ABSTRACT
Multidrug-resistant (MDR) Acinetobacter baumannii is increasingly more prevalent in nosocomial infections. Although in vitro susceptibility of A. baumannii to minocycline is promising, the in vivo efficacy of minocycline has not been well established. In this study, the in vivo activity of minocycline was evaluated in a neutropenic murine pneumonia model. Specifically, we investigated the relationship between minocycline exposure and bactericidal activity using five A. baumannii isolates with a broad range of susceptibility (MIC ranged from 0.25 mg/liter to 16 mg/liter). The pharmacokinetics of minocycline (single dose of 25 mg/kg of body weight, 50 mg/kg, 100 mg/kg, and a humanized regimen, given intraperitoneally) in serum and epithelial lining fluid (ELF) were characterized. Dose linearity was observed for doses up to 50 mg/kg and pulmonary penetration ratios (area under the concentration-time curve in ELF from 0 to 24 h [AUCELF,0–24]/area under the concentration time curve in serum from 0 to 24 h [AUCserum,0–24]) ranged from 2.5 to 2.8. Pharmacokinetic-pharmacodynamics (PK-PD) index values in ELF for various dose regimens against different A. baumannii isolates were calculated. The maximum efficacy at 24 h was approximately 1.5-log-unit reduction of pulmonary bacterial burdens from baseline. The AUC/MIC ratio was the PK-PD index most closely correlating to the bacterial burden (r2 = 0.81). The required AUCELF,0–24/MIC for maintaining stasis and achieving 1-log-unit reduction were 140 and 410, respectively. These findings could guide the treatment of infections caused by A. baumannii using minocycline in the future. Additional studies to examine resistance development during therapy are warranted.
KEYWORDS: Gram-negative bacteria, tetracyclines
INTRODUCTION
Acinetobacter baumannii is an opportunistic pathogen that mostly affects patients with compromised immune function. It is commonly implicated in infections of the respiratory tract, bloodstream, urinary tract, and skin. In recent years, the prevalence of multidrug-resistant (MDR) A. baumannii isolates has been increasing (1). Many commonly used antimicrobial agents are ineffective, and increased mortality was reported in patients infected with MDR A. baumannii (2, 3). Minocycline is a semisynthetic derivative of tetracycline that has a broad spectrum of activity against Gram-positive and Gram-negative bacteria. Compared to other tetracyclines, it has excellent penetration into tissues and a long elimination half-life (4, 5). Conventionally, minocycline has not been used as a first-line agent in Gram-negative bacterial infections. However, the shortage of new effective antibiotics against MDR A. baumannii has motivated us to reevaluate the utility of minocycline. Despite good in vitro results of minocycline against MDR A. baumannii (6), satisfactory clinical response was not consistently seen in patients treated with minocycline (7).
Although the typical dosing regimen of minocycline is 200 mg/day, 400 mg given intravenously (i.v.) every 12 h (q12h) with a 800-mg loading dose has been used in humans for acute spinal cord injury (8). A higher minocycline daily dose may be necessary for infections caused by MDR A. baumannii. However, the in vivo efficacy of minocycline has not been well established, and the rationale of minocycline dosing regimen design needs to be further substantiated. In this study, the relationship between minocycline exposure and bactericidal activity was studied in a neutropenic murine pneumonia model. The minocycline exposure at the infection site was correlated to systemic exposure. We expect these findings will be used to optimize the dosing regimens of minocycline for more-favorable therapeutic outcomes.
RESULTS
Susceptibility and clonality assessment.
The known tetracycline resistance mechanisms and susceptibilities of the A. baumannii isolates to various antibiotics are shown in Table 1. Cross-resistance was observed between minocycline and doxycycline. The isolates were found to belong to three clonally diverse groups (data not shown).
TABLE 1.
