ABSTRACT
Various procalcitonin ranges have been established to guide antimicrobial therapy; however, there are no data that establish whether the initial procalcitonin value can determine the likelihood of a positive culture result. This study aimed to establish if the initial procalcitonin value, on clinical presentation, has a positive predictive value for any positive culture result. This was a retrospective study of 813 medical intensive care unit patients. Data collected included patient demographics, procalcitonin assay results, sources of infection, culture results, and lengths of stay. Patients were excluded if they were immunocompromised. The primary outcome of this study was to determine a procalcitonin value that would predict any positive culture. Secondary outcomes included the sensitivity, specificity, positive predictive value, and negative predictive value for procalcitonin. After exclusions, a total of 519 patient charts were reviewed to determine the impact of the initial procalcitonin value on culture positivity. In our analyses, the receiver operating characteristic values were 0.62 for all cultures, 0.49 for pulmonary infections, 0.43 for urinary tract infections, and 0.78 for bacteremia. A procalcitonin value of 3.61 ng/ml was determined to be the threshold value for a positive blood culture result (prevalence, 4%). For bacteremia, the sensitivity of procalcitonin was 75%, the specificity was 72%, the positive predictive value was 20%, and the negative predictive value was 97%. Procalcitonin was a poor predictor of culture positivity. An initial procalcitonin value of less than 3.61 ng/ml may be useful in predicting whether bacteremia is absent. Procalcitonin should not be used as the only predictor for determining initiation of antibiotic therapy.
KEYWORDS: biomarkers, diagnostics, procalcitonin, sepsis
INTRODUCTION
Sepsis is a leading cause of death and disability in the United States (1, 2). Mortality from sepsis increases with each hour that anti-infective therapy is delayed (3). The impetus for promptly initiating appropriate broad-spectrum antimicrobial therapy must be weighed against the concern for reducing unnecessary antimicrobial therapy and potential harms, such as drug resistance, Clostridium difficile infections, and adverse drug effects (3). The lack of a specific test to assist clinicians in early decision-making regarding initiation and discontinuation of antimicrobial therapy for severe infection and presumed sepsis has led to expanded interest in inflammatory biomarkers, such as procalcitonin (PCT) (4–12).
Previous trials evaluating PCT have used value ranges to guide clinical decisions within the construct of initiating and discontinuing antimicrobial therapy using clinical criteria (4, 5, 7–10, 12–14). By using serial measurements and established cutoff values in intensive care units (ICUs), these studies have shown that PCT values guide antimicrobial therapy (13–18). However, no reports have described the utility of using initial PCT levels to guide upstream anti-infective therapy decision-making or the predictive ability of PCT values in the context of objective criteria of infection, such as culture positivity. Such data would provide clinicians with greater confidence in presuming the presence or absence of an infection when determining initial therapy. This study aimed to establish if a single initial PCT value can predict the result of a positive culture or the source of infection. The primary outcome of this study was to determine a PCT value that would predict any positive culture. Secondary outcomes included the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of PCT.
RESULTS
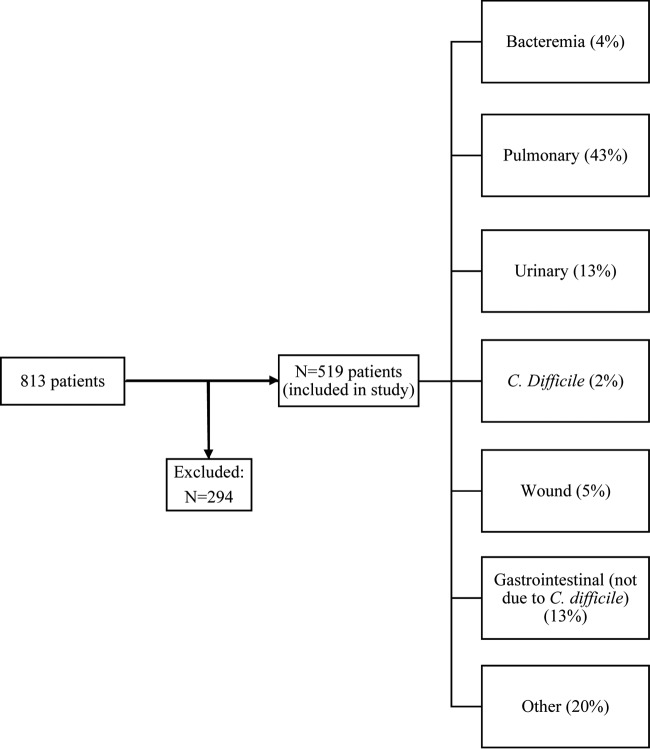
Over the 23-month study period, 813 patients met the inclusion criteria (Fig. 1). Of these, 294 patients were excluded due to their immunocompromised status. The remaining 519 patients (48% female) were included for study analyses. The median age was 59 years (interquartile range [IQR], 48–71 years). Pulmonary infections were the most common (43%) among patients in the study.
