ABSTRACT
The need for new antimicrobials to treat bacterial infections has led to the use of type II fatty acid synthesis (FASII) enzymes as front-line targets. However, recent studies suggest that FASII inhibitors may not work against the opportunist pathogen Staphylococcus aureus, as environmental fatty acids favor emergence of multi-anti-FASII resistance. As fatty acids are abundant in the host and one FASII inhibitor, triclosan, is widespread, we investigated whether fatty acid pools impact resistance in clinical and veterinary S. aureus isolates. Simple addition of fatty acids to the screening medium led to a 50% increase in triclosan resistance, as tested in 700 isolates. Moreover, nonculturable triclosan-resistant fatty acid auxotrophs, which escape detection under routine conditions, were uncovered in primary patient samples. FASII bypass in selected isolates correlated with polymorphisms in the acc and fabD loci. We conclude that fatty-acid-dependent strategies to escape FASII inhibition are common among S. aureus isolates and correlate with anti-FASII resistance and emergence of nonculturable variants.
KEYWORDS: antibiotic resistance, infection, persistence, nondetectable resistance, fatty acids, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is a leading cause of a wide range of infections that can affect numerous host organs and cause a range of effects from mild symptoms to severe and life-threatening diseases. Acquisition of antimicrobial resistance, likely due to overuse of antibiotics, is the principal cause of S. aureus drug resistance (1). A class of new-generation antimicrobials in intensive development uses the type II fatty acid synthesis (FASII) pathway as an antibacterial target (2–5) to treat S. aureus infections (6–8). A widely used biocide, triclosan (5-chloro-2-[2,4-dichlorophenoxy]phenol; commercialized as Irgasan or Microban), is a prototype for further anti-FASII development (4, 9, 10).
The utility of FASII inhibitors was questioned when several Gram-positive bacteria were shown to be refractory to FASII inhibitors in the presence of exogenous fatty acids, making FASII enzymes dispensable (11, 12). Both free and complexed fatty acids are abundant in the host (13, 14), which would facilitate FASII bypass. Reservoirs and invasion sites of clinical and community-acquired staphylococci (i.e., skin, nares, gut, blood, and organs) are naturally rich in fatty acids (13–16), and triclosan is present in the environment and in human body fluids (17, 18). This combination could favor FASII bypass via emergence of triclosan-resistant variants, including fatty acid auxotrophs. Fatty-acid-dependent isolates escape detection on standard isolation media, thereby confounding diagnosis and treatment.
Although S. aureus was at first considered sensitive to FASII inhibitors, our recent studies demonstrated benefits from environmental fatty acids for emergence of resistant mutants (19). The question of the survival of such mutants in a clinical context remains in debate (6, 7, 12, 19). It is therefore essential to determine whether FASII bypass, as characterized mainly in vitro, is relevant to natural staphylococcal populations.
In this work, we examined a large panel of clinical and veterinary S. aureus isolates for the extent of fatty acid impact on triclosan resistance and culturability. Our results demonstrate that isolates using fatty-acid-dependent strategies to escape FASII inhibition are common. Fatty-acid-containing medium gives a more accurate assessment of triclosan resistance in a clinical setting and facilitates identification of pathogens that escape detection by conventional approaches.
RESULTS
Fatty acid supplementation reveals a frequent class of triclosan-resistant S. aureus among clinical and veterinary isolates.
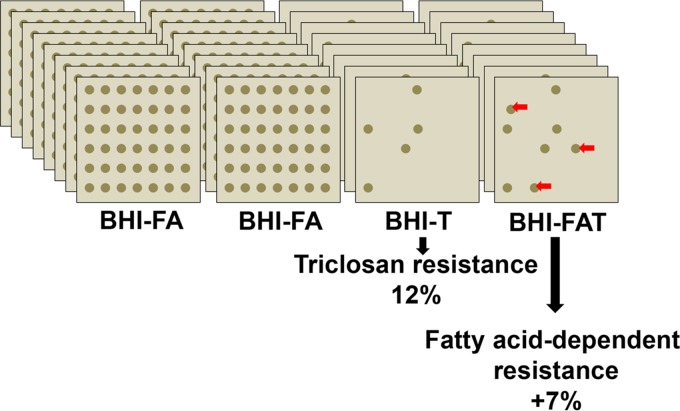
A four-plate screen using media with or without fatty acids (brain heart infusion [BHI], BHI-triclosan [BHI-T], BHI-fatty acids [BHI-FA], and BHI-fatty acids-triclosan [BHI-FA-T]) (Fig. 1) was set up to search for S. aureus isolates that require fatty acids for triclosan resistance. The fatty acids C14:0, C16:0, and C18:1 were chosen mainly as being predominant in two representative environments of localized and systemic infections, skin and serum (20, 21). Human clinical and veterinary mastitis isolates were scored for triclosan resistance (Tr) and fatty-acid-dependent triclosan resistance (FA-Tr). Eighty-five (12%) of the 695 tested isolates were scored as triclosan resistant (with growth on BHI, BHI-T, and BHI-FA-T plates), consistent with previous reports of high proportions of triclosan-resistant staphylococci among hospital strains (22, 23). Remarkably, the screen identified 49 isolates (7%) for which triclosan resistance was revealed or markedly enhanced on BHI-FA-T compared to BHI-T (scored as FA-Tr), raising the total proportion of triclosan-resistant isolates to 19%, i.e., a 58% increase. FA-Tr isolates were from diverse clinical or veterinary origins, and no overall correlation was observed between the FA-Tr phenotype and sample sources (see Table S2 in the supplemental material). spa typing was performed on FA-Tr S. aureus isolates to confirm their identification and determine whether they occurred within restricted S. aureus subgroups (see Table S3 in the supplemental material). FA-Tr isolates were distributed among various clonal and spa types, including the common multilocus sequence typing (MLST) clonal types (CC5, CC8, and CC45) (24). FA-Tr clones were also present in the emerging clonal type CC398 (25) and in less common clonal types. Broader screening studies will be needed to determine whether FA-Tr clones preferentially occur in any clonal groups.
FIG 1.
