ABSTRACT
Schistosomiasis, a major neglected tropical disease, affects more than 250 million people worldwide. Treatment of schistosomiasis has relied on the anthelmintic drug praziquantel (PZQ) for more than a generation. PZQ is the drug of choice for the treatment of schistosomiasis; it is effective against all major forms of schistosomiasis, although it is less active against juvenile than mature parasites. A pyrazino-isoquinoline derivative, PZQ is not considered to be toxic and generally causes few or transient, mild side effects. Increasingly, mass drug administration targeting populations in sub-Saharan Africa where schistosomiasis is endemic has led to the appearance of reduced efficacy of PZQ, which portends the selection of drug-resistant forms of these pathogens. The synthesis of improved derivatives of PZQ is attracting attention, e.g., in the (i) synthesis of drug analogues, (ii) rational design of pharmacophores, and (iii) discovery of new compounds from large-scale screening programs. This article reviews reports from the 1970s to the present on the metabolism and mechanism of action of PZQ and its derivatives against schistosomes.
KEYWORDS: cytochromes P450, enantiomers, metabolism, praziquantel, schistosomiasis
INTRODUCTION
Schistosomiasis, a major neglected tropical disease, is considered the most important helminthic disease of humanity in terms of morbidity and mortality rates. More than 200 million people are infected worldwide, and 600 million are at risk of infection (1, 2). Control strategies have been employed to block transmission and reduce the disease burden, including mass and targeted chemotherapy, improvements to sanitation, modification of the environment, and the use of molluscicides (3, 4). However, schistosomiasis remains a major public health problem, especially in rural regions of sub-Saharan Africa (2). The infection is caused by three main species of blood flukes, Schistosoma haematobium in Africa and the Middle East, S. mansoni in Africa and South America, and S. japonicum in China and the Philippines, and two less common ones, S. intercalatum in Africa and S. mekongi in Southeast Asia (5). Moreover, recent outbreaks reveal the reemergence of urogenital schistosomiasis in southern Europe (6). Additionally, infection with S. haematobium is classified as a group I biological carcinogen by the International Agency for Research in Cancer of the World Health Organization (WHO) (7). Table 1 summarizes the species that commonly infect humans, the geographical ranges of endemicity, and the major disease symptoms (5, 7, 8).
TABLE 1.
Schistosoma species, regions of prevalence, and major signs and symptoms of schistosomiasis
| Species | Regions of prevalence | Pathology, symptoms, signs |
|---|---|---|
| S. mansoni | Africa, Middle East, Caribbean, South America | Liver/periportal fibrosis, hepatomegaly, intestinal fibrosis, diarrhea |
| S. japonicum | China, Southeast Asia (Philippines, Indonesia) | Blood in stool, portal hypertension, hepatomegaly, intestinal fibrosis, diarrhea, blood in stool, CNSa complications |
| S. mekongi | Cambodia, Lao People's Democratic Republic | Same as for S. japonicum |
| S. hematobium | Africa, Middle East, southern Europe (Corsica, France) | Urogenital tract fibrosis, female genital schistosomiasis, bladder cancer, renal failure, infertility |
CNS, central nervous system.
Male and female schistosomes dwell in copula within the mesenteric veins (S. mansoni, S. japonicum) or the venous plexus (S. haematobium) of the human host, laying hundreds to thousands of fertilized eggs per day, depending on the species. The eggs traverse the intestinal wall (e.g., S. mansoni) or the bladder wall (S. haematobium) and exit the host to the external environment in feces or urine, respectively. However, many eggs are retained in host tissues, where they induce inflammation, granuloma, and fibrosis. In the external environment, the eggs hatch when they reach freshwater, releasing a free-living larva, the miracidium, that is ciliated and seeks to infect the obligate intermediate host, a snail. Within the snail, the parasite undergoes cycles of asexual reproduction through mother and daughter sporocyst stages, eventually shedding thousands of cercariae into the water. The cycles of asexual reproduction of the parasite within the snail require from 4 to 6 weeks before cercariae are released. The cercaria is the infectious developmental stage for humans and other mammals. After penetrating the skin, the cercariae shed the tail and the juvenile larvae, termed schistosomula, migrate within the circulatory system, reaching the lungs, the liver, and finally the portal venous system or the venous system that drains the pelvic organs, depending on the species, where the parasite fully matures. Adult S. mansoni worms migrate to the superior mesenteric veins, S. japonicum worms migrate to the inferior mesenteric and superior hemorrhoidal veins, and S. haematobium worms migrate to the vesical plexus and veins draining the ureters, bladder, and other pelvic organs. Male and female schistosomes mate, produce eggs, and thus complete the developmental cycle (Fig. 1) (6).
FIG 1.
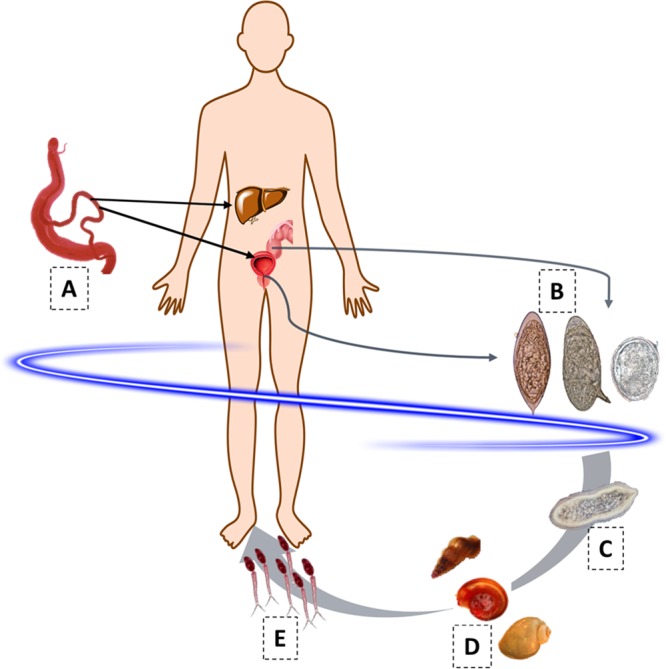
The developmental cycle of S. mansoni, S. haematobium, and S. japonicum. Stages: A, paired adult worms (larger male enfolding slender female); B, eggs (left to right, S. haematobium, S. mansoni, and S. japonicum); C, ciliated miracidium; D, intermediate host snails (left to right, genera Oncomelania, Biomphalaria, and Bulinus); E, cercariae (infective stage).
The infection clinically progresses from an immediate phase to acute and chronic stages (7, 9–13). The initial phase is typically characterized by an acute, pruritic, maculopapular eruption at the site of cercarial skin penetration within the first 24 h after exposure. This may last several days, may occur even with zoonotic schistosome species that do not usually mature in humans, and is also known as cercarial dermatitis or swimmer's itch. Acute schistosomiasis (Katayama fever) is a systemic hypersensitivity reaction to the migrating schistosomula that occurs a few weeks to months after a primary infection. The disease starts suddenly with fever, fatigue, myalgia, malaise, nonproductive cough, eosinophilia, and patchy infiltrates on chest radiography. Abdominal symptoms develop later, following the migration and residence of the mature worms in the blood vessels of the intestines and bladder. Most persons recover spontaneously from the acute stage after 2 to 10 weeks, but some develop a persistent and more serious disease with weight loss, dyspnea, diarrhea, diffuse abdominal pain, toxemia, hepatosplenomegaly, and a widespread rash (7, 9, 10). During chronic or advanced schistosomiasis, which can persist for decades in the absence of treatment, the gastrointestinal and urogenital tracts are affected, leading to hepatosplenic and pelvic organ diseases and other complications, including portal and pulmonary hypertension, abdominal ascites, upper gastrointestinal varices and hemorrhage, infertility, and increased risk of HIV-1 transmission (Table 2) (10–13).
TABLE 2.
Clinical phases of schistosomiasis and its associated symptomsa
| Clinical phase | Symptoms |
|---|---|
| Immediate | Acute, pruritic, maculopapular eruption at site of cercarial skin penetration within 1 day following exposure |
| Acute | Systematic hypersensitivity reaction against migrating schistosomula, fever, fatigue, myalgia, malaise, nonproductive cough, eosinophilia, patchy infiltrates, weight loss, dyspnea, diarrhea, diffuse abdominal pain, toxemia, hepatosplenomegaly, widespread rash |
| Chronic | Affects gastrointestinal and urogenital tracts, leading to hepatosplenic and pelvic organ diseases, portal and pulmonary hypertension, abdominal ascites, upper gastrointestinal varices and hemorrhage, female genital schistosomiasis, infertility, increased risk of HIV-1 transmission, and squamous cell carcinoma of the bladder |
The paucity of information on new derivatives of praziquantel (PZQ1) is curious, especially since not only is schistosomiasis one of the major neglected tropical diseases but infection with S. haematobium is a biological carcinogen (14). Neglect of the latter undoubtedly relates to the lack of reliable rodent models of urogenital schistosomiasis. Nonetheless, the design of novel, rational compounds with potential antischistosomal activity is hindered by the absence of the definitive mode of the antischistosomal action of PZQ1. Although investigation of novel PZQ1 derivatives apparently continues, there is not a wealth of information available on the mode of drug action. Here, we review recent developments on derivatives of PZQ1, including activity and metabolites, as well as modes of action and drug resistance. We believe that review of this information will be beneficial for the identification of novel antischistosomal drugs and new drug targets.