Susceptibilities of A. baumannii isolates
| Isolate | Source | Tetracyline resistance mechanism(s) | MIC (mg/liter)a |
|||
|---|---|---|---|---|---|---|
| Minocycline | Doxycycline | Imipenem | Amikacin | |||
| AB BAA 747 | Laboratory | Wild-type | 0.25 | 0.25 | 0.25 | 2 |
| AB 7283 | Clinical | Moderate overexpression of adeB | 0.5 | 0.5 | 128 | 128 |
| AB 1261 | Clinical | Moderate overexpression of adeB | 1 | 0.5 | 128 | 128 |
| AB 1129 | Clinical | adeABC and adeIJK overexpressed | 4 | 4 | 8 | 32 |
| AB 7416 | Clinical | tetB; moderate overexpression of adeB | 16 | 128 | 16 | >512 |
Values in bold type indicate a resistant phenotype as defined by the Clinical and Laboratory Standards Institute (CLSI).
Minocycline pharmacokinetic study.
The initial estimation of area under the concentration-time curve from 0 to 24 h (AUC0–24) in serum and the best-fit maximum concentration of drug in serum (Cmax) suggested that the pharmacokinetics of 25-mg/kg and 50-mg/kg doses were within the linear range. Also, the daily dose of 50 mg/kg was found to be comparable to the human equivalent AUC reported in a previous study (9). Therefore, the total daily dose was split into five doses to mimic a humanized regimen. Briefly, 18 mg/kg of minocycline was given at 0 h to achieve the serum Cmax similar to that in humans, and four supplemental doses (11 mg/kg, 9 mg/kg, 8 mg/kg, and 4 mg/kg given at 4 h, 9 h, 14 h, and 22 h, respectively) were given to maintain the serum concentrations around the target pharmacokinetic profile reported in humans (4).
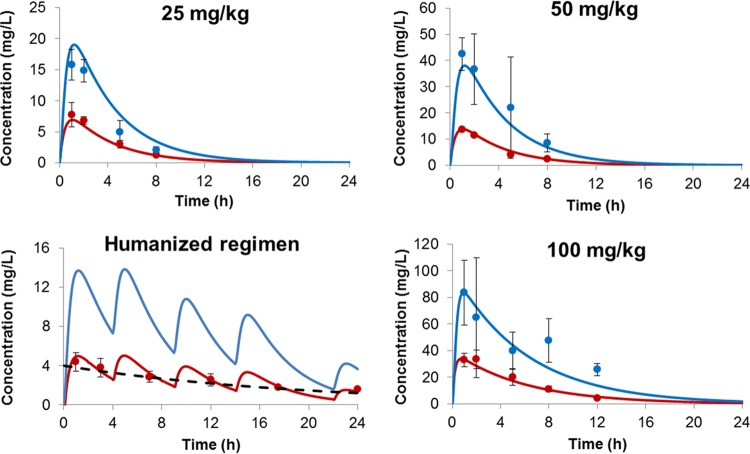
The data from these three dosing regimens were comodeled. The best-fit minocycline concentration-time profiles of different dosing regimens are shown in Fig. 1. The profiles of minocycline in both serum (r2 = 0.977) and epithelial lining fluid (ELF) (r2 = 0.952) were captured satisfactorily (Fig. 2). The elimination half-life in serum was 2.6 h. The serum AUC0–24 values were 34 mg · h/liter, 68 mg · h/liter, and 63 mg · h/liter for 25 mg/kg, 50 mg/kg, and the humanized regimen, respectively; while the ELF AUC0–24s were 94 mg · h/liter, 189 mg · h/liter, and 175 mg · h/liter, respectively. The pulmonary penetration ratio of minocycline was 2.8.
FIG 1.
Serum and ELF minocycline concentration-time profiles. The observed data are shown as means ± standard deviations (SDs) (error bars). Red closed circles are observed serum data; blue closed circles are observed ELF data; red solid lines depict best-fit serum profiles; blue solid lines depict best-fit ELF profiles; black dashed line represents the target serum profile in humans.
FIG 2.