Screening strategy to assess fatty-acid-dependent triclosan resistance among clinical and veterinary S. aureus isolates (FA-Tr). Plates contained BHI medium with the indicated fatty acid (FA) and triclosan (T) additives. The fatty acids were C14:0, C16:0, and C18:1 (170 μM each); triclosan was used at 0.25 μg/ml. Isolates were spotted in the same order on each plate. Those that grew on BHI-FA-T but did not grow, or grew poorly, on BHI-T were scored as FA-Tr and selected for further study. In total, ∼700 isolates were scored using this screen.
These results establish the frequent existence of clinical and veterinary staphylococci whose triclosan resistance requires the presence of exogenous fatty acids. They show that triclosan resistance among medically relevant strains is underestimated due to inappropriate screening conditions.
Three types of fatty-acid-dependent triclosan resistance.
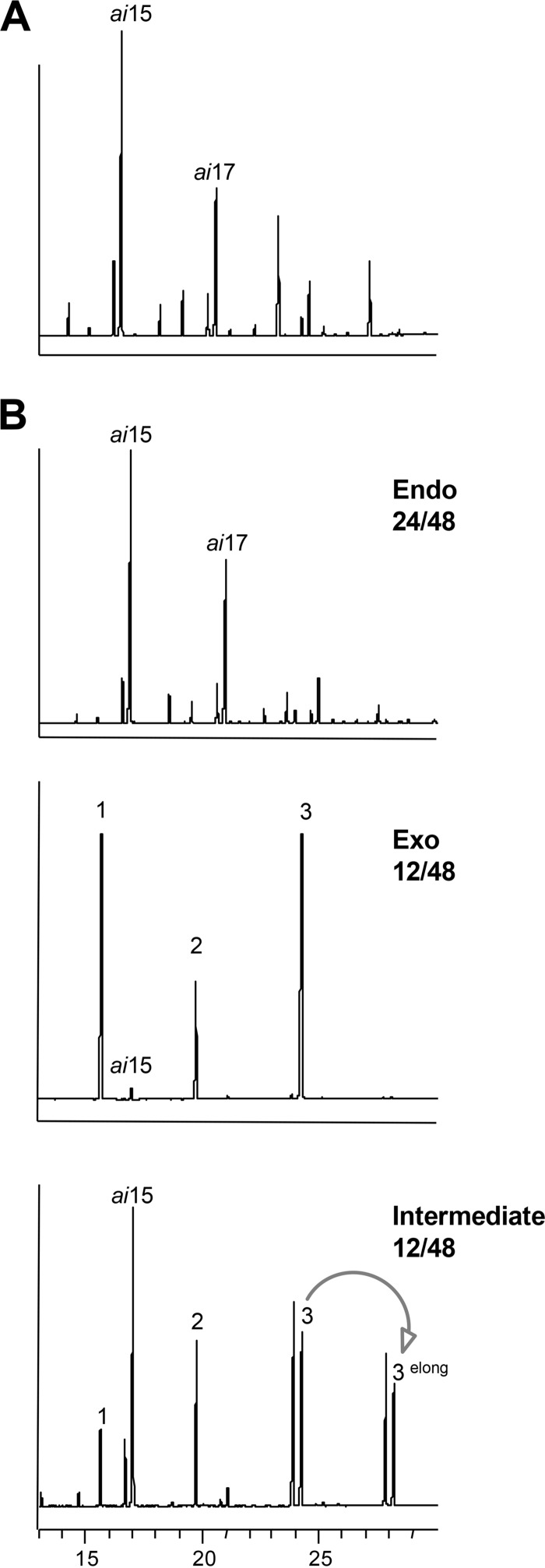
S. aureus strains grown in nonselective BHI medium synthesize mainly branched-chain fatty acids, comprising ai15 and ai17 (Fig. 2A). In contrast, primary cultures of 48 FA-Tr isolates grown in BHI-FA-T revealed three types of fatty acid profiles (Fig. 2B): endogenous fatty acids (Endo) (24 isolates), strictly exogenous fatty acids (Exo) (12 isolates), and mixed profiles (intermediate) (12 isolates). In general, Exo isolates grew more slowly than intermediate or Endo isolates in BHI-FA-T medium (the respective optical density at 600 nm [OD600] ranges were 3 to 6, 7 to 11, and 8 to 16 under selective conditions). We noted that FA-Tr strains having Exo profiles were isolated mainly from two fatty acid-rich niches: human skin and cattle mastitis (Table S2) (20, 26). These observations establish that FASII bypass using available fatty acids is relevant to triclosan resistance in clinical and veterinary isolates.
FIG 2.
Three types of FA-Tr S. aureus isolates. (A) Representative fatty acid profile of S. aureus isolates grown on nonselective BHI liquid medium. (B) Fatty acid profiles of exogenous (Exo), endogenous (Endo), and combined Exo and Endo (intermediate) fatty acid profiles of FA-Tr isolates grown in the presence of 3 fatty acids and triclosan (see the legend Fig. 1). The major endogenous fatty acids, anteiso ai15 and ai17, are indicated. Exogenous fatty acids in medium are as follows: 1, C14:0; 2, C16:0; and 3, C18:1. The arrow indicates elongation via FASII (in this case, C18:0 [endogenous] and C18:1 [3elong] are extended to C20:0 and C20:1), which suggests that triclosan is not fully inhibitory as tested; in some cases, higher triclosan concentrations shifted profiles to fully exogenous. Note that profiles scored as Exo contain fewer than 2% anteiso forms; above this threshold, they were scored as intermediate.
Anti-FASII resistance of S. aureus isolates varies according to the fatty acid environment.
We asked whether a better match between the fatty acids used in screenings and those from the natural environment would influence triclosan resistance and fatty acid incorporation. For example, an isolate from human sputum (Trsa 180) was initially scored as triclosan sensitive but was triclosan resistant with an Exo profile when grown with fatty acids more closely related to its initial biotope (27) (Fig. 3). Four additional FA-Tr isolates switched from an intermediate to an Exo profile when grown with fatty acids more closely matching those of their initial biotope. We therefore speculate that the FASII bypass frequencies in natural niches may be greater than estimated in this study.
FIG 3.