A SINGLE DRUG FOR TREATMENT AND CONTROL OF SCHISTOSOMIASIS
The pyrazino-isoquinoline derivative PZQ1 (Fig. 2) was developed by Bayer in the 1970s and shown to be active against parasitic flatworms, including schistosomes. Remarkably, treatment and control of schistosomiasis have relied only on this drug for over 40 years (15–17). In animal tests, PZQ1 showed minimal toxicity (18) and no genotoxic risks (19) were detected in assays for mutagenicity (20). The few observations that suggested accumulation of potentially mutagenic metabolites may have been abnormalities among otherwise overwhelming evidence indicating that PZQ1 is a safe drug (21). Generally, PZQ1 induces only mild and transient side effects, if any. The frequency and intensity of these effects are correlated with the intensity of infection, and the most severe side effects of bloody diarrhea or edematous urticaria observed in areas with high intensities of infection may be due to the release of antigens and other metabolites by dying worms (22, 23).
FIG 2.
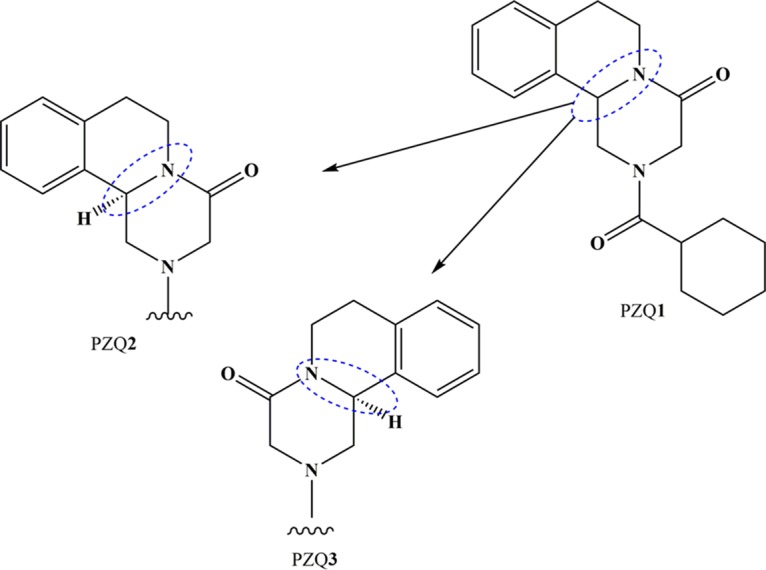
Enantiomers of PZQ1 and biologically active (R-PZQ, PZQ2) and inactive (S-PZQ, PZQ3) isomers.
During the past few years, the renewed acknowledgment of the burden imposed by schistosomiasis has led to the implementation of mass drug administration (MDA) programs for the control and possible elimination of the this major human helminthiasis, yet the WHO recently reported that less than one-third of individuals who required “preventive chemotherapies” received treatment (24). PZQ1 has been widely used since 2006 through “preventive chemotherapy” programs distributing the drug to school age children or at-risk populations, depending on prevalence rates. In 2010, 34 million people received PZQ1, mostly in sub-Saharan Africa (16). It has been estimated that by 2018, as many as 235 million people will have been treated with PZQ1, a projected use of 645 million tablets of PZQ1 (25). Also, PZQ1 is effective in the treatment of hypertension due to chronic schistosomiasis (10). This continues to be a key drug in the treatment of schistosomiasis and, indeed, most other fluke and cestode infections (17).
According to the Biopharmaceutics Classification System and the Biopharmaceutics Drug Disposition Classification System, PZQ1 is a class II drug that displays a high ability to permeate tissues and low solubility (0.4 mg/ml) and proceeds through extensive metabolism (discussed below) (26, 27) via hydroxylation of the absorbed drug to inactive metabolites, such that only minimal concentrations contact the parasites within the blood system. Currently, PZQ1 is distributed as a racemate that includes equivalent proportions of the biologically active R-PZQ (PZQ2, Fig. 2) and inactive S-PZQ (PZQ3, Fig. 2) (28) enantiomers, the consequence of which is that half the PZQ1 dose is pharmacologically inactive. This requires the use of a 600-mg tablet to provide a final dose of 40 mg/kg. Moreover, PZQ3 probably contributes to the unpleasant taste of PZQ1. These disadvantages contribute to inefficient treatment of school age children, since children frequently avoid swallowing the medicine because of its less-than-pleasant taste (28). Meyer et al. (29) investigated the bitterness value of enantiomers in regard to additional incentives for low-cost production of pure active PZQ1 (29). Indeed, the pure enantiomer of PZQ2 can probably be synthesized economically (30, 31). Among these variants, however, PZQ1 presents other disadvantages, such as decreased or complete absence of activity against juvenile schistosomes (32, 33). Accordingly, a complete cure is not reliably achieved with a single dose of PZQ1, particularly given that reinfection is routine (8, 34).
Despite decades of extensive use, much remains unknown about PZQ1, in particular, its exact mode of action, its in vivo metabolism, and its molecular target(s). Herein, these aspects are reviewed along with prospective derivatives of PZQ1.
PZQ PHARMACOKINETICS
Although PZQ1 has been employed for decades, few pharmacokinetic studies have been performed with humans (26), although a study carried out with healthy volunteers demonstrated that absorption of PZQ1 is relatively fast (time to maximum concentration of drug in serum [Tmax], 2.0 to 2.6 h) and nearly complete (>80%), which demonstrates an extensive first-pass effect (35). The systemic bioavailability of PZQ1 is low and varies considerably among individuals. After the administration of 40 mg/kg to a healthy adult, the half-life (t1/2) was reported to range from 2.2 to 8.9 h and the area under the curve (AUC) was reported to range from 2,100 to 5,400 ng h/ml. Oral drugs display higher pharmacokinetic variability than drugs administered by the parenteral route, which is explained by the blood flow at the absorption site, the absorptive surface area, the transit time, and the gastric pH (36). These factors are also influenced by concurrent food intake; the bioavailability of PZQ1 increases with concomitant food administration. Following the administration of 1,800 mg (∼25 mg/kg for a 70-kg body weight) to healthy adults, the AUC from 0 to 8 h was 2.7-fold higher with a fatty diet and ∼4 times as high with a high-carbohydrate diet (37). The effect of food on bioavailability may also be due to changes in hepatic flow, altered cytochrome P450 (CYP) expression in response to the diet, or changes in the first-pass metabolism of PZQ1 (38, 39). The bioavailability of PZQ1 has also been analyzed during schistosomiasis. Comparing the bioavailability of PZQ1 in healthy volunteers and infected people after the administration of 40 mg/kg, the Cmax (the maximum or peak concentration) and AUC were 1.7- and 4.2-fold higher in patients, the Tmax was 0.6 times shorter, and the t1/2 was 5.2 times longer (40). PZQ1 is mainly concentrated in the liver and kidneys. Concentrations higher than those in plasma occur in the lungs, pancreas, adrenal glands, pituitary gland, and salivary glands (41). However, the volume of distribution is not known (41). In addition, PZQ1 binds to proteins (∼80% exclusively to albumin). Hence, nutritional status and other factors, including chronic inflammation, influence the levels of the free drug (35, 42).
INSIGHTS INTO METABOLISM OF PZQ
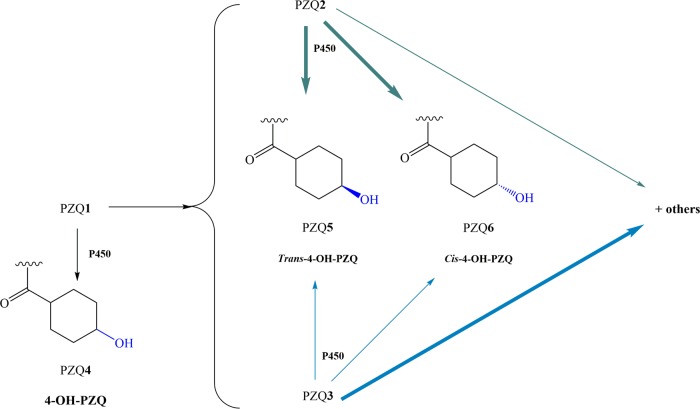
As noted, PZQ1 is mainly metabolized to PZQ2 and PZQ3, which in turn breaks down into various mono- or dihidroxy metabolites and S-trans- and S-cis-4-OH-PZQ, while PZQ2 is metabolized to R-trans-4-OH-PZQ or R-cis-4-OH-PZQ (Fig. 3) (43–45). Since higher drug concentrations in plasma and slightly longer half-lives are achieved with metabolites than with PZQ1, the metabolites likely contribute to the drug's antischistosomal activity (46). In fact, in vitro studies using PZQ2 and PZQ3 and its major metabolites against S. mansoni developmental stages (newly transformed schistosomula and adult worms) demonstrated that PZQ2 and its metabolites exhibit 100- and 1,000-fold higher activities than their S counterparts. These findings confirm that PZQ2 is the main effector, whereas PZQ3 and its metabolites do not contribute significantly to the drug's antischistosomal activity (15). Nonetheless, metabolites of PZQ1 are less active than the parent drug (47). Although the enzymes that metabolize PZQ1 are not fully known, PZQ1 is primarily metabolized by CYP 3A and to a lesser extent by CYP 2D6 (35). Several studies have been performed to clarify the metabolic profile of PZQ1, as well as the enzymes involved and the identities of the phase II metabolites (48–53).