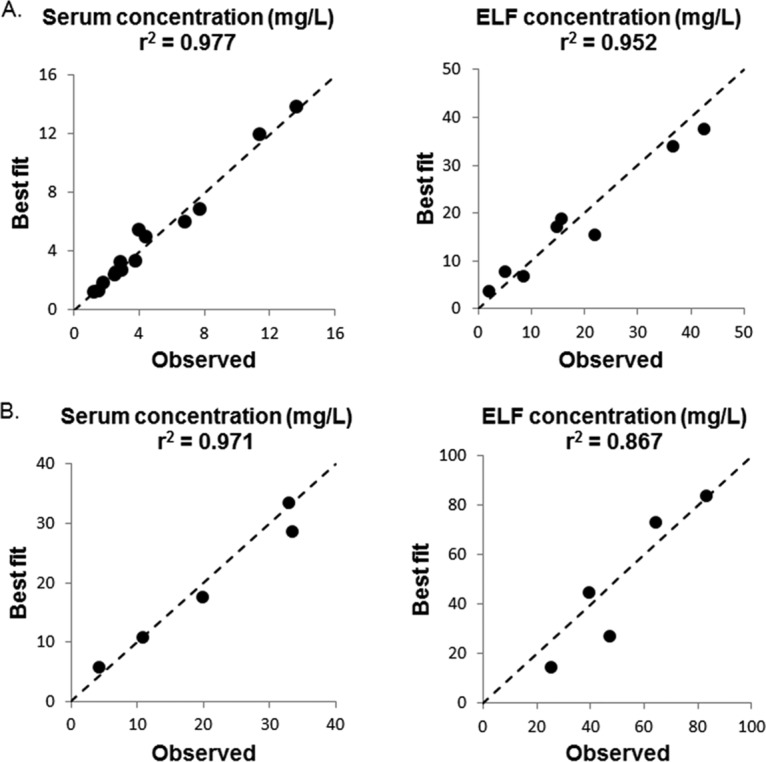
Correlation between observed and best-fit PK data. (A) 25 mg/kg, 50 mg/kg, and humanized regimen. (B) 100 mg/kg. The dashed line is the line of identity (i.e., y = x).
Compared to 50 mg/kg, the AUC0–24 of the 100-mg/kg dose observed was more than 3 times higher. Therefore, the concentration-time profiles of 100 mg/kg were analyzed separately (shown in Fig. 1). The r2 values were 0.971 and 0.867 for serum and ELF concentration-time profiles, respectively (Fig. 2). The elimination half-life was prolonged (3.9 h), suggesting saturable clearance for the 100-mg/kg dose. The AUC0–24s were 227 mg · h/liter and 564 mg · h/liter for serum and ELF, respectively. The pulmonary penetration ratio was 2.5.
Minocycline in vivo PD study.
The baseline bacterial burdens in lung tissues ranged from 7.75 to 8.18 log CFU/g. In the absence of treatment, the tissue burdens increased to 8.60 to 9.65 log CFU/g in 24 h. All treatment groups infected with the minocycline-resistant isolate (AB 7416) showed results similar to those of the no-treatment control group, while the other susceptible/intermediate isolates were suppressed to various extents.
PK-PD correlation.
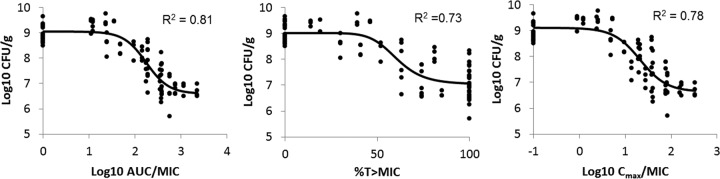
Looking at all the data collectively, the relationships between minocycline PK-PD indices in ELF and bacterial tissue burden at 24 h are shown in Fig. 3. The strongest relationship was observed when the tissue burdens were correlated with AUCELF,0–24/MIC (r2 = 0.81). Using the best-fit parameters, the required AUCELF,0–24 h/MIC for maintaining stasis was 140, and the required AUCELF,0–24/MIC for achieving 1-log-unit reduction was 410.