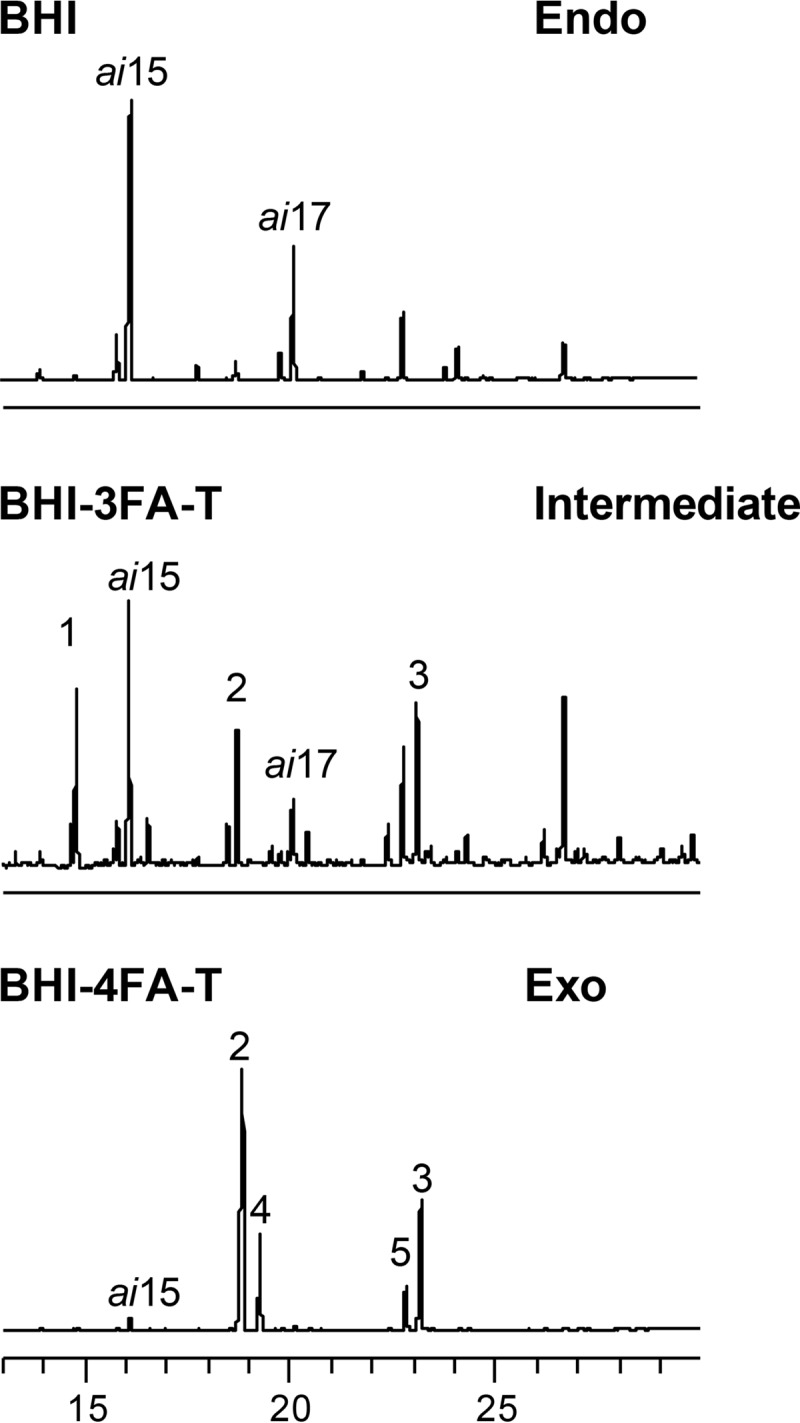
Triclosan resistance varies according to the nature of available exogenous fatty acids and their incorporation. Sputum isolate Trsa 180 was initially scored as triclosan sensitive using the four-plate test. The isolate was resuspended in 0.9% NaCl directly from the BHI agar plate and retested for growth on BHI and BHI-FA-T plates containing either the 3 fatty acids (3FA) as used for Fig. 2 or 4 fatty acids (4FA) comprising sputum fatty acids in physiological proportions (see below). Growth was confluent on 4FA-triclosan solid medium, while growth on 3FA-triclosan was poor. Colonies were recovered directly from plates for fatty acid profile determinations. The major endogenous fatty acids, ai15 and ai17 are indicated. Exogenous fatty acids in the medium are as follows: 1, C14:0; 2, C16:0; 3, C18:1; 4, C16:1; and 5, C18:0. The global fatty acid concentration was 500 μM. For 3FA, the amounts of the fatty acids were equimolar. For 4FA, proportions were 69, 3, 14, and 13%, respectively, for C16:0, C16:1, C18:0, and C18:1.
Genetic determinants of FA-Tr resistance.
Half of the FA-Tr isolates produced endogenous fatty acids despite the presence of triclosan. The well-known FASII routes for triclosan resistance in clinical isolates alter the triclosan target FabI via (i) mutations in fabI or (ii) the presence of sh-fabI (a fabI allele that is horizontally transferred from Staphylococcus haemolyticus) (10, 22, 28). Among 31 Endo isolates identified in this work, 7 carried fabI polymorphisms previously associated with triclosan resistance (see Table S4 in the supplemental material), and 24 carried the additional sh-fabI gene (Table S4). Both mechanisms were previously associated with triclosan resistance not requiring exogenous fatty acids. The mechanism for exogenous fatty-acid-dependent resistance in these Endo isolates remains to be investigated.
Exo isolates (10 tested isolates) carried wild-type fabI sequences and lacked secondary fabI copies, as expected in a case of FASII bypass. In vitro-selected FASII bypass in S. aureus was associated with mutations mapping to the acc and fabD genes (6, 19, 29). Among 10 analyzed Exo isolates, 6 carried polymorphisms in accC, accD, or accB, including stop codons (Table 1). A stop codon in accD putatively leads to a truncated protein lacking a domain needed for both enzymatic and regulatory activities (30). Nonauxotrophy of these isolates might be due to supplementary accBC copies (which are present in numerous staphylococcal genomes) and/or to partial accD read-through (e.g., the accD stop in Trsa 36 corresponds to a single-nucleotide deletion in a stretch of six adenines). About half of the Exo isolates studied here carried fabD polymorphisms, some of which mapped adjacent to a catalytic residue of the active site (Table 1) (31). Two isolates had the FabDG196R mutation (the more common amino acid at position 196 [aa 196] is glycine) that we recently showed to be responsible for FASII bypass (19). In conclusion, the acc and/or fabD polymorphism identified among FA-Tr clinical and veterinary isolates is likely responsible for the observed capacity to elude FASII inhibition when fatty acids are available.