FIG 3.
PZQ1 is metabolized by CYP 450, resulting in PZQ4 as the main product and other minor enantiomers such as PZQ5 and PZQ6. In addition, enantiomers of PZQ1 also undergo metabolism. Bold green and blue arrows indicate major metabolites of PZQ enantiomers PZQ2 and PZQ3, respectively. PZQ2 is mainly metabolized into trans- and cis-4-OH-PZQ (PZQ6), whereas PZQ3 is mainly metabolized into other mono- or dihydroxylated forms of PZQ1 and, to a less extent, into trans- and cis-4-OH-PZQ (PZQ5, PZQ6). P450 enzymes perform these transformations.
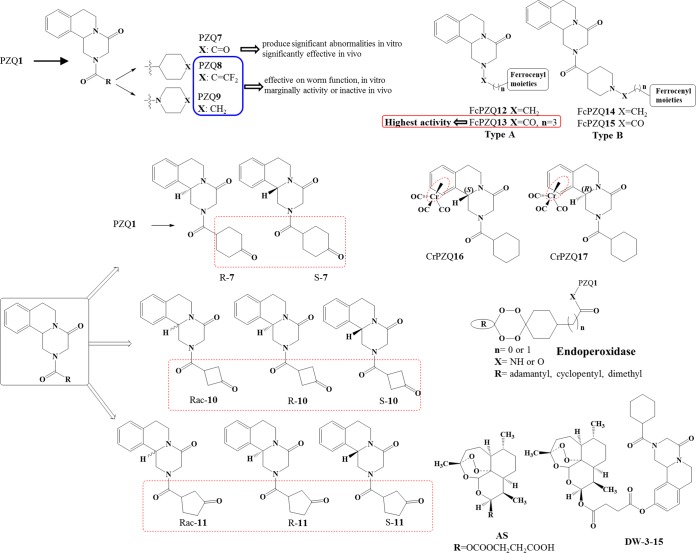
Development of new PZQ derivatives might be a good strategy to circumvent the major drawbacks of current PZQ1 therapy. Substantial investigation has been directed to the design of different types of PZQ derivatives. Through the years, several PZQ derivatives have been developed and assessed via in vitro and in vivo studies mainly against S. mansoni and S. japonicum. Design of urea and amide derivatives (Fig. 4) led to a moderate reduction of worm motility in vitro, but generally, this activity was not observed in vivo. However, one derivative of these series, PZQ7, stood out in regard to significant activity in vivo, which may derive from its in vivo reduction to a trans-cyclohexanol metabolite, PZQ4 (54). With this structure in mind, chiral PZQ derivatives (PZQ10 and PZQ11) were developed, as well as chiral PZQ7. Racemic PZQ10 and PZQ11 had modest activity against S. japonicum in vitro, while their enantiomers (S and R) failed to display any activity, with the exception of racemic PZQ7 and its enantiomers. Overall, all chiral PZQ derivatives display modest activity in vivo compared to that of PZQ1. Intriguingly, the size of the ring with a carbonyl group in these derivatives had an appreciable impact on R isomers, increasing their activity, but had almost no effect on S isomers (55). Using organometallic moieties such as ferrocenyl (already present in anticancer, antibacterial, and antimalarial drugs [56–58]) Fc-PZQ derivatives (types A and B, Fig. 4) displayed anthelmintic activity in the micromolar range but were considerably less active than PZQ1 (59). Upon alteration of the organometallic moiety to Cr(CO)3 (CrPZQ16, CrPZQ17, Fig. 4), the derivatives exhibited marked activity against S. mansoni in vitro; however, they exerted low activity in vivo (60). This fact might be related to the metabolite liability of these derivatives, resulting in less-active metabolites (Fig. 5), and due to protein binding or distribution (61). Following a different design strategy, a combination of artesunate (AS) and PZQ1 led to new derivatives that incorporate these two moieties, DW-3-15. Because of the complementary effect of these two drugs (AS is more effective against schistosomula and juvenile worms, whereas PZQ is effective against adult worms), it was expected that this derivative would demonstrate potential broad-spectrum antischistosomal activity (62, 63). In fact, biological evaluation of DW-3-15 (Fig. 4) proved that the complementary functions of AS and PZQ1 were more effective than PZQ1 alone. Therefore, this might encourage rational drug design by combining pharmacophore moieties of discrete bioactive compounds with dual modes of action (62, 63). In comparison to DW-3-15, it was expected that endoperoxide-PZQ derivatives would have increased bioavailability (since their molecular weight was less than that of DW-3-15) and thereby improved antischistosomal activity. Although these derivatives presented good efficacy against adult S. mansoni worms in vitro, their activity was lower than that of DW-3-15. Moreover, potential action in vitro did not translate to impressive killing in vivo (64). The diminished activity of endoperoxidase derivatives might be associated with intrinsic aspects of their in vivo metabolism.
FIG 4.
Structures of diverse PZQ derivatives developed and assessed for activity against schistosomes.
FIG 5.
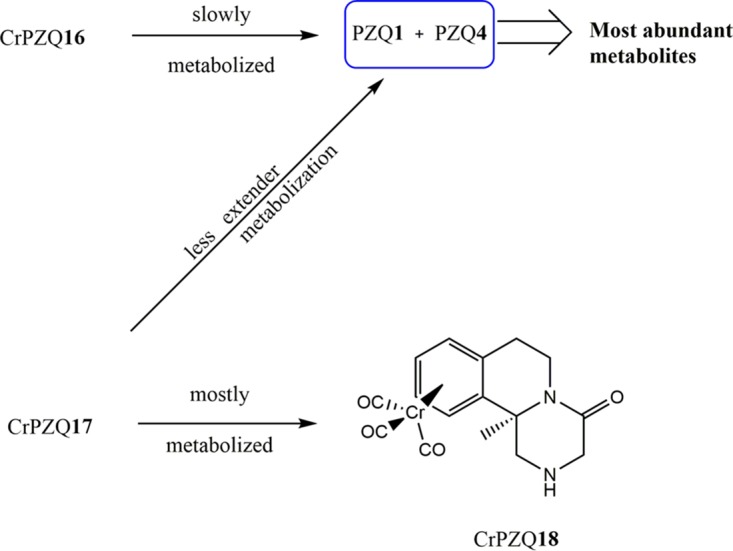
Metabolism of Cr-PZQ derivatives in vitro by human liver microsomes.
Apparently, the positions of chemical modifications played an important role in the compounds' activity. It seems that linkage through the metabolically liable cyclohexyl might not afford active derivatives, e.g., organometallic moieties. Moreover, PZQ derivatives have generally not achieved improved activity compared to that of the parent drug. Furthermore, in most cases, the promising in vitro activity of candidate drugs cannot be extrapolated to good in vivo activity since their pharmacokinetics and metabolic profiles are key determinants of their in vivo efficacy (53). Much remains to be done to develop an improved and effective derivative of PZQ1.
HOW DOES PZQ KILL SCHISTOSOMES?
Despite many years of use and the treatment of many millions of people, the mechanism(s) of action of PZQ1 has not been established yet. However, the early effects exerted by PZQ on the schistosome have been summarized under three main headings, (i) calcium influx into the whole parasite, (ii) muscle contraction, and (iii) surface modifications (65). It is tempting to link these phenomena into a single thread, assuming that calcium influx is the key event, which in turn induces muscle contraction and alterations to the tegument (65, 66). The correlation between increased intracellular Ca2+ and muscular contractions in schistosomes exposed to PZQ has been known for decades. However, how PZQ1 disrupts homeostasis in schistosomes remains largely unknown. Diverse studies have focused on the phenomenon (Table 3). Initially, it was hypothesized that PZQ1 affects Ca2+ influx through voltage-operated calcium channels (67–73). However, in subsequent studies, it was shown that calcium accumulation by itself, as measured in parasites maintained in vitro, may not explain the schistosomicidal activity of PZQ1 (71, 72).
TABLE 3.
