Abstract
Changes in the mesolimbic dopamine (DA) system are implicated in a range of neuropsychiatric conditions including addiction, depression and schizophrenia. Dysfunction of the neuroimmune system is often comorbid with such conditions and affects similar areas of the brain. The goal of this study was to use positron emission tomography with the dopamine D2 antagonist tracer, 11C-raclopride, to explore the effect of acute immune activation on striatal DA levels. DA transmission was modulated by an oral methylphenidate (MP) challenge in order to reliably elicit DA elevation. Elevation in DA concentration due to MP was estimated via change in 11C-raclopride binding potential from the baseline scan. Prior to the post-MP scan, subjects were pre-treated with either the immune activator lipopolysaccharide (LPS) or placebo (PBO) in a cross-over design. Immune activation was confirmed by measuring tumor necrosis factor alpha (TNFα), interleukin (IL)-6 and IL-8 concentration in plasma. Eight healthy subjects were scanned four times each to determine the MP-induced DA elevation under both LPS and PBO pre-treatment conditions. MP-induced DA elevation in the striatum was significantly greater (P<0.01) after LPS pre-treatment compared to PBO pre-treatment. Seven of eight subjects responded similarly. This effect was observed in the caudate and putamen (P<0.02), but was not present in ventral striatum. DA elevation induced by MP was significantly greater when subjects were pre-treated with LPS compared to PBO. The amplification of stimulant-induced DA signaling in the presence of systemic inflammation may have important implications for our understanding of addiction and other diseases of DA dysfunction.
Introduction
Interaction between the immune and nervous systems in health and disease is an area of growing research focus. The immune system plays a key role in classic neuroimmune diseases such as multiple sclerosis;1 however, genetic, imaging, postmortem and animal data also suggest that immune–brain interactions influence the pathophysiology of a broad range of neurological and psychiatric diseases, including neurodegenerative diseases,2 epilepsy,3 addiction,4, 5 depression,6, 7 schizophrenia8, 9 and autism.10 The exact role of immune–brain interactions in these diseases is not fully understood; however, it is well established that experimental manipulation of systemic inflammation produces effects on the human brain. Functional magnetic resonance imaging (fMRI) studies involving induced neuroinflammation have demonstrated increased neural activity in areas implicated in social pain11 and a blunted behavioral response to reward.12, 13 Using the same methods to induce neuroinflammation, Hannestad et al.14 observed an increase in glucose metabolism in the insula and a decrease of glucose metabolism in the cingulate using 18F-flurodeoxyglucose (FDG) positron emission tomography (PET). Systemic inflammation is known to produce behavioral effects such as change in sleep and appetite, fatigue, anhedonia and depression that are consistent with the roles of these brain regions.12, 15, 16 Elevated plasma concentration of inflammatory mediators is associated with these symptoms as well.17, 18, 19 Depressed patients with higher levels of inflammatory markers have been found to have less functional connectivity in reward areas such as the ventral striatum and ventromedial prefrontal cortex.20 It has also been shown that systemic inflammation can cause widespread activation of microglia in the human brain.21 The regions affected by experimental systemic inflammation in imaging studies overlap substantially with regions that are involved in neurologic (basal ganglia, insula and temporal cortex) or psychiatric disorders (cingulate, medial frontal cortex and superior frontal cortex),22 and regions that show reduced gray matter in psychiatric diseases (anterior insula/dorsal cingulate network).23
Immune activation can be reliably elicited with lipopolysaccharide (LPS), an agonist of the toll-like receptor 4 (TLR4). LPS-induced activation of TLR4 causes systemic inflammation,14 depression-like behavior,15, 16 activation of microglia21 and changes in neuronal activity.12 In humans, systemic inflammation has an effect on the response of areas involved in reward (ventral striatum and substantia nigra).12, 13 Although the effects of systemic inflammation on behavior, symptoms and gross measures of brain function (for example, fMRI and FDG PET) have been characterized, much less is known about the molecular events that mediate these effects. One potential mediator of the effects of systemic inflammation on behavior is dopamine (DA). fMRI and 18F-FDG do not have the specificity to definitively determine DA involvement.