TABLE 1.
Acc and FabD changes in FA-Tr isolates that bypass triclosan inhibition by incorporating exogenous fatty acids
| Isolate | Relevant AccABCD variationa | Relevant FabD variationa |
|---|---|---|
| Trsa 275 | Wild type | Wild type |
| MT8099 | AccC stop aa 445 | Wild type |
| P12 | Wild type | FabDE235Vb |
| P1113 | Wild type | FabDG196Dc |
| P1127 | AccDD268Nd | FabDG196D |
| P146 | AccDK35Ne stop aa 137 | FabDS71Lf |
| P176 | AccBK188Eg | Wild type |
| MT8348 | AccC stop aa 117 | Wild type |
| Trsa 36i | AccD stop aa 111 | Wild type |
| MT8343 | Wild type | FabDL192Ih |
Variants are also present in sequenced genomes reported in public databases (from NCBI, as of 1 January 2015), as indicated for the particular variation. Amino acid differences that were outside conserved regions and present in hundreds of sequenced genomes were not considered.
Thirty-six strains.
FabD variants at position 196 were recently shown to account for FASII bypass (19).
Twenty-two strains.
Fourteen strains.
Thirteen strains.
Eight strains.
Ten strains.
Poor growth on BHI medium.
Clinical staphylococci include triclosan-resistant fatty acid auxotrophs.
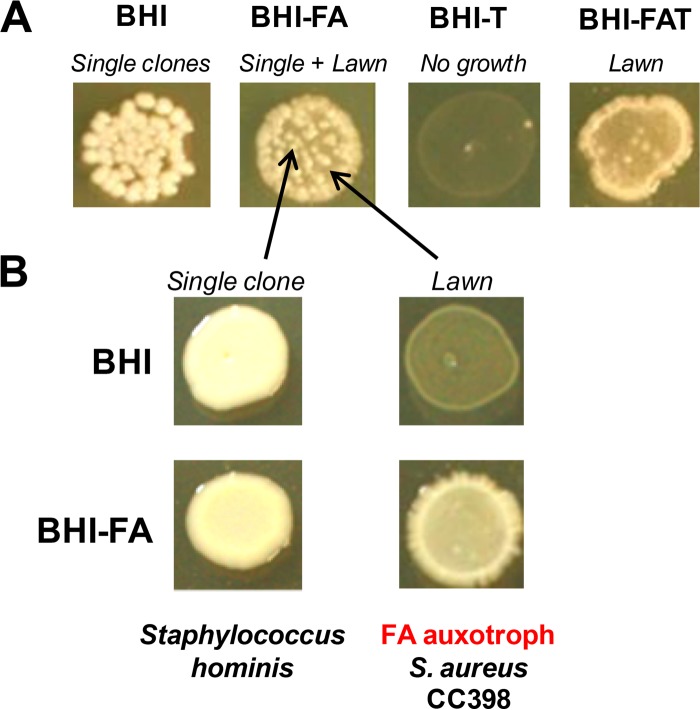
To search for fatty acid auxotrophs, primary clinical specimens from 103 patients were plated directly on fatty-acid-containing medium that is semiselective for S. aureus growth (see Materials and Methods). Colonies were then repatched on BHI, BHI-T, BHI-FA, and BHI-FA-T plates to identify fatty acid auxotrophs. Four fatty acid auxotrophs were recovered in the screen as growing on fatty-acid-containing media (BHI-FA and BHI-FA-T) but not on BHI nor BHI-T (Table 2). Two were identified as S. aureus and had Exo profiles even in the absence of triclosan. One S. aureus auxotroph was isolated from a bronchial aspirate in which staphylococci were not detected by routine identification. The second S. aureus auxotroph was recovered from a burn wound; in this case, the sample contained a mixed population of staphylococci (a lawn and distinct colonies), of which the predominant population (i.e., the lawn) appeared only on fatty-acid-containing media (Fig. 4). Analysis of potential genetic determinants revealed AccB and AccC variants in both S. aureus auxotrophs and a FabD variant in one of them (Table 2). Two non-S. aureus fatty acid auxotrophs were also detected: a Staphylococcus hominis isolated from sputum and a Corynebacterium isolate closely related to the lipid-requiring species Corynebacterium accolens (32) (95% 16S rRNA identity) from a bronchoalveolar lavage specimen.
TABLE 2.
Fatty acid auxotrophy in hidden clinical isolates
| Isolate | Source | Species assignment | Relevant AccABCD variation | Relevant FabD variation | Routine hospital identification |
|---|---|---|---|---|---|
| HP6D | Burn | S. aureus CC398 | AccC stop aa 94, AccBA60V,S56N | FabDP191T | S. aureus, S. epidermidis |
| HP7 | Bronchial aspirate | S. aureus CC398 | AccC stop aa 94, AccBA60V,S56N | Wild type | Streptococci, Neisseria |
| HP9 | Sputum | Staphylococcus hominis (98% 16S rRNA identity) | NDa | ND | None |
| HP8 | Bronchoalveolar lavage specimen | Corynebacterium accolens (95% 16S rRNA identity) | ND | ND | S. aureus, Enterobacter cloacae |
ND, not determined.
FIG 4.
A hidden FA-Tr S. aureus fatty acid auxotroph in a burn patient. Primary clinical samples were plated directly on a semiselective plate (HiChrome agar), to which oleic acid (C18:1) (500 μM) was added. (A) The FA-Tr isolate was then identified using the four-plate screen (for the method, see Fig. 1). (B) The isolate comprises a S. aureus fatty acid auxotroph (CC398 clonal type) requiring fatty acids for growth. Auxotrophy was confirmed by Exo profiles on both BHI-FA and BHI-FA-T. The minor Staphylococcus population (appearing as single large colonies in BHI-FA) is assigned as Staphylococcus hominis, which is reported to be a commensal and opportunist pathogen.
The inclusion of fatty acids in primary screening media thus uncovers a new class of clinical staphylococcal fatty acid auxotrophs that are viable but nonculturable (VBNC). Fatty acid auxotrophs were previously selected in the laboratory using FabI inhibitors in the presence of fatty acids (19, 29). The present results lead us to suggest that triclosan may select for successful FASII bypass variants in the human host.