Key reports and findings focused on the mechanism of action of PZQ
| Study design | Finding(s) | Reference(s) |
|---|---|---|
| Exposure of S. mansoni males in vitro | Muscular paralysis and rapid Ca2+ influx, removal of Ca2+ inhibited PZQ effect, tegument blebbing and disruption | 67, 68 |
| VOCCa β subunits expressed in Xenopus oocytes | Schistosome β subunits associated with drug sensitivity | 69 |
| Block VOCC, exposing schistosome to cytochalasin D | Inhibition of Ca2+ channels suppressed schistosomal activity | 70, 71 |
| Suppression of Dugesia japonica Ca2+ channel subunits by RNA interference | Suppression of Ca2+ channels of amputated parasite in two heads leads to inhibition of regeneration of two heads and tail | 72, 73 |
| Transcriptional response of S. mansoni to heat shock | >600 genes upregulated as possible targets of PZQ; schistosomes undergo oxidative-stress-like transcriptomic response | 74 |
| Gene expression in adult and juvenile S. mansoni cultured in PZQ | Juvenile schistosomes show enhanced transcriptomic elasticity | 75 |
| RNA interference-based silencing of CamKII | CamKII mitigated effect of PZQ by stabilizing Ca2+ fluxes within parasite muscles and tegument and might play role in mode of action | 76, 77 |
| Mass spectrometric characterization of surface lipids of schistosomes | Distinct chemical markers in female vs male responses to PZQ; PZQ may inhibit sphingomyelinase activity, impairing reproduction in females, whereas PZQ may impair activity of Na+/K+-ATPase in males | 78, 79 |
VOCC, voltage-operated calcium channels.
High-throughput transcriptomic approaches have been employed to address the refractory/susceptible nature of the developmental stages of schistosomes in terms of PZQ1 activity (73–77). These studies revealing genes that might be evolved in aerobic metabolism and cytosolic calcium regulation, suggesting that schistosomes undergo a transcriptomic response similar to that seen during oxidative stress (74). Moreover, it was demonstrated that CamKII (calcium/calmodulin-dependent protein kinase type II) appears to play a key role in the mode of action of PZQ1 and hence might be considered a promising novel drug target (76, 77). The use of mass spectrometry techniques revealed the existence of chemical markers that are distinct according to sex after drug exposure. Apparently, PZQ1 alters the conformation of the usual surface double lipid bilayer that surrounds schistosomes (78). Perhaps PZQ1 inhibits sphingomyelinase activity and thereby impairs reproduction by impeding the continuous release of eggs (79, 80).
Despite these efforts to understand how PZQ1 acts, the molecular targets remain elusive. Although from a medical point of view, how the drug acts might not be important as long as the drug is efficacious, the mechanism of action is relevant to improvement of the efficacy of new PZQ1 derivatives.
IS PZQ RESISTANCE IMMINENT?
Reliance on PZQ1 raises legitimate concerns about selection for PZQ resistance (65). MDA never reaches all of the infected people in a community, and so, the worm population remaining after treatment is not composed solely of resistant worms; there will still be a susceptible population that, in turn, reduces the likelihood of resistance (81–83). Whereas widespread drug resistance has not been proved, researchers have identified field and experimental isolates that exhibit significantly reduced susceptibility. These findings could portend the emergence of resistance to PZQ1 in schistosomes. Over the years, evidence of resistance to PZQ1 has been widely reviewed and remains controversial (81, 83–87). Moreover, the criteria used to classify a schistosome strain PZQ resistant are also controversial (81, 82). Here, we present an overview and synthesis of findings on this topic and also highlight potential mechanisms of drug resistance.
EXPERIMENTALLY INDUCED PZQ RESISTANCE
Attempts to induce resistance to PZQ1 in the laboratory were reported as early as the 1970s and have continued until the present, mostly focused on S. mansoni (88–90). Table 4 highlights some of the key studies (from our viewpoint) that attempt to demonstrate the appearance of resistance to PZQ. A hallmark study by Fallon and Doenhoff (91) published in 1994 demonstrated that S. mansoni developed resistance to PZQ1 over the course of several subcurative multiple doses of PZQ1 in mice; by the seventh generation of PZQ1 pressure, the population of schistosomes was 93% resistant to three PZQ1 doses of 300 mg/kg, a dose that killed 89% of the control schistosomes. Ismael and colleagues (92) studied the effect of PZQ1 at 300 and 500 mg/kg on successive generations of S. mansoni worms in mice and observed that at low subcurative doses, resistance to therapeutic doses of the drug appeared after several generations of the treatment regimen (92). More recently, Couto et al. (93) reported a novel method to induce resistance to PZQ1 in S. mansoni. Snails infected with schistosomes were treated successively with PZQ1 at 100 mg/kg for 5 consecutive days. Subsequently, mice were infected with cercariae released from the snails and treated with PZQ1 at 200, 400, or 800 mg/kg. This method is effective for inducing resistance of S. mansoni to PZQ1 in the laboratory and is far less costly and labor intensive than some other approaches mentioned above (93). Other studies have reported the generation of resistance to PZQ1 in S. japonicum, assayed in adult worms, cercariae, and miracidia (94). In contrast, we are not aware of reports describing experimental induction of resistance to PZQ1 in S. haematobium. Finally, it is also worth noting that the mode of action of the drug would be expected to be altered in strains that are insensitive to PZQ1 (81).
TABLE 4.
Key studies and findings focused on experimentally induced PZQ resistance in different Schistosoma species
| Strain(s) | Study design | Treatment | Findings/outcomes | Reference(s) |
|---|---|---|---|---|
| S. mansoni WW and LE | Miracidia from feces of infected patients were used to infect B. glabrata to obtain cercaria (strain WW), which were used to infect mice; infected mice were treated with antischistosomal drugs; sensitivities of strains WW and LE to drugs were compared | Hycanthone, 80 and 20 mg/kg; niridazole, 100 and 50 mg/kg/day for 5 days; oxamniquine, 100 and 50 mg/kg, 1 dose | Hycanthone altered oogram pattern of 100% of mice infected with strain LE; hycanthone did not affect oogram pattern of mice infected with strain WW; strain WW was more resistant to niridazole and oxamniquine | 88, 89 |
| S. mansoni Brazilian | Mice were infected with S. mansoni obtained from infected individual, they were treated with different antischistosomal drugs during the time of embryological development of genital organs of schistosomula of both sexes | Oxamniquine, 50 mg/kg, 1 dose; oltipraz, 60 mg/kg daily for 5 days; PZQ, 50 mg/kg for 5 days | All treated groups had larger percentages of worms in liver and portal vein and significantly lighter parasite loads than control group, high rates of worm reduction, low rates of surviving worms, and 100% had changed oogram pattern; failure to induce resistance in Brazilian strain | 90 |
| S. mansoni Egyptian | S. mansoni infected mice were treated with subcurative different doses of PZQ after 6 wk p.i.;a eggs produced by worms that survived to treatment were used to infect snails | PZQ, 3 × 300 mg/kg | S. mansoni subjected to drug pressure may develop resistance to schistosomicidal drugs after relatively few passages; first demonstration of resistance to PZQ | 91 |
| Miracidia obtained from eggs from infected patient were used to infected mice; eggs produced by worms that survive to treatment 6 wk p.i. were used to infect snails and mice of the following generations | PZQ, 300 or 500 mg/kg | Subcurative dose of PZQ led to development of resistance to therapeutic dose of PZQ in following generations | 92 | |
| S. mansoni LE | Infected B. glabrata snails were treated with PZQ; after treatment, cercariae obtained from these snails (LE-PZQ isolate) and susceptible LE strains were used to infect mice that were treated p.i. | B. glabrata, 3 × 100 mg/kg, 5 consecutive days; infected mice, 45 days p.i., 200, 400, 800 mg/kg | Experimental model of development of resistance to S. mansoni using infected snails; mean no. of worms recovered from group of mice infected with LE-PZQ isolate treated with 200 and 400 mg/kg was significantly higher than that from mice infected with LE strain with same treatment; in vitro, worms of LE-PZQ isolates were also less susceptible to PZQ | 93 |
| S. japonicum | Mice were infected with isolates from two distinct regions, PZQ-susceptible isolates and PZQ-induced isolates, and then treated with PZQ; cercariae and miracidia of different isolates were exposed to PZQ solution, and morphological alterations were observed | Infected mice, 35 days p.i., 0,37.5,75, 150, 300, and 600 mg/kg; cercariae and miracidia, 10−5, 5 × 10−6, 5 × 10−7, and 10− M | PZQ-resistant isolates of S. japonicum were established in mice with subcurative doses of PZQ by artificial selection in laboratory; drug resistance might be exhibited by different developmental stages (miracidia, cercaria, adult worms); established PZQ ED50s for different developmental stages | 94 |
p.i., postinfection.
PZQ RESISTANCE IN THE FIELD
Reports of field resistance or therapeutic failure of PZQ are listed in Table 5. Most field surveys of resistance to PZQ1 focus on S. mansoni. Reduced susceptibility to PZQ1 has been widely found in foci of endemicity, notably in Africa, including Egypt and Senegal. An extremely low cure rate (18%) was reported in Senegal (95); however, it was suggested that failure of PZQ1 therapy occurs because of factors other than drug resistance, including very intense transmission and the presence of PZQ-refractory juvenile worms (immature parasites) (96). In Egypt, eggs obtained from treated and uncured patients gave rise to schistosomes (S. mansoni) that showed 3- to 5-fold lower sensitivity to PZQ1 (97). In fact, in vitro measurements of PZQ1 susceptibility correlated well, in some cases, with the drug dose producing 50% of the maximal effect (ED50), as determined in murine infections, further indicating that factors in the worms themselves were responsible for the reduced susceptibility of these isolates to PZQ1 (98). Studies carried out 10 years later in the same area failed to show any hint of resistance to PZQ1 (99).