In rodents, acute systemic immune activation with LPS produced increases in DA.24 Similarly, in nonhuman primates, interferon-alpha (IFNα) administration caused an increase in stimulated DA release after 2 weeks, but a reversal of this effect was found after 4 weeks.25 Levodopa, a DA precursor, was found to restore deficits in DA elevation caused by chronic inflammation.26 These data suggest that acute or short-term increases in systemic inflammation lead to an increase in DA, while chronic inflammation has the opposite effect.
PET with 11C-raclopride, a DA D2 receptor antagonist, has been used reliably to detect elevation of striatal DA after administration of methylphenidate (MP), a DA reuptake inhibitor.27, 28, 29, 30, 31 Similarly, 11C-raclopride has also been used to characterize the mesolimbic DA system in disorders where DA dysfunction is implicated: smoking dependence,32 addiction,33 depression,34 schizophrenia35 and attention-deficit/hyperactivity disorder.36 In the present study, we examined the effects of LPS administration on MP-induced DA elevation. To our knowledge, this is the first study in humans to measure changes in DA after experimental activation of the immune system.
Materials and methods
Design overview
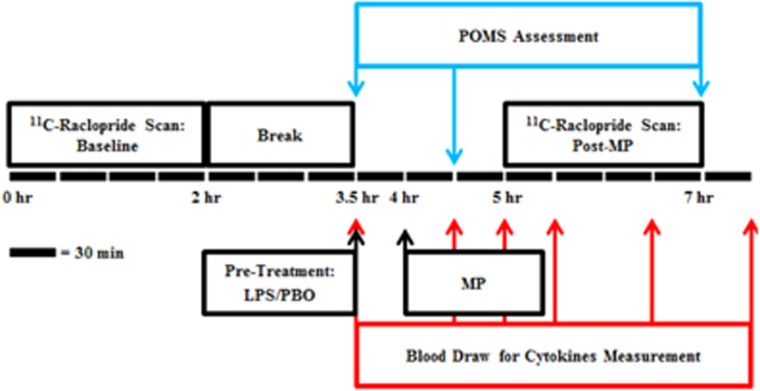
This study was approved by Yale University's Human Investigation Committee, Radioactive Drug Research Committee, and Radiation Safety Committee. Eight healthy subjects (four women and four men, mean age 31.3±11.2 years) without significant medical issues, current or recent smoking or other nicotine use, and personal or family history of psychiatric disorders (including substance misuse) were recruited for this study. Eligibility was confirmed by a detailed clinical interview, medical history, a physical exam, screening labs, urine cotinine and various assessments, including the Beck Anxiety Inventory, Hamilton Depression Rating Scale and Cognitive Emotion Regulation Questionnaire. Informed consent was obtained prior to the performance of any study procedures. Each subject underwent a structural MRI scan on a Trio 3T MR scanner (Siemen's/CTI, Knoxville, TN, USA). Subjects then participated in two PET scan sessions on two separate days. Each scan session consisted of a pair of PET scans: a baseline scan and a post-MP scan. Sessions began with a baseline 120 min 11C-raclopride scan. Subjects then rested for ~90–120 min. Following the break, a pre-treatment of either LPS (0.8 ng kg−1) or saline placebo (PBO) was administered intravenously. After 30 min, subjects received oral MP (40 mg). The post-MP 11C-raclopride scan was then performed 60 min after MP administration (90 min after LPS/PBO pre-treatment) for 120 min (Figure 1). Subjects returned at least 1 week later for a second scan session that followed the same sequence (baseline scan, break, pre-treatment, MP administration, post-MP scan) but with the opposite pre-treatment condition (LPS/PBO). The order of pre-treatment was randomized and both subjects and experimenters were blinded to the condition until after data processing was completed. The dose of LPS was selected based on previous studies that produced changes in brain activity12, 14 and induced fatigue.15, 16 The dose of MP was determined using previous studies that reported a robust change in raclopride signal.29, 30
Figure 1.
Schematic depiction of a session containing two 11C-raclopride PET scans: baseline and post MP. Each subject underwent two of these sessions with different pre-treatments: LPS or placebo. The order of the sessions was randomized. Blood sampling and POMS questions were performed periodically (denoted by arrows) from the start of pre-treatment until the end of the post-MP scan. LPS, lipopolysaccharide; MP, methylphenidate; PET, positron emission tomography; PBO, placebo; POMS, Profile of Mood States.