DISCUSSION
Our recent work demonstrated that fatty acids enable in vitro emergence of infectious S. aureus mutants that are resistant to bacterial FASII inhibitors, including triclosan (19). In this study, we demonstrate that FASII bypass, using environmental fatty acids, is clinically relevant and appears to be a common triclosan resistance strategy in S. aureus. We show that fatty-acid-dependent bypass of this FASII inhibitor accounts for resistance among clinical and veterinary isolates, thus attesting to competitiveness and potential success of such strains as pathogens in the host. Our results further indicate that previous determinations of triclosan resistance among clinical strains have been underestimated. The proportion of currently undetected resistance may be even higher, as fatty acid combinations that favor resistance might vary according to the site of infection.
FA-Tr clinical and veterinary isolates that incorporate exogenous fatty acids comprise a common class of anti-FASII-resistant S. aureus. FASII bypass is attributed to polymorphisms in acc and/or fabD genes, including a fabD mutation previously identified as responsible for FASII bypass and resistance (19). Most isolates of this class were from superficial skin wounds in humans and mastitis cases in cows. Both infection sites are fatty acid rich (13, 26).
This study also uncovers a new class of unculturable staphylococci among clinical and veterinary isolates, which are anti-FASII resistant and require fatty acids for growth. A previous report suggested that fatty acid auxotrophs would be noninfectious based on the behavior of constructed S. aureus acc mutants in a mouse model (6). In contrast to those conclusions, this study establishes that at the least, fatty acid auxotrophs (i) are maintained in in vivo reservoirs and (ii) define a new category of VBNC pathogens that would escape detection in current routine identification screens. Other Gram-positive bacteria may also generate infectious fatty acid auxotrophs, as exemplified here by identification of S. hominis, a coagulase-negative species, and a Corynebacterium isolate closely related to benign C. accolens species, whose presence is a predictor of S. aureus nasal carriage (32, 33). To date, identification of fatty acid auxotrophs among clinical isolates is rare; recently, an Enterococcus faecalis auxotroph was isolated from an umbilical exudate (34), and an S. aureus strain mutated in the FabF fatty acid synthase was isolated from a patient with septic arthritis (35). A small-colony variant of S. aureus isolated in vitro was restored to normal growth by fatty acid supplementation, indicating a possible FASII defect (36). Taken together, these findings suggest that fatty acid auxotrophs, not systematically searched, may be common in the host. Our work suggests a straightforward means for their detection by including fatty acids in the screening medium.
We recently showed that S. aureus FASII bypass variants isolated in triclosan and fatty acid selections were cross-resistant to a second FabI inhibitor, AFN-1252 (renamed Debio 1452 [2, 3]); inversely, AFN-1252-resistant mutants were also triclosan resistant (19). A drug related to Debio 1452 is being tested in phase II clinical trials to treat skin infections (ClinicalTrials.gov registration no. NCT02426918). Based on our findings, AFN-1252 treatment would expectedly be ineffective in patients harboring FASII bypass isolates, including fatty acid auxotrophs.
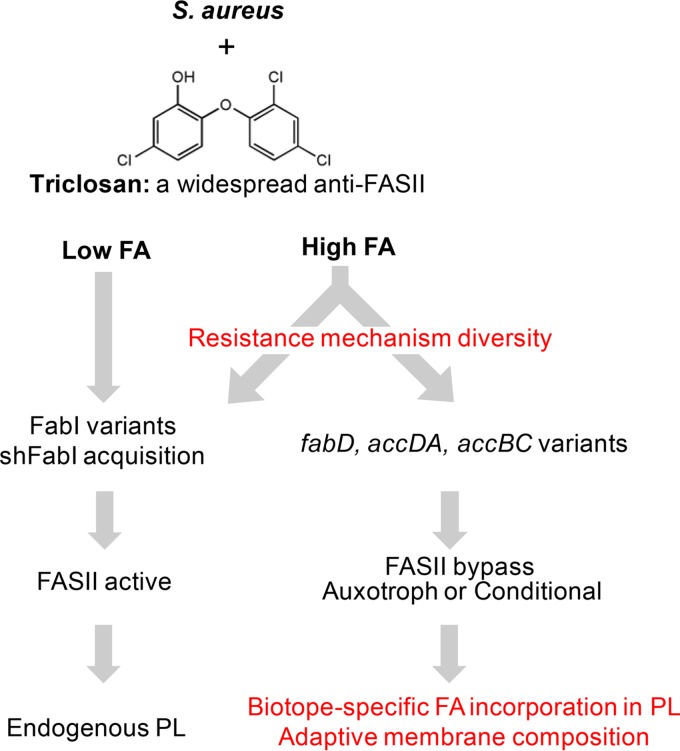
In conclusion, the fatty-acid-dependent strategy of anti-FASII resistance is frequent and likely relevant to infection, and it may favor diverse mechanisms of resistance (Fig. 5). Emergence of resistance may be due to the presence of both triclosan (37) and fatty acids in numerous host biotopes (19). Two distinct resistance classes are discerned: (i) alterations in FabI, the triclosan target, lead to variants that produce membranes with endogenous membrane fatty acid composition, and (ii) alterations in alternative FASII genes, e.g., fabD and accDA accBC, lead to flexible membrane fatty acids whose composition varies according to the host biotope (Fig. 5). These results raise caveats on the use of FASII inhibitors for sustainable antimicrobial treatment and argue for an even more restrictive use of triclosan than advised in recent European and American legislations (see Commission implementing decision [EU] 2016/110, https://www.federalregister.gov/articles/2016/09/06/2016-21337/safety-and-effectiveness-of-consumer-antiseptics-topical-antimicrobial-drug-products-for).
FIG 5.
Contribution of fatty acid (FA)-rich environments to diversity of S. aureus anti-FASII resistance. Intensive use of triclosan has led to its accumulation in numerous environments, including FA-rich animal biotopes. High-FA environments may select for diverse resistance mechanisms, including those that require exogenous fatty acids. They comprise triclosan-resistant fabI variants that were identified independently of FA (22, 23, 41–43); our results show that resistance frequencies are even greater in FA-rich environments. FA-rich environments favor emergence of FASII bypass mutants affected in initiation genes fabD and accDA accBC (6, 19, 29); the present work shows that FASII bypass is frequent among clinical isolates and correlates with genetic polymorphisms in fabD and acc. Fatty acid auxotrophs (nonculturable without fatty acids) and normally growing isolates constitute this novel clinical group of resistant staphylococci. Anti-FASII resistance under low-FA conditions leads to endogenous phospholipid (PL) membranes. FASII bypass under high-FA conditions leads to a flexible membrane composition that varies according to what fatty acids are available in each biotope.