TABLE 5.
Key reports of field resistance or therapeutic failure of PZQ
| Species | Country | Yr | Sample size | Treatment(s) | Outcome measure | Reference |
|---|---|---|---|---|---|---|
| S. mansoni | Senegal | 1991 | 422 | 40 mg/kg | 12 wk after treatment, cure rate only 18%, antigen detection positive in 90% of individuals; low cure rates may be due to intense transmission and/or development immune responses | 95 |
| Egypt | 1994 | 1,607 | 1 dose of 40 mg/kg, 3 successive doses of 40, 40 and 60 mg/kg | 1–2.4% of villagers treated could not be completely cured of infection, and 3 of every 1,000 treated villagers may harbor parasites that can tolerate high doses of PZQ; extensive use of PZQ has not resulted in dramatic change in its efficacy | 97 | |
| 2005 | 1,405 | Compared with results obtained in 1994 in same villages, decreased prevalence and intensity of infections; first treatment resulted in normal cure rate (73–92%); after 3 successive doses, no uncured patients; drug failure did not increase over 10 years of therapeutic pressure in these villages | 99 | |||
| S. hematobium | Malawia | 1995 | 1 | ∼40 mg/kg (30% was spit up) | 3 doses of PZQ necessary to cure infection; concomitant Giardia lamblia infection might have caused malabsorption of drug; repeated courses of therapy may be necessary to cure infection, and both parasite and host factors should be considered if infection persists; 7 treatments necessary to eliminate eggs from parasites | 100 |
| Senegala | 2006 | 2 | 40 mg/kg | Repeated standard treatment failed to clear infection | 102 | |
| S. japonicum | Sichuan Province, China | 1985 | 185 | 2 × 40 mg/kg | Low no. of treatment failures (only 1 remained infected); good compliance with treatment; PZQ remains effective against this schistosome | 106 |
| 2010 | 584 | 6 wk after treatment, 95% had no detectable eggs, 3% were still excreting eggs, and 2nd dose of drug was given; no detectable eggs were observed 6 wk after 2nd dose; efficacy of PZQ still high; no evidence of resistance detected | 108 | |||
| 2012 | 43 | Single oral dose of 40 mg/kg, 30 mg/kg for 2 days | 6 wk after second treatment, eggs not found were found in infected villagers; no evidence of resistance | 110 |
Travelers from this area of endemicity.
As noted, there is no evidence of S. haematobium resistance to PZQ1. However, some studies have reported failures of treatment to cure infections with this species (100–102). For example, Alonso et al. (102) described the case of two Spanish travelers with urogenital schistosomiasis in whom repeated standard treatment (a single 40-mg/kg dose of PZQ1) failed to clear the infection. Sabah et al. (103) hypothesized that people coming from areas where schistosomiasis is not endemic may lack an immunological component that has been shown to contribute to the activity of PZQ1 in experimental animals. Emergence of resistance of S. japonicum to PZQ1 has also received attention (101–105). However, despite large-scale and repeated use, the current efficacy of PZQ1 remains unchanged and it is highly effective at a curative dosage (a single dose of 40 mg/kg) in the main areas of China where schistosomiasis is endemic (106–108). Seto et al. (106) conducted a cross-sectional survey, in which the efficacy of PZQ1 was evaluated in 33 villages in Sichuan Province, where the prevalence of infection was found to be 5.7%. Of 3,269 persons tested, 185 were infected. The infected persons were treated two times with a 40-mg/kg dose of PZQ1, and only one remained infected, findings that support the notion that PZQ1 remains effective for the treatment of infection with S. japonicum in China.
Insensitive measurement of infection burdens may lead to overestimation of PZQ1 efficacy and thereby confound attempts to discriminate between reduced PZQ1 susceptibility and drug resistance. Diagnostic techniques for schistosomiasis are time-consuming, and many epidemiological assessments rely on microscopic observation of viable eggs in urine (S. haematobium) and feces (S. mansoni, S. japonicum) (109, 110). However, fluctuation of egg output in urine or stool occurs routinely, negatively influencing the sensitivity of the assay in the absence of repeated sampling (111). New diagnostic techniques such as egg detection by PCR aim to improve sensitivity, but the sampling limitations persist (112, 113). Despite the development of new tools for diagnosis (reviewed in reference 114), there remains a need for better diagnostics, both in the field and in the clinic. In addition to the importance of improvements for clinical diagnosis, advances in diagnostic tools are also critical in programs targeting elimination by MDA and for the development and assessment of new drugs and vaccines (3).
MECHANISM OF PZQ RESISTANCE
In the absence of the exact mechanism of action of PZQ1, the mechanism of drug resistance in schistosomes also remains unclear (115). However, the likely nature of the mechanism of PZQ resistance has been described, such as induction of ATP-binding cassette (ABC) transporters (ABC transporters are proteins involved in the transport of toxins and xenobiotics). Several members of this family, like P-glycoprotein (Pgp) and multidrug resistance (MDR)-associated proteins (MRPs) represent two classes of these MDR transporters (116, 117). ABC transport protein homologues from S. mansoni are known, i.e., SmMRP1 (orthologue of MRP1) and SMDR2 (orthologue of Pgp) (106). Juvenile schistosomes express ∼2.5-fold higher basal levels of SMDR2 and SmMRP1 than adults, higher levels of SMRD2 RNA are seen in females than in males, and higher SmMRP1 levels are seen in males than in females (118). Furthermore, SMRD2 is modulated by PZQ1, suggesting that PZQ1 is also a substrate for SMRD2 (119). Transcriptomic analysis reveals increasing levels of transcripts encoding the ABC transporters SMDR1, SmMRP1, SmMRD2, and SMDR3 in juveniles exposed to PZQ1 in vitro, supporting the notion that ABC transporters participate in resistance to PZQ1 in schistosomes (75). Guglielmo et al. (120) developed a series of PZQ NO-donors furoxans that are worthy of investigation in view of their potential activity against PZQ-resistant schistosomes. Involvement of Ca2+ channel changes in resistance to PZQ has been widely described (121). Nonetheless, whether these phenomena are responsible for drug action or represent downstream consequences has not been established (83, 122).
CONCLUDING REMARKS
Because of its efficacy, safety, cost, and indeed the lack of alternatives, PZQ1 has remained the drug of choice for schistosomiasis treatment and transmission control for >40 years (15, 16). Yet PZQ1 has drawbacks, including inactivity against juvenile schistosomes. Moreover, reliance on a single drug for the treatment of a disease with the global public significance of schistosomiasis risks facilitating the development and spread of drug resistance, especially since reduced susceptibility has occurred frequently both in the field and in the laboratory. A pressing need for new interventions has arisen, including novel compounds with modes of action discrete from those of PZQ1 and methods to detect the appearance and spread of resistance to PZQ1 (123). Despite the novel structures of several derivatives of PZQ1, most are sufficiently efficacious to warrant closer investigation in clinical trials. In addition, understanding the mechanism of action of PZQ1 and its metabolism is critical since this information would facilitate the elucidation of novel targets and/or lead to improvements in the efficacy of this essential and singular medicine.
ACKNOWLEDGMENTS
We acknowledge support from the Fundação para a Ciência e Tecnologia (FCT, Portugal) and FEDER (European Union) through UID/MULTI/04378/2013 and project grant IF/00092/2014 and from award R01CA164719 from the National Cancer Institute (NCI), National Institutes of Health (NIH). We thank FCT for the IF2014 position (N.V.) and Pest-OE/AGR/UI0211/2011 and Strategic Project UI211 (J.M.C.C). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the FCT, the NCI, or the NIH.