Two additional subjects participated in a single scan session with only LPS pre-treatment without MP to assess the effect of LPS alone on DA levels. This session consisted of a baseline scan, break, pre-treatment with LPS and a post-LPS scan.
PET scans
Subjects were permitted a light breakfast (without caffeine) prior to scanning. An intravenous catheter was placed for 11C-raclopride injection and periodic blood sampling. Blood pressure, heart rate and temperature were monitored by medical staff to ensure subject well-being. 11C-raclopride was synthesized using methods previously described in Langer et al.37 The radiotracer was injected intravenously as a bolus containing a mean activity of 17.4±3.4 mCi and a mean mass of 0.92±0.35 μg. Neither injected activity nor mass was significantly different between baseline, MP+PBO, and MP+LPS scans (P=0.83 and P=0.68, respectively) as characterized by a single factor analysis of variance. Subjects were placed on the scanner bed with their head centered at the field of view; a 9 min transmission scan (68Ge rods, 511 keV source) was obtained for attenuation correction. PET scans were acquired for 120 min on an ECAT HR+ PET scanner (Siemen's/CTI) that has a spatial resolution of 4.3–8.3 mm FWHM.38
Blood and behavior analyses
Samples of venous blood (10 ml) were drawn at 30, 90 and 150 min post-MP administration to assess MP concentration. Additional 10 ml venous blood samples were drawn at 0, 60, 90, 120, 180 and 240 min post LPS injection (Figure 1) to measure concentrations of the cytokines: tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6) and IL-8. Blood samples were centrifuged for 10 min and the plasma stored at −80 °C. Plasma samples were assayed for MP (Nathan Kline Institute, Orangeburg, NY, USA) and cytokines (Yale Cancer Center Immune Monitoring Lab, New Haven, CT, USA).
The Profile of Mood States questionnaire (POMS39) was administered to all subjects at 0, 60 and 210 min after pre-treatment with LPS or PBO. POMS is a Likert scale containing various mood states that are each rated from 0 to 4. For this analysis, we examined the ‘fatigued' mood state because, based on previous studies by our group, this symptom was reliably induced by LPS alone.15, 21
Image analysis
PET emission data were collected in 3D. Two-dimensional sinograms were created with Fourier rebinning. Data were binned into time frames of 6 × 0.5, 3 × 1, 2 × 2 and 22 × 5 min. Data were corrected for attenuation, scatter, dead time, detector sensitivity and randoms. Images were reconstructed with ordered subset expectation maximization using 4 iterations and 16 subsets (in-house software) at a voxel size of 2.1 mm × 2.1 mm × 2.4 mm and image volume of 128 × 128 × 63 voxels. PET images were co-registered to the subject's respective MR scan (FMRIB's Software Library40) and then non-linearly registered to a MR template in a common space (Bioimage Suite41, 42). ROIs were extracted using automated anatomic labeling43 (AAL) in order to generate regional time activity curves. The whole striatum, caudate, and putamen ROIs were extracted using the AAL atlas. The ventral striatum ROI was based on Martinez et al.44 Time activity curves were fitted with the simplified reference tissue model45 using the cerebellum as a reference region in order to calculate non-displaceable binding potential (BPND; abbreviated here as BP). Test–retest reliability of BP was assessed by comparing BP values for the caudate, putamen and ventral striatum from each of the two baseline scans for each subject using interclass correlation and a Wilcoxon signed-rank test. Change in BP was calculated between baseline and post MP for both LPS and PBO pre-treatment conditions:
ΔBP was compared between conditions for each ROI using the Student's paired t-test (two-tailed) to produce P-values (P<0.05 considered significant, uncorrected). BP was also calculated at the voxel level for each subject and used to construct mean, voxel-wise ΔBP images for each condition. T-tests were applied voxel by voxel to detect differences in ΔBP by condition. In order to examine potential relationships between MP concentration, cytokine concentration, ΔBP and change in POMS scores, the Pearson correlation coefficients (and associated P-values) were used.