FIG 3.
Correlation of PK-PD indices in ELF and tissue burden at 24 h. Each data point represents an observation from a single animal. In view of the logarithmic scale used, AUC/MIC values were input as 1 and Cmax/MIC values were input as 0.1 for placebo controls. Black solid lines depict the best-fit profiles.
DISCUSSION
The shortage of new effective antibiotics against MDR A. baumannii prompted us to maximize the effectiveness of the currently available drugs, such as minocycline. With a good understanding of the drug exposures and bacterial susceptibilities likely to be encountered, a PK-PD model could facilitate the optimal use of minocycline.
In vitro susceptibility results of minocycline are promising. Denys et al. (10) reported susceptibility data of Gram-negative bacteria in the United States between 2005 and 2011. In that study (n = 883), the susceptibility rate of MDR Acinetobacter isolates to minocycline was 72.1%, whereas these isolates were found to be resistant to most of the other drugs examined (10). In addition, the pharmacokinetics of minocycline in animals and humans has been characterized previously. In horses, the elimination half-life was 7.7 ± 1.9 h (mean ± standard deviation [SD]) after a single 2.2-mg/kg i.v. injection (11). A prolonged half-life (11.5 ± 3.2 h [mean ± SD]) at steady state was observed when the dose was increased to 4 mg/kg q12h (12). The observation of nonlinear pharmacokinetics reported with high doses was consistent with our findings from the current study (100 mg/kg in mice) and might be due to saturation of drug metabolism (13). The elimination half-life in humans ranged from 12 h to 18 h, and the area under the concentration-time curve from 0 h to infinity (AUC0–∞) in serum ranged from 70 to 86 mg · h/liter after 200 mg was given intravenously (4). Up to 800 mg of minocycline daily has been given intravenously in a clinical trial for acute spinal cord injury (8); however, there is little known thus far about the dose linearity of minocycline in humans. A prospective study examining the safety, tolerability, and pharmacokinetics of minocycline (single and multiple ascending doses) in heathy adults has been planned (ClinicalTrials.gov identifier NCT02802631).
Free drug exposures in serum are commonly used in PK-PD studies. In the current study, we found that minocycline concentrations in ELF were higher than those observed in serum. Subsequently, the PK-PD analysis was performed with unbound minocycline concentrations in ELF (i.e., the site of infection), as they were deemed to be more relevant to therapeutic outcomes of pneumonia. Furthermore, serum protein binding of the tetracyclines may not be as straightforward as one might anticipate. Tigecycline was previously shown to exhibit atypical serum protein binding; the fraction of binding was higher with increasing total drug concentration in serum (14). The serum protein binding of minocycline was found be dependent on the experimental conditions. However, our preliminary data showed that the levels of protein binding to minocycline in human and mouse sera were comparable at different concentrations (15). Since the ELF exposure of minocycline achieved in humans after a clinical dose is not available, the total drug concentration-time profiles in serum was mimicked, and we assumed that pulmonary penetration ratios of minocycline in humans and mice were similar.
Using three different bacterial isolates, regimens with the same daily dose (single dose of 50 mg/kg, 25 mg/kg every 12 h, and humanized regimen) showed similar in vivo efficacy. Since these dosing regimens have similar AUC0–24 values but different Cmax and percentage of time above the MIC (T%>MIC), AUCELF,0–24/MIC was expected be the PK-PD index best correlating to the bacterial burden. Using a wide range of minocycline exposure and bacterial susceptibility (23 dose regimen-bacterium combinations in total), our initial finding was subsequently substantiated by the results of the full PK-PD analysis.