REFERENCES
- 1.World Health Organization. 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee: technical report series 912, p 2–5. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/42588/1/WHO_TRS_912.pdf?ua=1. [PubMed] [Google Scholar]
- 2.Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffenq LE, Fèvre EM, Fürst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ, Naghavi M. 2014. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Secor WE. 2014. Water-based interventions for schistosomiasis control. Pathog Glob Health 108:246–254. doi: 10.1179/2047773214Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross AGP, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. 2002. Schistosomiasis. N Engl J Med 346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 6.Berry A, Fillaux J, Martin-Blondel G, Boissier J, Iriart X, Marchou B, Magnaval JF, Delobel P. 2016. Evidence for a permanent presence of schistosomiasis in Corsica, France 2015. Euro Surveill 21:pii=30100. doi: 10.2807/1560-7917.ES.2016.21.1.30100. [DOI] [PubMed] [Google Scholar]
- 7.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological Agents. 2012. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilby KJ, Gilchrist SE, Ensom MHH. 2013. A review of the pharmacokinetic implications of schistosomiasis. Clin Pharmacokinet 52:647–656. doi: 10.1007/s40262-013-0055-8. [DOI] [PubMed] [Google Scholar]
- 9.Gryseels B, Polman K, Clerinx J, Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 10.Papamatheakis DG, Mocumbi AOH, Kim NH, Mandel J. 2014. Schistosomiasis-associated pulmonary hypertension. Pulm Circ 4:596–611. doi: 10.1086/678507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gryseels B. 2012. Schistosomiasis. Infect Dis Clin North Am 26:383–397. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kjetland EF, Leutscher PD, Ndhlovu PD. 2012. A review of female genital schistosomiasis. Trends Parasitol 28:58–65. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Chofle AA, Jaka H, Koy M, Smart LR, Kabangila R, Ewings FM, Mazigo HD, Johnson WD Jr, Fitzgerald DW, Peck RN, Downs JA. 2014. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect Dis 14:303. doi: 10.1186/1471-2334-14-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brindley PJ, da Costa JM, Sripa B. 2015. Why does infection with some helminths cause cancer? Trends Cancer 1:174–182. doi: 10.1016/j.trecan.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews P. 1985. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol Ther 29:129–156. doi: 10.1016/0163-7258(85)90020-8. [DOI] [PubMed] [Google Scholar]
- 16.Caffrey CR. 2015. Schistosomiasis and its treatment. Future Med Chem 7:675–676. doi: 10.4155/fmc.15.27. [DOI] [PubMed] [Google Scholar]
- 17.Cioli D, Pica-Mattoccia L. 2003. Praziquantel. Parasitol Res 90(Suppl 1):S3–S9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 18.Frohberg H. 1984. Results of toxicological studies on praziquantel. Arzneimittelforschung 34:1137–1144. [PubMed] [Google Scholar]
- 19.Montero R, Ostrosky P. 1997. Genotoxicity activity of praziquantel. Mutat Res 387:123–139. doi: 10.1016/S1383-5742(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 20.Kramers PGN, Gentile JM, Gryseels BJM, Jordan P, Katz N, Mott KE, Mulvihill J, Seed JL, Frohberg H. 1991. Review of the genotoxicity and carcinogenicity of antischistosomal drugs: is there a case for a study of mutation epidemiology? Report of a task group on mutagenic antischistosomals. Mutat Res 257:49–89. [DOI] [PubMed] [Google Scholar]
- 21.Doenhoff MJ, Coles GC, Pica-Mattoccia L, Wheatcroft-Franclow K. 2009. Chemotherapy and drug resistance in schistosomiasis, fascioliasis and tapeworm infections, p 629–646. In Mayers D. (ed), Antimicrobial drug resistance: mechanism of drug resistance, vol 1 Springer, New York, NY. doi: 10.1007/978-1-59745-180-2_45. [DOI] [Google Scholar]
- 22.Polderman AM, Gryssels B, Gerold JL, Mpamila K, Manshande JP. 1984. Side effects of praziquantel in the treatment of Schistosoma mansoni in Maniema, Zaire. Trans R Soc Trop Med Hyg 78:752–754. doi: 10.1016/0035-9203(84)90007-5. [DOI] [PubMed] [Google Scholar]
- 23.Berhe N, Gundersen SG, Abebe F, Birrie H, Medhin G, Gemetchu T. 1999. Praziquantel side effects and efficacy related to Schistosoma mansoni egg loads and morbidity in primary school children in northeast Ethiopia. Acta Trop 72:53–63. doi: 10.1016/S0001-706X(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 2006. Preventive chemotherapy in human helminthiasis, coordinated use of antihelminthic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/43545/1/9241547103_eng.pdf. [Google Scholar]
- 25.World Health Organization. 2013. Sustaining the drive to overcome the global impact of neglected tropical diseases. Second WHO report on neglected tropical diseases. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/80245/1/WHO_HTM_NTD_2013.2_eng.pdf. [Google Scholar]
- 26.Lindenberg M, Kopp S, Dressman JB. 2004. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm 58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Benet LZ, Broccatelli F, Oprea TI. 2011. BDDCS applied to over 900 drugs. AAPS J 13:519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olliaro P, Delgado-Romero P, Keiser J. 2014. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemother 69:863–870. doi: 10.1093/jac/dkt491. [DOI] [PubMed] [Google Scholar]
- 29.Meyer T, Sekljic H, Fuchs S, Bothe H, Schollmeyer D, Miculka C. 2009. Taste a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Negl Trop Dis 3:e357. doi: 10.1371/journal.pntd.0000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woelfle M, Seerden JP, de Gooijer J, Pouwer K, Olliaro P, Todd MH. 2011. Resolution of praziquantel. PLoS Negl Trop Dis 5:e1260. doi: 10.1371/journal.pntd.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woelfle M, Olliaro P, Todd MH. 2011. Open science is a research accelerator. Nat Chem 3:745–748. doi: 10.1038/nchem.1149. [DOI] [PubMed] [Google Scholar]
- 32.Xiao S, Catto BA, Webster LT. 1985. Effects of praziquantel on different developmental stages of Schistosoma mansoni. J Infect Dis 151:1130–1137. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- 33.Pica-Mattoccia L, Cioli D. 2004. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol 34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Bühring KU, Diekmann HW, Müller H, Garbe A, Nowak H. 1978. Metabolism of praziquantel in man. Eur J Drug Metab Pharm 3:179–190. doi: 10.1007/BF03189504. [DOI] [Google Scholar]
- 35.Bohnert J, Gan LS. 2013. Plasma protein binding—from discovery to development. J Pharm Sci 102:2953–2994. doi: 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- 36.Undevia SD, Gomez-Abuin G, Ratain MJ. 2005. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer 5:447–458. doi: 10.1038/nrc1629. [DOI] [PubMed] [Google Scholar]
- 37.Castro N, Medina R, Sotelo J, Jung H. 2000. Bioavailability of praziquantel increases with concomitant administration of food. Antimicrob Agents Chemother 44:2903–2904. doi: 10.1128/AAC.44.10.2903-2904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson GR. 1997. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev 27:129–159. doi: 10.1016/S0169-409X(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 39.Murray M. 2006. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr Drug Metab 7:67–81. doi: 10.2174/138920006774832569. [DOI] [PubMed] [Google Scholar]
- 40.Mandour ME, el Turabi H, Homeida MH, el Sadig T, Ali HM, Bennet JL, Leahey WJ, Harrow DW. 1990. Pharmacokinetics of praziquantel in healthy volunteers and patients with schistosomiasis. Trans R Soc Trop Med Hyg 84:389–393. doi: 10.1016/0035-9203(90)90333-A. [DOI] [PubMed] [Google Scholar]
- 41.Andrews P, Thomas H, Pohlke R, Seubert J. 1983. Praziquantel. Med Res Rev 3:147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 42.Ali BH. 2006. A short review of some pharmacological, therapeutic and toxicological properties of praziquantel in man and animals. Pak J Pharm Sci 19:170–175. [PubMed] [Google Scholar]
- 43.Lacy CF, Armstrong LL, Goldman MP, Lance LL. 2011. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals, 20th ed Lexi-Comp, Inc., Hudson, OH. [Google Scholar]
- 44.Lerch C, Blaschke G. 1998. Investigation of stereoselective metabolism of praziquantel after incubation with rat liver microsomes by capillary electrophoresis and liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 708:267–275. doi: 10.1016/S0378-4347(97)00638-5. [DOI] [PubMed] [Google Scholar]
- 45.Melo AJB, Iamamoto Y, Maestrin APJ, Smith JRL, Santos MD, Lopes ND, Bonato PS. 2005. Biomimetic oxidation of praziquantel catalysed by metalloporphyrins. J Mol Catal A Chem 226:23–31. doi: 10.1016/j.molcata.2004.09.015. [DOI] [Google Scholar]
- 46.Meier H, Blaschkle G. 2001. Investigation of praziquantel metabolism in isolated rat hepatocytes. J Pharm Biomed Anal 26:409–415. doi: 10.1016/S0731-7085(01)00417-4. [DOI] [PubMed] [Google Scholar]