MATERIALS AND METHODS
Ethics statement.
S. aureus isolates were from patients hospitalized from 2012 to 2014 in Percy Military Hospital (Clamart, France) and were provided anonymously after isolation. Additional S. aureus isolates were from previously published collections. Specimens (sputum, biopsy samples, skin samples, etc.) were collected during the usual care of patients, and the collections were approved by the hospital ethics committee.
Bacterial isolates and handling.
A collection of 695 S. aureus isolates (629 human clinical and 66 cow mastitis isolates) was screened for triclosan resistance in the absence or presence of fatty acids (FA-Tr phenotypes). Isolates were obtained from Percy Military Hospital (482 isolates), the Harmony collection (91 isolates) (38), and our laboratory collection (122 isolates). Isolates other than those from the Harmony collection were from French patients. The Harmony collection comprises methicillin-resistant S. aureus (MRSA) isolates representative of epidemic or otherwise common nosocomial clones collected between 1981 and 1998 from 11 European countries. We do not have information on exposure of patients or farm animals to triclosan. However, triclosan has had widespread use worldwide since the 1970s, making exposure likely in all individuals (18). Human isolates with specified sample origins (381) were from healthy carriers (42) and patients suffering from infections corresponding to superficial (177) and deep wound (14) exudates, surgical drains (10), bone biopsy samples (57), pulmonary samples (sputum, bronchial aspirate, or pleural fluids) (39), otorhinolaryngological samples (7), or blood cultures (35). S. aureus was isolated after purification on mannitol salt plates according to routine procedure.
To screen for isolates that grew only in the presence of fatty acids (i.e., fatty acid auxotrophs), primary samples (133) of diverse origins were collected from 103 patients (Percy Military Hospital; see Table S1 in the supplemental material). They were spread directly on fatty-acid-containing plates (see below). The same patient samples were analyzed by routine clinical tests for bacterial identification (mannitol salt agar [Chapman medium] for S. aureus).
Screening for fatty-acid-dependent triclosan resistance.
Four types of agar plates were prepared to identify the triclosan resistance status of isolates: brain heart infusion (BHI), BHI-triclosan (BHI-T), BHI-fatty acids (BHI-FA), and BHI-fatty acids-triclosan (BHI-FA-T). Triclosan was used at 0.25 μg/ml. BHI-FA and BHI-FA-T comprised an equimolar mix (170 μM) [each] fatty acids found in human skin (20) and serum (21): myristic acid (C14:0), palmitic acid (C16:0), and oleic acid (C18:1) (Sigma-Aldrich). Harmony and laboratory collection isolates were spotted directly (5 μl) from thawed culture stocks on the four types of medium. For isolates received on fresh slants from Percy Hospital, colonies were resuspended in 200 μl of 0.9% NaCl before spotting. Plates were incubated at 37°C for 20 h and then photographed. Isolates that grew on BHI-FA-T but grew poorly or not at all on BHI-T were scored as fatty acid dependent and triclosan resistant (FA-Tr) and studied further.
Fatty acid auxotroph screening test.
To identify fatty acid auxotrophs, primary clinical samples were isolated on a modified HiChrome agar medium (HiMedia Laboratories, supplied by Sigma) supplemented with oleic acid (C18:1) (500 μM), which is not toxic for S. aureus growth (39). Egg lecithin, an optional additive to HiChrome medium, was omitted to control the fatty acid content (egg lecithin comprises phosphatidyl ethanolamine lipid). Tellurite was added as per the supplier's recommendation to distinguish staphylococci, which give dark to black colonies, from other bacteria. A second plate contained added triclosan (0.25 μg · ml−1) to confirm triclosan resistance in primary samples, as expected for fatty acid auxotrophs. After 48 h of incubation, colonies (both tellurite stained and cream colored) present in the plate deprived of triclosan were subjected to the four-plate screen as described above to identify fatty acid auxotrophs. Those that grew only on fatty-acid-supplemented medium (BHI-FA and BHI-FA-T) were reisolated, stored, and tested for fatty acid profiles and spa typing (see Table S3 in the supplemental material).
Fatty acid analysis.
Aerated 4-ml bacterial cultures were prepared in BHI, BHI-FA, or BHI-FA-T. Whole-cell fatty acid profiles were determined as described previously (40), except that gas-liquid chromatography was performed in a split-splitless injection mode on an Autosystem XL chromatograph (PerkinElmer) equipped with a ZB-Wax (J&W Scientific) column (30 m by 0.25 mm by 0.25 μm; Phenomenex, France). Data were recorded and analyzed with a TotalChrom workstation (PerkinElmer). S. aureus fatty acid peaks were detected between 12 and 30 min of elution.
In some cases, fatty acid profiles shifted from exogenous to endogenous during strain reisolation and after storage. We classified clinical and veterinary isolates from fatty acid phenotypes determined in initial screenings, which reflected their primary potentialities.
fabI, fabD, accBC, and accDA sequencing.
Chromosomal DNA was extracted according to commonly used techniques. Briefly, S. aureus cell suspensions were lysed by treatment with 100 μg · ml−1 lysostaphin (Sigma) for 1 h at 37°C, followed by use of standard DNA preparation methods and PCR conditions. Primers (Eurogentec, Seraing, Belgium) for S. aureus fabI gene amplification were 5′-GATACAGAAAGGACTAAATCAAA-3′ and 5′-TTTCCATCAGTCCGATTATTATA-3′. In cases where these primers gave no product, we used primers 5′-TAGCCGTAAAGAGCTTGAA-3′ and 5′-ATATTTTCACCTGTAACGCCA-3′ to amplify the central part of the gene. PCR amplification using primers 5′-TGGCGAAGAAGTAGGCAATAT-3′ and 5′-GCAACAATACTACCACCGTT-3′ was done to screen for an S. haemolyticus fabI (sh-fabI) copy as reported previously (22). Primers for accBC genes were either 5′-ACGGGTAGATGAAAACAAAC-3′ and 5′-TCTTTTTCATCACGAGCAA-3′ or 5′-AGTTGTTCCTGGTAGTGACG-3′ and 5′-CCAGTGATGCCTTCGACTTC-3′. For accDA genes, primers were either 5′-ATTGCTAGCATGGTTAAAGATTTTTTTAATCGAAC-3′ and 5′-GTGCGGCCGCTTCTATATAAGAACCGATA-3′ or 5′-AACATTCAACAGTCAAACGA-3′ and 5′-AACTAATGTATTGAATTGATGTAAACG. Primers for fabD were 5′-GAAGGTACTGTAGTTAAAGCACACG-3′ and 5′-GCTTTGATTTCTTCGACTACTGCTT-3′.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Elise Borezée-Durant for valuable advice and discussion, to Charlotte Lebouleux for technical assistance, and to the entire MicrobAdapt team for stimulating discussion in the course of this work. We thank Didier Moissenet (Hôpital Trousseau, Paris) for his assistance in the course of this project.