FIG 1.
Flow chart depicting patient enrollment and culture results.
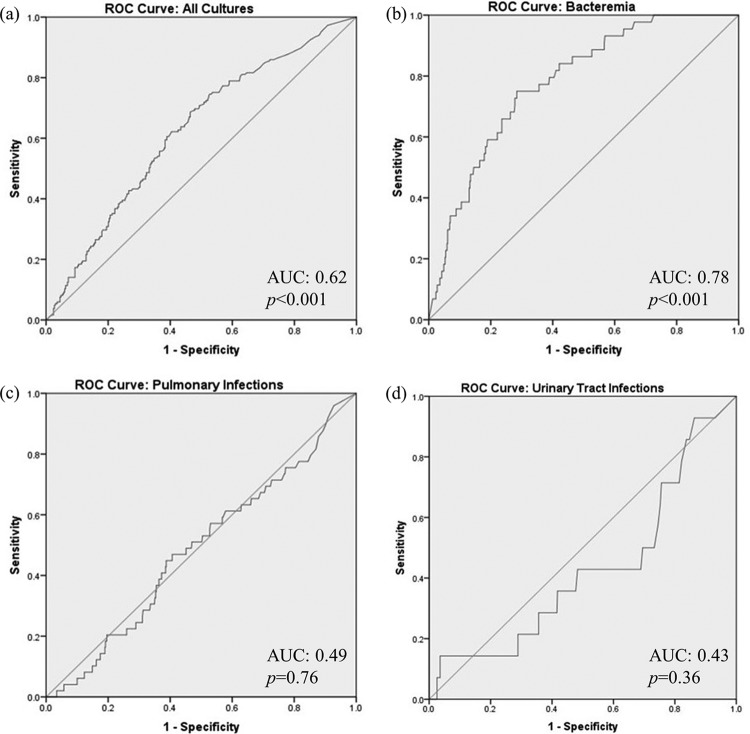
Of the different infection types analyzed (Fig. 2), bacteremia had the greatest area under the receiver operating characteristic (ROC) curve (AUC) value (0.78 [95% confidence interval (CI), 0.72–0.84]; P < 0.001) with a threshold PCT level of 3.61 ng/ml in determining culture positivity. Using our prevalence of bacteremia (4%), PCT testing had the following diagnostic characteristics: 75% sensitivity, 72% specificity, 20% PPV, and 97% NPV.
FIG 2.
ROC curve analysis: (a) all cultures; (b) bacteremia; (c) pulmonary infections; (d) urinary tract infections.
Patients with a positive culture from any source had a higher PCT level and acute physiology and chronic health evaluation II (APACHE II) score and a longer ICU/hospital length of stay (Table 1) than those with a negative culture. Although PCT levels performed poorly for predicting culture positivity in the setting of a pulmonary infection (AUC, 0.49 [95% CI, 0.40–0.56]; P = 0.76), respiratory culture positivity was associated with prolonged ICU/hospital stays and higher PCT levels (Table 2).
TABLE 1.
Comparison of culture-negative and culture-positive patient data (n = 519)
| Patient characteristica | Value for group (median [IQR])b |
P | |
|---|---|---|---|
| Culture negative | Culture positive | ||
| Age (yr) | 61 (47–73) | 57 (49–67) | 0.25 |
| ICU LOS (days) | 4 (3–7) | 6 (3–12) | <0.001 |
| Hospital LOS (days) | 8 (5–15) | 13 (8–24) | <0.001 |
| APACHE II score | 20 (15–26) | 23 (17–27) | 0.01 |
| PCT value (ng/ml) | 0.74 (0.17–4.36) | 2.12 (0.57–11.42) | <0.001 |
ICU, intensive care unit; LOS, length of stay; APACHE II, acute physiology and chronic health evaluation II; PCT, procalcitonin.