Results
Blood and behavior analysis
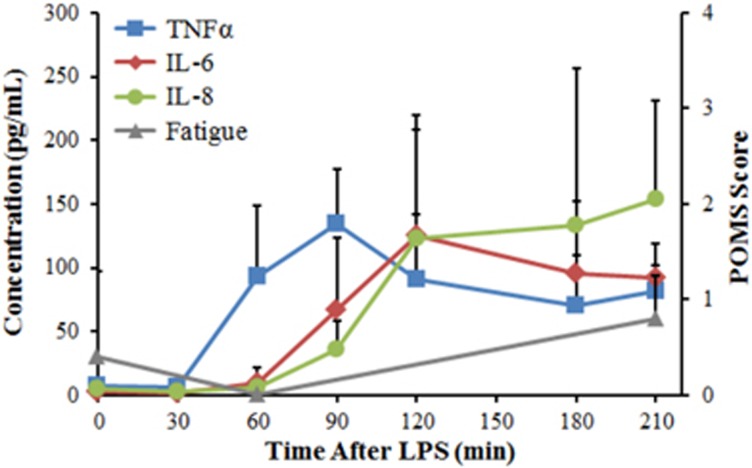
The mean concentration of MP in plasma during the scan was not different between the MP+LPS (12.7±7.4 ng ml−1) and MP+PBO (11.7±5.2 ng ml−1) conditions (P>0.6). MP concentration did not correlate with ΔBP in either the MP+PBO (r=0.5; P>0.2) or MP+LPS condition (r=0.4; P>0.3). Mean cytokine concentrations for TNFα, IL-6, and IL-8 (Figure 2) were consistent with previous LPS studies by our group15, 16 and others.12 The measured concentrations of cytokines were not correlated with ΔBPMP+LPS (r<0.5; P>0.2). POMS fatigue score did not change significantly from baseline in MP+LPS or MP+PBO conditions at 60 and 210 min post LPS or PBO administration (P>0.2). Change from baseline fatigue scores taken at the 60 or 210 min time points did not correlate with ΔBP in either the MP+PBO or MP+LPS condition (r<0.5; P>0.2). Concentration of MP did not correlate with cytokine concentrations or POMS scores (r<0.4; P>0.3).
Figure 2.
Mean cytokine concentration of TNFα (▪), IL-6 (♦) and IL-8 (•) in MP+LPS scans (n=8). The corresponding mean POMS score of fatigue is plotted on a secondary axis (▴). Error bars are shown as the standard deviation between subjects. IL, interleukin; LPS, lipopolysaccharide; MP, methylphenidate; POMS, Profile of Mood States; TNFα, tumor necrosis factor alpha.
Image analysis
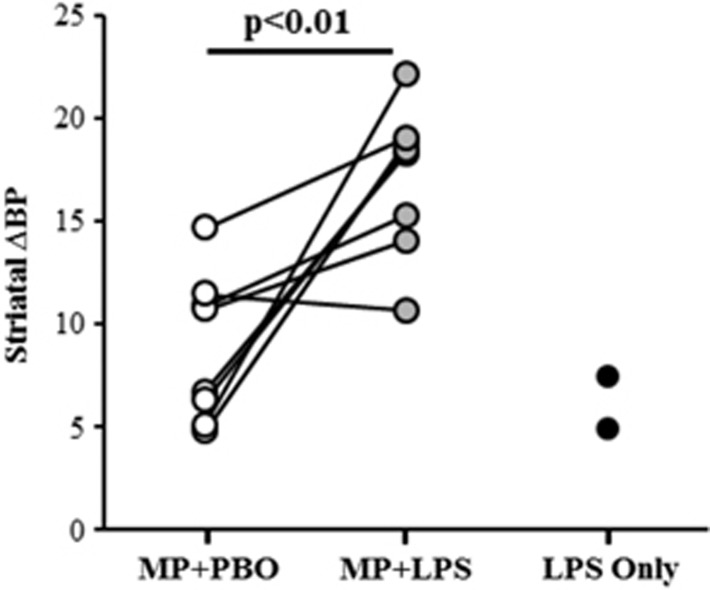
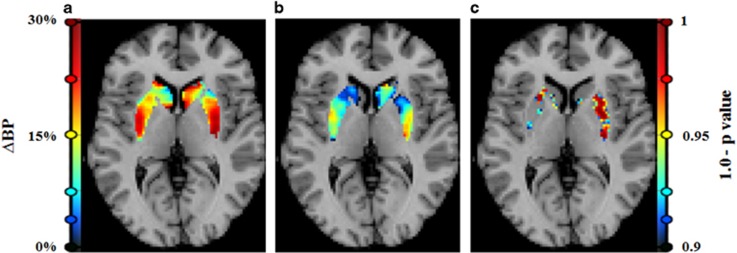
Baseline BP was not different between pre-treatment conditions in the striatum (P=0.82) or striatal sub-regions (ICC=0.95, Wilcoxon P=1.0). Intra-subject percent variability in baseline BP was 5.2%. Whole striatum ΔBP was greater in the MP+LPS condition (ΔBPMP+LPS=17.1±3.6) compared to the MP+PBO condition (ΔBPMP+PBO=8.8±3.6). This finding was consistent: seven of eight subjects exhibited greater ΔBPMP+LPS compared to ΔBPMP+PBO in whole striatum (P=0.007), shown in Figure 3. Striatal results were robust enough to survive Bonferroni correction for 6 independent measurements (left/right caudate, putamen, and