This project was supported by funding from the ANR, the French Research Agency (FattyBact project), and the Vaincre la Mucoviscidose (cystic fibrosis) foundation. C.M. was awarded a PhD grant from the French Ministry.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02515-16.
REFERENCES
- 1.Carey DE, McNamara PJ. 2014. The impact of triclosan on the spread of antibiotic resistance in the environment. Front Microbiol doi: 10.3389/fmicb.2014.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan N, Awrey D, Bardouniotis E, Berman J, Yethon J, Pauls HW, Hafkin B. 2013. In vitro activity (MICs and rate of kill) of AFN-1252, a novel FabI inhibitor, in the presence of serum and in combination with other antibiotics. J Chemother 25:18–25. doi: 10.1179/1973947812Y.0000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flamm RK, Rhomberg P, Kaplan N, Jones RN, Farrell DJ. 2015. Activity of Debio1452, a FabI inhibitor with potent activity against S. aureus and coagulase-negative Staphylococcus spp., including multidrug-resistant strains. Antimicrob Agents Chemother 59:2583–2587. doi: 10.1128/AAC.05119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escaich S, Prouvensier L, Saccomani M, Durant L, Oxoby M, Gerusz V, Moreau F, Vongsouthi V, Maher K, Morrissey I, Soulama-Mouze C. 2011. The MUT056399 inhibitor of FabI is a new antistaphylococcal compound. Antimicrob Agents Chemother 55:4692–4697. doi: 10.1128/AAC.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiebel J, Chang A, Shah S, Lu Y, Liu L, Pan P, Hirschbeck MW, Tareilus M, Eltschkner S, Yu W, Cummings JE, Knudson SE, Bommineni GR, Walker SG, Slayden RA, Sotriffer CA, Tonge PJ, Kisker C. 2014. Rational design of broad spectrum antibacterial activity based on a clinically relevant enoyl-acyl carrier protein (ACP) reductase inhibitor. J Biol Chem 289:15987–16005. doi: 10.1074/jbc.M113.532804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons JB, Frank MW, Rosch JW, Rock CO. 2013. Staphylococcus aureus fatty acid auxotrophs do not proliferate in mice. Antimicrob Agents Chemother 57:5729–5732. doi: 10.1128/AAC.01038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balemans W, Lounis N, Gilissen R, Guillemont J, Simmen K, Andries K, Koul A. 2010. Essentiality of FASII pathway for Staphylococcus aureus. Nature 463:E3 (Discussion, 463:E4.) doi: 10.1038/nature08667. [DOI] [PubMed] [Google Scholar]
- 8.Parsons JB, Rock CO. 2011. Is bacterial fatty acid synthesis a valid target for antibacterial drug discovery? Curr Opin Microbiol 14:544–549. doi: 10.1016/j.mib.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A, Schiebel J, Yu W, Bommineni GR, Pan P, Baxter MV, Khanna A, Sotriffer CA, Kisker C, Tonge PJ. 2013. Rational optimization of drug-target residence time: insights from inhibitor binding to the Staphylococcus aureus FabI enzyme-product complex. Biochemistry 52:4217–4228. doi: 10.1021/bi400413c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiebel J, Chang A, Lu H, Baxter MV, Tonge PJ, Kisker C. 2012. Staphylococcus aureus FabI: inhibition, substrate recognition, and potential implications for in vivo essentiality. Structure 20:802–813. doi: 10.1016/j.str.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature 458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 12.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. 2010. Brief communication arising replying to: W. Balemans et al. Nature 462, 10.1038/nature08667 (2009). Nature 463:E4. doi: 10.1038/nature08668. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaides N, Apon JM. 1977. The saturated methyl branched fatty acids of adult human skin surface lipid. Biomed Mass Spectrom 4:337–347. doi: 10.1002/bms.1200040604. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Azuma A, Kuribayashi T, Sugihara H, Okuda S, Nakagawa M. 2003. Serum fatty acid levels, dietary style and coronary heart disease in three neighbouring areas in Japan: the Kumihama study. Br J Nutr 89:267–272. doi: 10.1079/BJN2002747. [DOI] [PubMed] [Google Scholar]
- 15.Syed AK, Ghosh S, Love NG, Boles BR. 2014. Triclosan promotes Staphylococcus aureus nasal colonization. mBio 5(2):e01015-13. doi: 10.1128/mBio.01015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegard IL, Wold AE. 2006. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res 59:96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 17.Ortega Morente E, Fernandez-Fuentes MA, Grande Burgos MJ, Abriouel H, Perez Pulido R, Galvez A. 2013. Biocide tolerance in bacteria. Int J Food Microbiol 162:13–25. doi: 10.1016/j.ijfoodmicro.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Dann A, Hontela A. 2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 19.Morvan C, Halpern D, Kénanian G, Hays C, Anba-Mondoloni J, Brinster S, Kennedy S, Trieu-Cuot P, Poyart C, Lamberet G, Gloux K, Gruss A. 2016. Environmental fatty acids enable emergence of infectious Staphylococcus aureus resistant to FASII-targeted antimicrobials. Nat Commun 7:12944. doi: 10.1038/ncomms12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni Raghallaigh S, Bender K, Lacey N, Brennan L, Powell FC. 2012. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br J Dermatol 166:279–287. doi: 10.1111/j.1365-2133.2011.10662.x. [DOI] [PubMed] [Google Scholar]