IQR, interquartile range.
TABLE 2.
Comparison of culture-negative and culture-positive pulmonary patient data (n = 222)
| Patient characteristica | Value for group (median [IQR])b |
P | |
|---|---|---|---|
| Culture negative | Culture positive | ||
| Age (yr) | 65 (50–78) | 56 (50–67) | 0.27 |
| ICU LOS (days) | 3 (2–7) | 6 (3–15) | <0.001 |
| Hospital LOS (days) | 8 (5–15) | 11 (7–21) | 0.02 |
| APACHE II score | 19 (15–26) | 23 (16–27) | 0.09 |
| PCT value (ng/ml) | 0.74 (0.17–3.41) | 2.07 (0.32–5.36) | 0.03 |
ICU, intensive care unit; LOS, length of stay; APACHE II, acute physiology and chronic health evaluation II; PCT, procalcitonin.
IQR, interquartile range.
DISCUSSION
This study highlights the potential role of initial PCT testing in predicting culture positivity from various sources of infection. The results suggest that PCT levels are not a useful biomarker for predicting culture positivity for infection from any source. However, in patients with bacteremia, a PCT value greater than 3.61 ng/ml appeared to be associated with blood culture positivity. The NPV (97%) for bacteremia would be useful in patients with a PCT value less than 3.61 ng/ml, which would be indicative of a low likelihood of a positive blood culture. This PCT level threshold might be an acceptable biomarker for future studies as a potential basis for withholding antibiotic treatment in patients with possible bacteremia until culture results are available, although this was not proven directly in our study. For infections from a pulmonary or urinary source (56% of infections studied in our population), PCT levels resulted in poor AUC ROC values (0.49 and 0.43, respectively) and therefore should not be used during the initial evaluation to determine initiation of antibiotic therapy for these types of infections.
In an era of escalating antimicrobial resistance and with a reported incidence of 30 to 50% inappropriate antimicrobial therapy (19–22), there is growing need to study biomarkers which might improve antimicrobial stewardship, especially during the initial patient care evaluation. A robust evidence-based approach to studying PCT in clinical care is currently lacking, as demonstrated by the grade 2C recommendation in the recent Surviving Sepsis Campaign guidelines (23). Two previous large clinical trials reported that measurement of serial PCT values resulted in fewer overall antibiotic days without a difference in number of deaths (13, 24). More recently, the Stop Antibiotics on Guidance of Prolactin Study (SAPS) confirmed that measuring PCT can help reduce the duration of antibiotic therapy, and this reduction is associated with a significant decrease in mortality (25). However, these trials have used PCT serially to determine duration of antimicrobial therapy. No studies preceding ours have examined the upstream utility of a single PCT measurement during the initial patient care evaluation in the context of culture positivity, which is a critical area for antimicrobial stewardship improvement.
Prior studies have compared PCT to other biomarkers, predominantly C-reactive protein (26–28). These studies have demonstrated a consensus that PCT may have a higher prognostic value for septic/bacteremic patients than does C-reactive protein (26–28). However, these other biomarkers are relatively inconsistent determinants for other sources of infection, with studies showing mixed results (26–28). Furthermore, no direct comparisons have been made between PCT levels and the clinical criteria used to determine the risk/severity of infections (e.g., systemic inflammatory response syndrome [SIRS] criteria). Our study serves to confirm prior evaluations regarding the utility of PCT in bacteremic patients and cautions against its use in determining initial antimicrobial therapy for other types of infection. Future areas of interest might be the study of biomarkers in comparison with rapid identification tests, such as those from BioFire (Salt Lake City, UT) and PNA FISH (Woburn, MA). Due to the costs associated with these rapid identification systems, their widespread use remains limited at this time.