- 47.Lima RM, Ferreira MA, de Jesus Ponte Carvalho TM, Dumet Fernandes BJ, Takayanagui OM, Garcia HH, Coelho EB, Lanchote VL. 2011. Albendazole-praziquantel interaction in healthy volunteers: kinetics disposition, metabolism and enantioselectivity. Br J Clin Pharmacol 71:528–535. doi: 10.1111/j.1365-2125.2010.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meister I, Ingram-Sieber K, Cowan N, Todd M, Robertson MN, Meli C, Patra M, Gasser G, Keiser J. 2014. Activity of praziquantel enantiomers and main metabolites against Schistosoma mansoni. Antimicrob Agents Chemother 58:5466–5472. doi: 10.1128/AAC.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meister I, Leonidova A, Kovač J, Durthaler U, Keiser J, Huwyler J. 2016. Development and validation of an enatioselective LC-MS/MS method for the analysis of the antihelminthic drug praziquantel and its main metabolite in human plasma, blood and dried blood spots. J Pharm Biomed Anal 118:81–88. doi: 10.1016/j.jpba.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Bathena SP, Alnouti Y. 2010. Metabolite profiling of praziquantel and is analogs during the analysis of in vitro metabolic stability using information-dependent acquisition on a hybrid triple quadrupole linear ion trap mass spectrometer. Drug Metab Pharmacokinet 25:487–499. doi: 10.2133/dmpk.DMPK-10-RG-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masimirembwa CM, Hasler JA. 1994. Characterisation of praziquantel metabolism by rat liver microsomes using cytochrome P450 inhibitors. Biochem Pharmacol 48:1779–1783. [DOI] [PubMed] [Google Scholar]
- 52.Li XQ, Bjorkman A, Andersson TB, Gustafsson LL, Masimirembwa CM. 2003. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol 59:429–442. doi: 10.1007/s00228-003-0636-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Fang Z-Z, Zheng Y, Zhou K, Hu C, Krausz KW, Sun D, Idle JR, Gonzalez FJ. 2014. Metabolic profiling of praziquantel enantiomers. Biochem Pharmacol 90:166–178. doi: 10.1016/j.bcp.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong Y, Chollet J, Vargas M, Mansour NR, Bickle Q, Alnouti Y, Huang J, Keiser J, Vennerstrom JL. 2010. Praziquantel analogs with activity against juvenile Schistosoma mansoni. Bioorg Med Chem Lett 20:2481–2484. doi: 10.1016/j.bmcl.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Dong L, Hu C, Zhao B, Yang C, Xia C. 2014. Development of chiral praziquantel analogues as potential drug candidates with activity to juvenile Schistosoma japonicum. Bioorg Med Chem Lett 24:4223–4226. doi: 10.1016/j.bmcl.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 56.Gasser G, Ott I, Metzler-Nolte N. 2011. Organometallic anticancer compounds. J Med Chem 54:3–25. doi: 10.1021/jm100020w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patra M, Gasser G, Metzler-Nolte N. 2012. Small organometallic compound as antibacterial agents. Dalton Trans 41:6350–6358. doi: 10.1039/c2dt12460b. [DOI] [PubMed] [Google Scholar]
- 58.Biot C, Castro W, Botté CY, Navarro M. 2012. The therapeutic potential of metal-based antimalarial agents: implications for the mechanism of action. Dalton Trans 41:6335–6349. doi: 10.1039/c2dt12247b. [DOI] [PubMed] [Google Scholar]
- 59.Patra M, Ingram K, Pierroz V, Ferrari S, Spingler B, Keiser J, Gasser G. 2012. Ferrocenyl derivatives of the anthelmintic praziquantel: design, synthesis, and biological evaluation. J Med Chem 55:8790–8798. doi: 10.1021/jm301077m. [DOI] [PubMed] [Google Scholar]
- 60.Patra M, Ingram K, Pierroz V, Ferrari S, Spingler B, Gasser RB, Keiser J, Gasser G. 2013. [(η(6)-Praziquantel)Cr(CO)3] derivatives with remarkable in vitro anti-schistosomal activity. Chem Eur J 19:2232–2235. doi: 10.1002/chem.201204291. [DOI] [PubMed] [Google Scholar]
- 61.Patra M, Ingram K, Leonidova A, Pierroz V, Ferrari S, Robertson MN, Todd MH, Keiser J, Gasser G. 2013. In vitro metabolic profile and in vivo antischistosomal activity studies of (η(6)-praziquantel)Cr(CO)3 derivatives. J Med Chem 56:9192–9198. doi: 10.1021/jm401287m. [DOI] [PubMed] [Google Scholar]
- 62.Duan WW, Qiu SJ, Zhao Y, Sun H, Qiao C, Xia CM. 2012. Praziquantel derivatives exhibit activity against both juvenile and adult Schistosoma japonicum. Bioorg Med Chem Lett 22:1587–1590. doi: 10.1016/j.bmcl.2011.12.133. [DOI] [PubMed] [Google Scholar]
- 63.Dong L, Duan W, Chen J, Sun H., Qiao C, Xia C. 2014. An artemisinin derivative of praziquantel as an orally active antischistosomal agent. PLoS One 9:e112163. doi: 10.1371/journal.pone.0112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Boissier J, Chen J-L, Yao H, Yang S, Rognon A, Qiao C. 2015. Design, synthesis and biological evaluation of praziquantel and endoperoxide conjugates as antischistosomal agents. Future Med Chem 7:713–725. doi: 10.4155/fmc.15.20. [DOI] [PubMed] [Google Scholar]
- 65.Cioli D, Pica-Mattoccia L, Basso A, Guidi A. 2014. Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol 195:23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Cioli D, Pica-Mattoccia L, Archer S. 1995. Antischistosomal drugs: past, present … and future? Pharmacol Ther 68:35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 67.Pax R, Bennet JL, Fetterer R. 1978. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedebergs Arch Pharmacol 304:309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 68.Harnett W, Kussel JR. 1986. Increased exposure of parasite antigens at surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology 93:401–405. doi: 10.1017/S0031182000051568. [DOI] [PubMed] [Google Scholar]
- 69.Kohn AB, Anderson PAV, Roberts-Misterly JM, Greenberg RM. 2001. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem 276:36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 70.Pica-Mattoccia L, Valle C, Basso A, Troiani AR, Vigorosi F, Liberti P, Festucci A, Cioli D. 2007. Cytochalasin D abolishes the schistosomicidal activity of praziquantel. Exp Parasitol 115:344–351. doi: 10.1016/j.exppara.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, Marcatto-Maggi A, Nobre-Santana S, Troiani A, Cioli D, Valle C. 2008. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp Parasitol 119:332–335. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Nogi T, Zhang D, Chan JD, Marchant JS. 2009. A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel β subunits: subversion of flatworm regenerative polarity. PLoS Negl Trop Dis 3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan JD, Agbedanu PN, Zamanian M, Gruba SM, Haynes CL, Day TA, Marchant JS. 2014. ‘Death and axes’: unexpected Ca2+ entry phenologs predict new anti-schistosomal agents. PLoS Pathog 10:e1003942. doi: 10.1371/journal.ppat.1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aragon AD, Imani RA, Blcakburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C. 2009. Towards an understanding of the mechanism of action of praziquantel. Mol Biochem Parasitol 164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hines-Kay J, Cupit PM, Sanchez MC, Rosenberg GH, Hanelt B, Cunningham C. 2012. Transcriptional analysis of Schistosoma mansoni treated with praziquantel in vitro. Mol Biochem Parasitol 186:87–94. doi: 10.1016/j.molbiopara.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.You H, McManus P, Hu W, Smout MJ, Brindley PJ, Gobert GN. 2013. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signaling pathway member CamKII. PLoS Pathog 9:e1003254. doi: 10.1371/journal.ppat.1003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rinaldi G, Loukas A, Brindley PJ, Irelan JT, Smout MJ. 2015. Viability of developmental stages of Schistosoma mansoni quantified with xCELLigence worm real-time motility assay (xWORM). Int J Parasitol Drugs Drug Resist 5:141–148. doi: 10.1016/j.ijpddr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schepers H, Brasseur R, Goormaghtigh E, Duquenoy P, Ruysschaert JM. 1988. Mode of insertion of praziquantel and derivatives into lipid membranes. Biochem Pharmacol 37:1615–1623. doi: 10.1016/0006-2952(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira MS, Oliveira RN, Oliveira DN, Esteves CZ, Allegretti SM, Catharino RR. 2015. Revealing praziquantel molecular targets using mass spectrometry imaging: an expeditious approach applied to Schistosoma mansoni. Int J Parasitol 45:385–391. doi: 10.1016/j.ijpara.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Hu W, Brindley PJ, McManus DP, Feng Z, Hang ZG. 2004. Schistosome transcriptomes: new insights into the parasite and schistosomiasis. Trends Mol Med 10:217–225. doi: 10.1016/j.molmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Fallon PG. 1998. Schistosome resistance to praziquantel. Drug Resist Updat 1:236–241. doi: 10.1016/S1368-7646(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 82.van Wyk JA. 2001. Refugia—overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J Vet Res 68:55–57. [PubMed] [Google Scholar]
- 83.Wang W, Wang L, Liang Y. 2012. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res 111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- 84.Cioli D. 2000. Praziquantel: is there real resistance and are there alternatives? Curr Opin Infect Dis 13:659–663. doi: 10.1097/00001432-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 85.Doenhoff MJ, Cioli D, Utzinger J. 2008. Praziquantel: mechanism of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 86.Greenberg RM. 2013. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology 140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. 2002. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg 96:465–469. doi: 10.1016/S0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 88.Katz N, Dias EP, Araújo N, Souza CP. 1973. Estudo de uma cepa humana de Schistosoma mansoni resistente a agente esquistosomicidas. Rev Soc Bras Med Trop 7:382–387. doi: 10.1590/S0037-86821973000600008. [DOI] [Google Scholar]