- 21.Hodson L, Skeaff CM, Fielding BA. 2008. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Ciusa ML, Furi L, Knight D, Decorosi F, Fondi M, Raggi C, Coelho JR, Aragones L, Moce L, Visa P, Freitas AT, Baldassarri L, Fani R, Viti C, Orefici G, Martinez JL, Morrissey I, Oggioni MR. 2012. A novel resistance mechanism to triclosan that suggests horizontal gene transfer and demonstrates a potential selective pressure for reduced biocide susceptibility in clinical strains of Staphylococcus aureus. Int J Antimicrob Agents 40:210–220. doi: 10.1016/j.ijantimicag.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Brenwald NP, Fraise AP. 2003. Triclosan resistance in methicillin-resistant Staphylococcus aureus (MRSA). J Hosp Infect 55:141–144. doi: 10.1016/S0195-6701(03)00222-6. [DOI] [PubMed] [Google Scholar]
- 24.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Mee-Marquet NL, Corvaglia A, Haenni M, Bertrand X, Franck JB, Kluytmans J, Girard M, Quentin R, Francois P. 2014. Emergence of a novel subpopulation of CC398 Staphylococcus aureus infecting animals is a serious hazard for humans. Front Microbiol 5:652. doi: 10.3389/fmicb.2014.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansson HL. 2008. Fatty acids in bovine milk fat. Food Nutr Res 52. doi: 10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu S, Lynn WS. 1978. Lipid composition of sputum from patients with asthma and patients with cystic fibrosis. Inflammation 3:27–36. doi: 10.1007/BF00917319. [DOI] [PubMed] [Google Scholar]
- 28.Furi L, Haigh R, Al Jabri ZJ, Morrissey I, Ou HY, Leon-Sampedro R, Martinez JL, Coque TM, Oggioni MR. 2016. Dissemination of novel antimicrobial resistance mechanisms through the insertion sequence mediated spread of metabolic genes. Front Microbiol 7:1008. doi: 10.3389/fmicb.2016.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons JB, Frank MW, Subramanian C, Saenkham P, Rock CO. 2011. Metabolic basis for the differential susceptibility of Gram-positive pathogens to fatty acid synthesis inhibitors. Proc Natl Acad Sci U S A 108:15378–15383. doi: 10.1073/pnas.1109208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meades G Jr, Benson BK, Grove A, Waldrop GL. 2010. A tale of two functions: enzymatic activity and translational repression by carboxyltransferase. Nucleic Acids Res 38:1217–1227. doi: 10.1093/nar/gkp1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong SK, Kim KH, Park JK, Jeong KW, Kim Y, Kim EE. 2010. New design platform for malonyl-CoA-acyl carrier protein transacylase. FEBS Lett 584:1240–1244. doi: 10.1016/j.febslet.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Bomar L, Brugger SD, Yost BH, Davies SS, Lemon KP. 2016. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio 7:e01725–01715. doi: 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan M, Pamp SJ, Fukuyama J, Hwang PH, Cho DY, Holmes S, Relman DA. 2013. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14:631–640. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota N, Kuzumoto K, Hidaka E, Yoshizawa K, Yumoto K, Oana K, Ogiso Y, Nakamura T, Kawakami Y. 2013. First isolation of oleate-dependent Enterococcus faecalis small-colony variants from the umbilical exudate of a paediatric patient with omphalitis. J Med Microbiol 62:1883–1890. doi: 10.1099/jmm.0.062752-0. [DOI] [PubMed] [Google Scholar]
- 35.Lin YT, Tsai JC, Yamamoto T, Chen HJ, Hung WC, Hsueh PR, Teng LJ. 2016. Emergence of a small colony variant of vancomycin-intermediate Staphylococcus aureus in a patient with septic arthritis during long-term treatment with daptomycin. J Antimicrob Chemother 71:1807–1814. doi: 10.1093/jac/dkw060. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan ML, Dye W. 1976. Growth requirements of some small-colony-forming variants of Staphylococcus aureus. J Clin Microbiol 4:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweizer HP. 2001. Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol Lett 202:1–7. doi: 10.1111/j.1574-6968.2001.tb10772.x. [DOI] [PubMed] [Google Scholar]
- 38.Cookson BD, Robinson DA, Monk AB, Murchan S, Deplano A, de Ryck R, Struelens MJ, Scheel C, Fussing V, Salmenlinna S, Vuopio-Varkila J, Cuny C, Witte W, Tassios PT, Legakis NJ, van Leeuwen W, van Belkum A, Vindel A, Garaizar J, Haeggman S, Olsson-Liljequist B, Ransjo U, Muller-Premru M, Hryniewicz W, Rossney A, O'Connell B, Short BD, Thomas J, O'Hanlon S, Enright MC. 2007. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J Clin Microbiol 45:1830–1837. doi: 10.1128/JCM.02402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons JB, Yao J, Frank MW, Jackson P, Rock CO. 2012. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bacteriol 194:5294–5304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Pargade V, Lamberet G, Gaudu P, Thomas F, Texereau J, Gruss A, Trieu-Cuot P, Poyart C. 2006. The group B Streptococcus NADH oxidase Nox-2 is involved in fatty acid biosynthesis during aerobic growth and contributes to virulence. Mol Microbiol 62:772–785. doi: 10.1111/j.1365-2958.2006.05406.x. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen LN, Larsen MH, Skovgaard S, Kastbjerg V, Westh H, Gram L, Ingmer H. 2013. Staphylococcus aureus but not Listeria monocytogenes adapt to triclosan and adaptation correlates with increased fabI expression and agr deficiency. BMC Microbiol 13:177. doi: 10.1186/1471-2180-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan F, Yan K, Wallis NG, Reed S, Moore TD, Rittenhouse SF, DeWolf WE Jr, Huang J, McDevitt D, Miller WH, Seefeld MA, Newlander KA, Jakas DR, Head MS, Payne DJ. 2002. Defining and combating the mechanisms of triclosan resistance in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother 46:3343–3347. doi: 10.1128/AAC.46.11.3343-3347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skovgaard S, Nielsen LN, Larsen MH, Skov RL, Ingmer H, Westh H. 2013. Staphylococcus epidermidis isolated in 1965 are more susceptible to triclosan than current isolates. PLoS One 8:e62197. doi: 10.1371/journal.pone.0062197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.