There are several limitations of this study. In this retrospective design, our population demonstrated a variability in infectious diagnoses, with skewing toward pulmonary infections (43%) and a relatively smaller population of patients with bacteremia (4%). Additionally, although PCT levels were measured on the first day of admission to our tertiary care center, patients may have received antimicrobial therapy prior to arrival (i.e., in the emergency department or prior to transfer from an outside institution). Nevertheless, the purpose of our study was to examine PCT values within the context of arrival to our medical ICU regardless of antibiotic use in the emergency department or an outside institution. This limitation may have been mitigated by early obtainment of culture and PCT levels within the first 24 h of admission to our institution's ICU. It is important to note that PCT results are available within 24 h at our institution. Since culture results are not available until after 48 h, PCT values are available before culture results. The PCT level increases within the first 2 to 4 h of a triggering event, peaks within 12 to 24 h, and has a half-life that varies between 24 and 35 h (29–32). Therefore, in our cohort, early antibiotic use was less likely to impact PCT values in patients admitted by our institution's emergency department but may have had a greater impact on transferring patients, for whom the duration to ICU admission was longer.
Conclusion.
Initial PCT results were poor predictors of culture positivity. However, an initial PCT level less than 3.61 ng/ml might be acceptable for predicting whether bacteremia is absent. This is supported by a negative predictive value of 97%. Routine use of PCT level in conjunction with clinical judgment can be useful for bacteremic patients, but our study cautions against its use to determine initial antimicrobial therapy for other sources of infection, particularly pulmonary and urinary sources.
MATERIALS AND METHODS
Population.
Institutional review board approval was granted from Rush University Medical Center for this retrospective cohort study using an existing database. Data from April 2013 to December 2014 were collected for patients admitted to the medical ICU at Rush University Medical Center. Patients were included if they were greater than 18 years of age and had a suspected infection as their admitting diagnosis and an associated bacterial culture, and their first PCT value was obtained within 24 h upon admission to the medical ICU. Patients were excluded if they were immunocompromised, which was defined as receiving immunosuppressive therapy (6-mercaptopurine, prednisone equivalent to greater than 5 mg/day, antithymocyte globulin, azathioprine, basiliximab, cyclophosphamide, cyclosporine, calcineurin inhibitors, everolimus, methotrexate, mycophenolate, rituximab, or sirolimus) or having a diagnosis of HIV or active cancer.
Data collected included age, gender, acute physiology and chronic health evaluation II (APACHE-II) score, first PCT value, ICU length of stay (LOS), hospital LOS, suspected and confirmed source of infection, culture result, and type of infection.
Statistical analysis.
The ability of PCT to predict a positive culture was evaluated by using receiver operating characteristic (ROC) curve methodology. The best cutoff value of PCT for a positive culture result was determined according to Youden's index method. The areas under the ROC curves (AUC) were reported with 95% confidence intervals (CIs) in addition to prevalence, sensitivity, specificity, PPV, and NPV. Nonparametric statistical analyses (Mann-Whitney U test) were applied to compare culture-positive and culture-negative data among various baseline characteristics. Values were reported as medians with interquartile ranges (IQR). Statistical analyses were conducted using the Statistical Program for Social Science (SPSS version 23, Chicago, IL, USA). Statistical significance was defined as a P value of <0.05.
ACKNOWLEDGMENTS
We declare no conflicts of interest.
All of the authors had access to the data, reviewed the manuscript, and approved this submission.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Linde-Zwirble WT, Angus DC. 2004. Severe sepsis epidemiology: sampling, selection, and society. Crit Care 8:222–226. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 4.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, Taneja S, Ray S. 2015. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care 30:218.e7–e12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. 2013. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 13:426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 6.Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. 2008. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 7.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, Schroeder S. 2009. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 13:R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Jeong TD, Lee W, Chun S, Min WK. 2015. Procalcitonin in the assessment of bacteraemia in emergency department patients: results of a large retrospective study. Ann Clin Biochem 52:654–659. doi: 10.1177/0004563214568685. [DOI] [PubMed] [Google Scholar]