- 89.Campos R, Moreira AA, Sette H Jr, Chamone DA, Da Silva LC. 1976. Hycanthone resistance in a human strain of Schistosoma mansoni. Trans R Soc Trop Med Hyg 70:261–262. [DOI] [PubMed] [Google Scholar]
- 90.Dias LCS, Olivier CE. 1986. Failure at inducing resistance to schistosomicidal drugs in a Brazilian human strain of Schistosoma mansoni. Rev Inst Med Trop São Paulo 28:352–357. doi: 10.1590/S0036-46651986000500010. [DOI] [PubMed] [Google Scholar]
- 91.Fallon PG, Doenhoff MJ. 1994. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg 51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 92.Ismail MM, Taha SA, Farghaly AM, el-Azony AS. 1994. Laboratory induced resistance to praziquantel in experimental schistosomiasis. J Egypt Soc Parasitol 24:685–695. [PubMed] [Google Scholar]
- 93.Couto FB, Coelho PMZ, Araújo N, Kusel JR, Katz N, Jannoti-Passos LK, Mattos ACA. 2011. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem Inst Oswaldo Cruz 106:153–157. doi: 10.1590/S0074-02762011000200006. [DOI] [PubMed] [Google Scholar]
- 94.Li HJ, Liang YJ, Dai JR, Wang W, Qu GL, Li YZ, Xing YT, Tao YH, Qian K, Jia Y, Yang KZ, Wei JY. 2011. Studies on resistance of Schistosoma to praziquantel XIV. Experimental comparison of susceptibility to praziquantel between praziquantel-resistant isolates and praziquantel-susceptible isolates of Schistosoma japonicum in stages of adult worms, miracidia and cercariae. Chin J Schistosomiasis Control 23:611–619. [PubMed] [Google Scholar]
- 95.Stelma FF, Talla I, Sow S, Kongs A, Niang M, Polman K, Deelder AM, Gryseels B. 1995. Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am J Trop Med Hyg 53:167–170. [DOI] [PubMed] [Google Scholar]
- 96.Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guissé F, Van Lieshout L, Faye D, Diop M, Ly A, Tchuem-Tcheunté LA, Engles D, Polman K. 2001. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop Med Int Health 6:864–873. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- 97.Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennet JL. 1996. Characterization of isolates of Schistosoma mansoni from Egyptian villages that tolerate high doses of praziquantel. Am J Trop Med Hyg 55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 98.William S, Botros S. 2004. Validation of sensitivity to praziquantel using Schistosoma mansoni worm muscle tension and Ca2+-uptake as possible in vitro correlates to in vivo ED50 determination. Int J Parasitol 34:971–977. doi: 10.1016/j.ijpara.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Botros S, Sayed H, Amer N, El-Ghannam M, Bennet JL, Day TA. 2005. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int J Parasitol 35:787–791. doi: 10.1016/j.ijpara.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Herwaldt BL, Tao LF, van Pelt W, Tsang VC, Bruce JI. 1995. Persistence of Schistosoma haematobium infection despite multiple courses of therapy with praziquantel. Clin Infect Dis 20:309–315. [DOI] [PubMed] [Google Scholar]
- 101.Silva IM, Pereira Filho E, Thiengo R, Ribeiro PC, Conceição MJ, Panasco M, Lenzi HL. 2008. Schistosomiasis haematobia: histopathological course determined by cystoscopy in a patient in whom praziquantel treatment failed. Rev Inst Med Trop Sao Paulo 50:343–346. doi: 10.1590/S0036-46652008000600006. [DOI] [PubMed] [Google Scholar]
- 102.Alonso D, Muñoz J, Gascón J, Vallo ME, Corachan M. 2006. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hyg 74:342–344. [PubMed] [Google Scholar]
- 103.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. 1985. Schistosoma mansoni: reduced efficacy of chemotherapy in infected T-cell-deprived mice. Exp Parasitol 60:348–354. doi: 10.1016/0014-4894(85)90041-4. [DOI] [PubMed] [Google Scholar]
- 104.Wang W, Liang YS. 2007. Progress on research of resistance of schistosome to praziquantel. Int J Med Parasit Dis 34:291–296, 300. [Google Scholar]
- 105.Wu W, Wang W, Huang YX. 2011. New insight into praziquantel against various developmental stages of schistosomes. Parasitol Res 109:1501–1507. doi: 10.1007/s00436-011-2670-3. [DOI] [PubMed] [Google Scholar]
- 106.Seto EYW, Wong BK, Lu D, Zhong B. 2011. Human schistosomiasis resistance to praziquantel in China: should we be worried? Am J Trop Med Hyg 85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang W, Dai JR, Li HJ, Shen XH, Liang YS. 2010. Is there reduced susceptibility to praziquantel in Schistosoma japonicum? Evidence from China. Parasitology 137:1905–1912. doi: 10.1017/S0031182010001204. [DOI] [PubMed] [Google Scholar]
- 108.Wang W, Dai JR, Li HJ, Shen XH, Liang YS. 2012. The sensitivity of Schistosoma japonicum to praziquantel: a field evaluation in areas with low endemicity of China. Am J Trop Med Hyg 86:834–836. doi: 10.4269/ajtmh.2012.11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lengeler C, Utzinger J, Tanner M. 2002. Questionnaires for rapid screening of schistosomiasis in sub-Saharan Africa. Bull World Health Organ 80:235–242. [PMC free article] [PubMed] [Google Scholar]
- 110.De Vlas SJ, Engels D, Rabello AL, Oostburg BF, Van Lieshout L, Polderman AM, Van Oortmarssen GJ, Habbema JD, Gryseels B. 1997. Validation of a chart to estimate true Schistosoma mansoni prevalence from simple egg counts. Parasitology 114:113–121. doi: 10.1017/S0031182096008207. [DOI] [PubMed] [Google Scholar]
- 111.ten Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L, van Lieshout L. 2008. Multiplex real-time PCR for detection and quantification of Schistosoma mansoni and S. haematobium infection in stool samples collected in northern Senegal. Trans R Soc Trop Med Hyg 102:179–185. doi: 10.1016/j.trstmh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Fung MS, Xiao N, Wang S, Carlton EJ. 2012. Field evaluation of a PCR test for Schistosoma japonicum egg detection in a low-prevalence region in China. Am J Trop Med Hyg 87:1053–1058. doi: 10.4269/ajtmh.2012.12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lier T, Simonsen GS, Wang T, Lu D, Haukland HH, Vennervald BJ, Hegstad J, Johansen MV. 2009. Real-time polymerase chain reaction for detection of low-intensity Schistosoma japonicum infections in China. Am J Trop Med Hyg 81:428–432. doi: 10.4269/ajtmh.2009.81.428. [DOI] [PubMed] [Google Scholar]
- 114.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. 2015. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 21:529–542. doi: 10.1016/j.cmi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 115.Cupit PM, Cunningham C. 2015. What is the mechanism of action of praziquantel and how might resistance strike? Future Med Chem 7:701–705. doi: 10.4155/fmc.15.11. [DOI] [PubMed] [Google Scholar]
- 116.Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. 2008. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol 24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 117.Bosch IB, Wang ZX, Tao LF, Shoemaker CB. 1994. Two Schistosoma mansoni cDNAs encoding ATP-binding cassette (ABC) family proteins. Mol Biochem Parasitol 65:351–256. doi: 10.1016/0166-6851(94)90085-X. [DOI] [PubMed] [Google Scholar]
- 118.Kasinathan RS, Morgan WM, Greenberg RM. 2010. Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRD1) in juvenile worms and in response to praziquantel. Mol Biochem Parasitol 173:25–31. doi: 10.1016/j.molbiopara.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasinathan RS, Goronga T, Messerli SM, Webb TR, Greenberg RM. 2010. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB J 24:128–135. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guglielmo S, Cortese D, Vottero F, Rolando B, Kommer VP, Williams DL, Fruttero R, Gasco A. 2014. New praziquantel derivatives containing NO-donor furoxans and related furazans as active agents against Schistosoma mansoni. Eur J Med Chem 84:135–145. doi: 10.1016/j.ejmech.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kohn AB, Roberts-Misterly JM, Anderson PAV, Khan N, Greenberg RM. 2003. Specific sites in the beta interaction domain of a schistosome Ca2+ channel β subunit are key to its role in sensitivity to the antischistosomal drug praziquantel. Parasitology 127:349–356. doi: 10.1017/S003118200300386X. [DOI] [PubMed] [Google Scholar]
- 122.Valle C, Troiani AR, Festucci A, Pica-Mattoccia L, Liberti P, Wolstenholme A, Francklow K, Doenhoff MJ, Cioli D. 2003. Sequence and level of endogenous expression of calcium channel β subunits in Schistosoma mansoni displaying different susceptibilities to praziquantel. Mol Biochem Parasitol 130:111–115. doi: 10.1016/S0166-6851(03)00171-3. [DOI] [PubMed] [Google Scholar]
- 123.Brindley PJ, Hotez PJ. 2013. Break out: urogenital schistosomiasis and Schistosoma haematobium infection in the post-genomic era. PLoS Negl Trop Dis 7:e1961. doi: 10.1371/journal.pntd.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]