The PK-PD model described the relationship between minocycline exposure and bactericidal activity quantitatively. The maximum efficacy observed was approximately 1.5-log-unit reduction of the bacterial burden from the baseline. The required AUCELF,0–24/MIC values for maintaining stasis or achieving 1-log-unit reduction of bacterial tissue burdens were also estimated. AUCELF,0–24 h of the humanized regimen (a clinical dose of 200 mg is given intravenously to humans) was 175 mg · h/liter. With a MIC of 0.25 mg/liter, the corresponding AUCELF,0–24/MIC (700) is expected to achieve more than 1-log-unit kill. However, the therapeutic outcomes will vary when the MICs are different. Using these estimates, minocycline dosing regimens could be optimized when the susceptibility of an A. baumannii isolate is known. Furthermore, it is also important to avoid selective amplification of the resistant subpopulation(s) during treatment. In the future, additional studies will be needed to evaluate resistance development during therapy and to correlate minocycline exposures to therapeutic outcomes of patients with infections caused by A. baumannii.
In conclusion, the pharmacokinetics of minocycline in mouse serum and ELF were characterized. We have also identified threshold target exposures for achieving different pharmacodynamic responses in A. baumannii infections.
MATERIALS AND METHODS
Chemicals and reagents.
Liquid chromatography-mass spectrometry (LC-MS)-grade water, methanol, and acetonitrile were purchased from EMD Millipore Corporation (Billerica, MA). Dimethyl sulfoxide (DMSO) was obtained from EM Science (Gibbstown, NJ). LC-MS-grade formic acid, minocycline hydrochloride, and doxycycline hyclate powder were purchased from Sigma-Aldrich (St. Louis, MO). Mouse serum was obtained from Equitech-Bio, Inc. (Kerrville, TX). The Ultra Clean microbial DNA isolation kit was from Mo Bio Laboratories, Inc. (Carlsbad, CA). The DNA Acinetobacter strain typing kit was a product of DiversiLab (Marcy I'Etoile, France), and Taq DNA polymerase was purchased from Bioline USA Inc. (Randolph, MA). The urea assay kit was from BioAssay Systems (Hayward, CA).
Bacterial isolates.
Five A. baumannii isolates (one laboratory wild-type isolate and four clinical isolates) with a wide range of minocycline MICs were used. Susceptibilities to minocycline, doxycycline, amikacin, and imipenem were determined by the broth dilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI). Pseudomonas aeruginosa strain ATCC 27853 (American Type Culture Collection, Manassas, VA) was used as a control strain. The clonal relatedness of the A. baumannii isolates was assessed by repetitive-element-based PCR (rep-PCR) (16, 17). Briefly, genomic DNA was isolated with a Ultra Clean microbial DNA isolation kit, and the DNA was used as the template for the Acinetobacter strain typing kit. The DNA fragments of rep-PCR products were separated by the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA) and compared by the DiversiLab software using the Pearson correlation coefficient (Bacterial Barcodes, Inc., Athens, GA).
Neutropenic murine pneumonia model.
A neutropenic murine pneumonia model was used to examine the in vivo bactericidal activity of minocycline. The experimental setup was as previously described (9, 18). Briefly, female Swiss Webster mice between 20 and 25 g (Harlan, Indianapolis, IN) were rendered neutropenic by two doses of intraperitoneal cyclophosphamide prior to the experiment (150 mg/kg on day −4 and 100 mg/kg on day −1). Anesthetized mice were inoculated with approximately 107 CFU of A. baumannii under laryngoscopic guidance. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Houston.
Infected animals were treated with different dosing regimens of minocycline approximately 2 h after infection. For reference, there was a no-treatment control group for each bacterial isolate. There were 80 animals given 23 dosing regimen-bacterium combinations in total (n ≥ 3 for each regimen). For reference, there was a placebo (i.e., no-treatment) control group for each bacterial isolate. The bacterial burdens in lung tissues were determined at 0 h (baseline) and 24 h after the first dose of minocycline as described elsewhere (9, 19). Briefly, after the animals were sacrificed by CO2 asphyxiation, the lung tissues were harvested and homogenized in sterile saline. Pulmonary bacterial burdens were determined by quantitative culture and normalized by the weight of the lung tissue.
Minocycline pharmacokinetic study.