- 9.Jeong S, Park Y, Cho Y, Kim HS. 2012. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin Chim Acta 413:1731–1736. doi: 10.1016/j.cca.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. 1993. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrol ED, Thomson AP, Hart CA. 2002. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents 20:1–9. [DOI] [PubMed] [Google Scholar]
- 12.Balcl C, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B. 2003. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care 7:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Régnier B, Brun-Buisson C, Chastre J, Wolff M; PRORATA Trial Group. 2010. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. 2013. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care 17:R115. doi: 10.1186/cc12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuetz P, Chiappa V, Briel M, Greenwald JL. 2011. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 171:1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 16.Schuetz P, Briel M, Christ-Crain M, Stolz D, Bouadma L, Wolff M, Luyt CE, Chastre J, Tubach F, Kristoffersen KB, Wei L, Burkhardt O, Welte T, Schroeder S, Nobre V, Tamm M, Bhatnagar N, Bucher HC, Mueller B. 2012. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 55:651–662. doi: 10.1093/cid/cis464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuetz P, Mueller B. 2014. Biomarker-guided de-escalation of empirical therapy is associated with lower risk for adverse outcomes. Intensive Care Med 40:141. doi: 10.1007/s00134-013-3139-x. [DOI] [PubMed] [Google Scholar]
- 18.Naffaa M, Makhoul BF, Tobia A, Kaplan M, Aronson D, Azzam ZS, Saliba W. 2014. Procalcitonin and interleukin 6 for predicting blood culture positivity in sepsis. Am J Emerg Med 32:448–451. doi: 10.1016/j.ajem.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Schuetz P, Suter-Widmer I, Chaudri A, Christ-Crain M, Zimmerli W, Mueller B; Procalcitonin-Guided Antibiotic Therapy and Hospitalisation in Patients with Lower Respiratory Tract Infections (ProHOSP) Study Group. 2011. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J 37:384–392. doi: 10.1183/09031936.00035610. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States; 2013. http://www.cdc.gov/drugresistance/threat-report-2013/ Accessed 1 June 2016.
- 21.Leung E, Weil DE, Raviglione M, Nakatani H; World Health Organization World Health Day Antimicrobial Resistance Technical Working Group. 2011. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ 89:390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack LA, Plachouras D, Gruhler H, and Sinkowitz-Cochran R. Transatlantic Taskforce on Antimicrobial Resistance (TATFAR). Summary of the modified Delphi process for common structure and process indicators for hospital antimicrobial stewardship programs; 2015. http://www.cdc.gov/drugresistance/pdf/summary_of_tatfar_recommendation_1.pdf Accessed 3 June 2016.
- 23.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen JU, Lundgren B, Hein L, Mohr T, Petersen PL, Andersen LH, Lauritsen AO, Hougaard S, Mantoni T, Bømler B, Thornberg KJ, Thormar K, Løken J, Steensen M, Carl P, Petersen JA, Tousi H, Søe-Jensen P, Bestle M, Hestad S, Andersen MH, Fjeldborg P, Larsen KM, Rossau C, Thomsen CB, Ostergaard C, Kjaer J, Grarup J, Lundgren JD. 2008. The Procalcitonin and Survival Study (PASS)—a randomised multi-center investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and pro-active diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infect Dis 13:91. doi: 10.1186/1471-2334-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC, Kemperman H, van den Elsen MJ, Schouten JA, Streefkerk JO, Krabbe HG, Kieft H, Kluge GH, van Dam VC, van Pelt J, Bormans L, Otten MB, Reidinga AC, Endeman H, Twisk JW, van de Garde EM, de Smet AM, Kesecioglu J, Girbes AR, Nijsten MW, de Lange DW. 2016. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 16:819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 26.Lisboa T, Seligman R, Diaz E, Rodriguez A, Teixeira PJ, Rello J. 2008. C-Reactive protein correlates with bacterial load and appropriate antibiotic therapy in suspected ventilator-associated pneumonia. Crit Care Med 36:166–171. doi: 10.1097/01.CCM.0000297886.32564.CF. [DOI] [PubMed] [Google Scholar]
- 27.Seligman R, Meisner M, Lisboa TC, Hertz FT, Filippin TB, Fachel JM, Teixeira JP. 2006. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Crit Care 10:R125. doi: 10.1186/cc5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmers JD, Singanayagam A, Hill AT. 2008. C-Reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 121:219–225. doi: 10.1016/j.jmjmed.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh B, Kennedy P, Kruger PS, Looke D, Jones M, Hall J, Barruel GR. 2009. Changes in serum procalcitonin and C-reactive protein following antimicrobial therapy as a guide to antibiotic duration in the critically ill: a prospective evaluation. Anaesth Intensive Care 37:20–26. [DOI] [PubMed] [Google Scholar]
- 30.Christ-Crain M, Müller B. 2007. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J 30:556–573. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 31.Kibe S, Adams K, Barlow G. 2011. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 66(Suppl 2):33–40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 32.Schuetz P, Albrich W, Mueller B. 2011. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]