematic review and meta-analysis. Int J Tuberc Lung Dis 20:819–826. doi: 10.5588/ijtld.15.0803. [DOI] [PubMed] [Google Scholar]
- 27.Prahl JB, Johansen IS, Cohen AS, Frimodt-Moller N, Andersen AB. 2014. Clinical significance of 2 h plasma concentrations of first-line anti-tuberculosis drugs: a prospective observational study. J Antimicrob Chemother 69:2841–2847. doi: 10.1093/jac/dku210. [DOI] [PubMed] [Google Scholar]
- 28.Kayhan S, Akgunes A. 2011. Therapeutic monitoring of isoniazid, rifampicin, ethambutol and pyrazinamide serum levels in the treatment of active pulmonary tuberculosis and determinants of their serum concentrations. Afr J Pharm Pharmacol 5:2035–2041. doi: 10.5897/AJPP11.511. [DOI] [Google Scholar]
- 29.Hemanth Kumar AK, Chandrasekaran V, Kiran Kumar A, Kawaskar M, Lavanya J, Swaminathan S, Ramachandran G. Food significantly reduces plasma concentrations of first-line anti-TB drugs. Indian J Med Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burhan E, Ruesen C, Ruslami R, Ginanjir A, Mangunnegoro H, Ascobat P, Donders R, van Crevel R, Aarnoutse R. 2013. Isoniazid, rifampin and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 57:3614. doi: 10.1128/AAC.02468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narita M, Hisada M, Thimmappa B, Stambaugh JJ, Ibrahim E, Hollender ES, Ashkin D. 2001. Tuberculosis recurrence: multivariate analysis of serum levels of tuberculosis drugs, human immunodeficiency virus status and other risk factors. Clin Infect Dis 32:515–517. doi: 10.1086/318490. [DOI] [PubMed] [Google Scholar]
- 32.Pasipanodya JG, McIlleron H, Burger A, Wash P, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heysell SK, Moore JL, Keller SJ, Houpt ER. 2010. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 16:1546–1553. doi: 10.3201/eid1610.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang KC, Leung CC, Yew WW, Kam KM, Yip CW, Ma CH, Tam CM, Leung EC, Law WS, Leung WM. 2008. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur J Clin Microbiol Infect Dis 27:467–472. doi: 10.1007/s10096-007-0454-6. [DOI] [PubMed] [Google Scholar]
- 35.Maze MJ, Paynter J, Chiu W, Hu R, Nisbet M, Lewis C. 2016. Therapeutic drug monitoring of isoniazid and rifampicin during anti-tuberculosis treatment in Auckland, New Zealand. Int J Tuberc Lung Dis 20:955–960. doi: 10.5588/ijtld.15.0792. [DOI] [PubMed] [Google Scholar]
- 36.Revised National Tuberculosis Control Programme. 2011. Annual status report, p 98–101. Central TB Division, Government of India, New Delhi, India. [Google Scholar]
- 37.Hemanth Kumar AK, Chandra I, Geetha R, Chelvi KS, Lalitha V, Prema G. 2004. A validated high performance liquid chromatography method for the determination of rifampicin and desacetyl rifampicin in plasma and urine. Indian J Pharmacol 36:231–233. [Google Scholar]
- 38.Hemanth Kumar AK, Sudha V, Ramachandran G. 2012. Simple and rapid liquid chromatography method for simultaneous determination of isoniazid and pyrazinamide in plasma. SAARC J TB Lung Dis HIV/AIDS 9:13–18. [Google Scholar]
- 39.Kuznetsov IB, McDuffie M, Moslehi R. 2009. A web-server for inferring the human N-acetyl transferase-2 (NAT2) enzymatic phenotype from NAT2 genotype. Bioinformatics 25:1185–1186. doi: 10.1093/bioinformatics/btp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuberculosis Research Centre. 2010. Standard operating procedure for mycobacteriology laboratory. National Institute for Research in Tuberculosis, Chennai, India: http://www.nirt.res.in/pdf/bact/SOP.pdf. [Google Scholar]
- 41.Santha T, Garg R, Frieden TR, Chandrasekaran V, Subramani R, Gopi PG, Selvakumar N, Ganapathy S, Charles N, Rajamma J, Narayanan PR. 2002. Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, south India, 2000. Int J Tuberc Lung Dis 6:780–788. [PubMed] [Google Scholar]