The single-dose pharmacokinetics of minocycline in mouse serum and epithelial lining fluid (ELF) were characterized. Minocycline was administered intraperitoneally in 67 animals 2 h after infection (by AB 1261). Serum or bronchoalveolar lavage (BAL) fluid samples were collected serially over time (n ≥ 3 for each time point). Blood samples were collected by cardiac puncture. BAL fluid samples were recovered through the trachea after 1 ml of saline was injected into the lungs. Minocycline concentrations were determined by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method as detailed below. The minocycline concentrations in ELF were calculated by correcting the minocycline concentration in BAL fluid samples for urea concentrations as described previously (20). The serum and ELF concentration-time profiles were comodeled by a modified two-compartmental model in ADAPT 5 (21; data not shown). Three regimens were initially evaluated: 25 mg/kg, 50 mg/kg, and 100 mg/kg. Based on the preliminary results, a humanized regimen mimicking the human serum concentration-time profile of minocycline (when a clinical dose of 200 mg is given intravenously to humans) was validated. The area under the curve (AUC) values of the serum and ELF concentration-time profiles were derived by integrating the best-fit instantaneous concentrations with respect to time. The pulmonary penetration ratio of minocycline was estimated by the AUC ratio of ELF to serum. In addition, using the best-fit parameters, various PK-PD indices (i.e., AUC/MIC, Cmax/MIC, and percentage of time above the MIC [T%>MIC]) for ELF profiles were calculated for different dosing regimens against different A. baumannii isolates (data not shown).
Minocycline assay.
Minocycline concentrations in serum and epithelial lining fluid samples were determined by a validated liquid chromatography-tandem mass spectrometry method. The samples for the standard curve were prepared by spiking minocycline into blank serum. Briefly, 10 μl of serum or ELF sample was mixed with 10 μl water (with 0.5% DMSO), 30 μl of internal standard (10 mg/liter doxycycline in water), and 170 μl acetonitrile (with 0.03% formic acid). The samples were centrifuged for 15 min at 18,000 × g. Aliquots (40 μl) of the supernatants were transferred into new tubes and evaporated under a stream of ambient air. After being reconstituted with 1 ml of 50% methanol, 5-μl portions of the samples were injected into the ultraperformance liquid chromatography (UPLC) system. The conditions and parameters of LC-MS/MS were described previously (9). The linear range of quantification was 0.0625 to 128 mg/liter. The intraday and interday variability of the assay method were <2.5% and <3.6%, respectively.
Data analysis.
The relationships between minocycline PK-PD indices (unbound drug exposures in ELF) and bacterial burden in lung tissues at 24 h were described by an inhibitory sigmoid maximum effect (Emax) model, as shown previously (22). The coefficient of determination was used to discriminate the PK-PD index most closely correlated to bactericidal activity. In addition, the best-fit parameters were also used to derive the required PK-PD exposures for maintaining stasis or achieving 1-log-unit reduction in the bacterial tissue burden at 24 h.
ACKNOWLEDGMENTS
This study was supported in part by the Medicines Company.
We thank David Griffith for providing minocycline (pharmaceutical grade) powder and isolate AB 1129.
V.H.T. is a consultant on the Advisory Board of Tetraphase Pharmaceuticals.