ventral striatum; P<0.05/6). Analysis by sub-region showed that mean ΔBPMP+LPS was greater than mean ΔBPMP+PBO in both the caudate (P<0.02) and the putamen (P<0.02), but not in the ventral striatum (P>0.8; see sub-region results in Table 1). Voxel-wise ΔBP images were consistent with ROI-level findings: significant differences were only found in voxels for which ΔBPMP+LPS>ΔBPMP+PBO (Figure 4). The percentage of voxels found to be significantly different between pre-treatment conditions was 34.3% in putamen, 23.5% in caudate and 5.5% in ventral striatum.
Figure 3.
ΔBP values in striatum from each subject in each condition. Seven of eight subjects exhibited greater ΔBP after MP+LPS administration (gray) versus MP+PBO (white; P<0.01). Line segments were added to indicate the same subject in different conditions. Preliminary results (n=2) of ΔBP after LPS alone are plotted in black in the right-most column. These subjects did not receive any other scan condition. BP, binding potential; LPS, lipopolysaccharide; MP, methylphenidate; PBO, placebo.
Table 1. Mean (standard deviation) change in binding potential from baseline in MP+LPS, MP+PBO, LPS pre-treatment conditions.
| ROI | Volume (mL) |
Mean ΔBP (n=8) |
P-value | Mean ΔBP (n=2) | |
|---|---|---|---|---|---|
| MP+LPS | MP+PBO | LPS | |||
| Striatum | 42.6 | 17.1 (3.6) | 8.8 (3.6) | 0.007 | 6.1 (1.8) |
| Putamen | 21.9 | 17.9 (5.0) | 9.5 (4.5) | 0.013 | 4.5 (3.2) |
| Left | 10.7 | 18.0 (5.4) | 9.7 (7.5) | 0.071 | 4.7 (0.1) |
| Right | 11.3 | 17.7 (5.6) | 9.1 (4.9) | 0.001 | 4.6 (5.6) |
| Caudate | 20.7 | 16.1 (5.4) | 7.7 (3.7) | 0.020 | 8.6 (0.2) |
| Left | 10.2 | 13.9 (7.8) | 4.8 (9.2) | 0.099 | 9.6 (3.3) |
| Right | 10.5 | 18.5 (5.3) | 11.0 (11.6) | 0.058 | 7.3 (3.7) |
| Ventral striatum | 2.9 | 12.7 (8.1) | 11.4 (9.3) | 0.809 | 1.7 (5.4) |
| Left | 1.4 | 16.6 (7.1) | 11.5 (11.4) | 0.409 | 3.9 (7.5) |
| Right | 1.5 | 7.3 (13.8) | 10.8 (13.5) | 0.574 | −1.3 (2.9) |
Abbreviations: ΔBP, change in binding potential; LPS, lipopolysaccharide; MP, methylphenidate; PBO, placebo.
Figure 4.