REFERENCES
- 1.Keen EF III, Murray CK, Robinson BJ, Hospenthal DR, Co EM, Aldous WK. 2010. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect Control Hosp Epidemiol 31:728–732. doi: 10.1086/653617. [DOI] [PubMed] [Google Scholar]
- 2.Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ. 2007. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis 26:793–800. doi: 10.1007/s10096-007-0371-8. [DOI] [PubMed] [Google Scholar]
- 3.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control 41:e59–. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe A, Anzai Y, Niitsuma K, Saito M, Yanase K, Nakamura M. 2001. Penetration of minocycline hydrochloride into lung tissue and sputum. Chemotherapy 47:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Castanheira M, Mendes RE, Jones RN. 2014. Update on Acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin Infect Dis 59(Suppl 6):S367–S373. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 7.Goff DA, Bauer KA, Mangino JE. 2014. Bad bugs need old drugs: a stewardship program's evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin Infect Dis 59(Suppl 6):S381–S387. doi: 10.1093/cid/ciu593. [DOI] [PubMed] [Google Scholar]
- 8.Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. 2012. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 9.Bowers DR, Cao H, Zhou J, Ledesma KR, Sun D, Lomovskaya O, Tam VH. 2015. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother 59:2720–2725. doi: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denys GA, Callister SM, Dowzicky MJ. 2013. Antimicrobial susceptibility among Gram-negative isolates collected in the USA between 2005 and 2011 as part of the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.). Ann Clin Microbiol Antimicrob 12:24. doi: 10.1186/1476-0711-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata S, Yamashita S, Kurosawa M, Kuwajima M, Hobo S, Katayama Y, Anzai T. 2010. Pharmacokinetics and tissue distribution of minocycline hydrochloride in horses. Am J Vet Res 71:1062–1066. doi: 10.2460/ajvr.71.9.1062. [DOI] [PubMed] [Google Scholar]
- 12.Schnabel LV, Papich MG, Divers TJ, Altier C, Aprea MS, McCarrel TM, Fortier LA. 2012. Pharmacokinetics and distribution of minocycline in mature horses after oral administration of multiple doses and comparison with minimum inhibitory concentrations. Equine Vet J 44:453–458. doi: 10.1111/j.2042-3306.2011.00459.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelis HJ, De Leenheer AP. 1982. Metabolism of minocycline in humans. Drug Metab Dispos 10:142–146. [PubMed] [Google Scholar]
- 14.Mukker JK, Singh RP, Derendorf H. 2014. Determination of atypical nonlinear plasma-protein-binding behavior of tigecycline using an in vitro microdialysis technique. J Pharm Sci 103:1013–1019. doi: 10.1002/jps.23872. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Tran BT, Tam VH. 25 February 2017. The complexity of minocycline serum protein binding. J Antimicrob Chemother doi: 10.1093/jac/dkx039. [DOI] [PubMed] [Google Scholar]
- 16.Tam VH, Chang KT, LaRocco MT, Schilling AN, McCauley SK, Poole K, Garey KW. 2007. Prevalence, mechanisms, and risk factors of carbapenem resistance in bloodstream isolates of Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 58:309–314. doi: 10.1016/j.diagmicrobio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Syrmis MW, O'Carroll MR, Sloots TP, Coulter C, Wainwright CE, Bell SC, Nissen MD. 2004. Rapid genotyping of Pseudomonas aeruginosa isolates harboured by adult and paediatric patients with cystic fibrosis using repetitive-element-based PCR assays. J Med Microbiol 53:1089–1096. doi: 10.1099/jmm.0.45611-0. [DOI] [PubMed] [Google Scholar]
- 18.Tam VH, Ledesma KR, Schilling AN, Lim TP, Yuan Z, Ghose R, Lewis RE. 2009. In vivo dynamics of carbapenem-resistant Pseudomonas aeruginosa selection after suboptimal dosing. Diagn Microbiol Infect Dis 64:427–433. doi: 10.1016/j.diagmicrobio.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch EB, Guo B, Chang KT, Cao H, Ledesma KR, Singh M, Tam VH. 2013. Assessment of antimicrobial combinations for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. J Infect Dis 207:786–793. doi: 10.1093/infdis/jis766. [DOI] [PubMed] [Google Scholar]
- 20.He J, Abdelraouf K, Ledesma KR, Chow DS, Tam VH. 2013. Pharmacokinetics and efficacy of liposomal polymyxin B in a murine pneumonia model. Int J Antimicrob Agents 42:559–564. doi: 10.1016/j.ijantimicag.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA. [Google Scholar]
- 22.Lim TP, Ledesma KR, Chang KT, Hou JG, Kwa AL, Nikolaou M, Quinn JP, Prince RA, Tam VH. 2008. Quantitative assessment of combination antimicrobial therapy against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 52:2898–2904. doi: 10.1128/AAC.01309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]