Images created from SRTM modeling at the voxel level. Analyses were performed on images aligned to a common space and averaged: (a) mean ΔBPMP+LPS image, (b) mean ΔBPMP+PBO image and (c) significance image created by paired t-testing ΔBP between drug conditions at each voxel. Voxel intensity is calculated as (1.0−P-value). Voxel values are only displayed where (1.0−P-value)>0.90. In this two-tailed t-test, no voxels were found to be significant for ΔBPMP+LPS<ΔBPMP+PBO. The sparsity of significant differences on the left side of the brain is likely due to greater observed variability in the MP+PBO condition in left ROIs (see Table 1 for ROI level values). BP, binding potential; LPS, lipopolysaccharide; MP, methylphenidate; PBO, placebo; SRTM, simplified reference tissue model.
In the two subjects who did not receive MP, the mean ΔBPLPS=6.1±1.8 in whole striatum (Figure 3 and Table 1). Cytokine levels for these two control subjects were consistent with the eight subjects who received the MP+LPS condition. POMS fatigue scores in the LPS alone condition increased by 1 in one subject and by 2 in the other subject.
Discussion
In the striatum, BP at baseline was significantly greater than BP post MP in both the MP+PBO condition (P<0.0005) and in MP+LPS condition (P<0.0005; Supplementary Figure 1). ΔBP due to MP+PBO was comparable to that found in other studies using similar MP doses and routes of administration.29, 30 The effects we observed (either due to MP+PBO or MP+LPS) were consistently greater than the calculated test–retest variability in baseline BP in our data (5.2%) as well as in other studies.27, 31
Post-MP scans were initiated 90 min after LPS administration and image acquisition continued for 120 min, a time period during which the peak effects of LPS on blood cytokine levels,12, 15, 16 neuronal function (glucose metabolism in 18F-FDG PET14 or BOLD signal in fMRI11, 46) and behavioral effects (fatigue and other sickness symptoms) occur.12, 15, 16, 21, 47 Plasma analyses confirmed that cytokines were elevated as anticipated post LPS administration in a manner consistent with prior studies (Figure 2).12, 15, 16
Four subjects underwent the LPS pre-treatment session before the PBO pre-treatment session and four subjects received pre-treatment sessions in the opposite order. No significant difference in ΔBPMP+LPS was observed between subjects whether LPS was given first or PBO was given first. The same result was found in ΔBPMP+PBO.
In previous studies, LPS administration produced a clear effect on fatigue;15, 16 however, in this study we did not observe fatigue when LPS was co-administered with MP. We speculate that MP partially counteracts LPS-induced behavioral effects through a central (brain) mechanism, rather than a peripheral mechanism, as MP had no effect on circulating levels of cytokines (r<0.4; P>0.3). This decoupling of LPS-induced behavioral effects (fatigue and other sickness symptoms) and immune effects (blood cytokine levels) in the presence of MP is a novel result which indicates that blood cytokine concentrations may not be reliable indicators of the effects of systemic inflammation on brain-related symptoms. The potential effect of elevated cytokines in blood on the brain will depend on the susceptibility of the brain to these influences. In this study, it appears that MP had an effect on the brain that prevented the typical feeling of fatigue induced by LPS. Since both LPS alone and MP alone produce similar increases in DA, our data suggest that striatal DA levels do not have a direct relationship with fatigue. It is possible that DA in other brain regions is implicated in fatigue, or that other effects of MP counteracted fatigue (for example, the inhibition of norepinephrine uptake).48
Prior studies have suggested a cross sensitization between stressors and DA changes.49, 50 But, there appear to be differences between the effects of short- and long-term neuroimmune activation. In rodents, LPS induced an increase in DA concentration, an effect which peaked 2 h after LPS administration.24 Mice acutely pre-treated with LPS and cocaine displayed greater locomotion in a behavioral task compared to pre-treatment with cocaine alone.51 In nonhuman primates, 2 weeks of IFNα (a cytokine involved in inflammatory signaling) caused an increase in amphetamine-stimulated DA release over amphetamine alone.25 Our findings are consistent with prior studies, namely, stimulated DA elevation was enhanced with an acute dose of LPS compared to placebo. Studies of chronic inflammation have not found a synergistic effect of inflammation on DA activity. Capuron et al.52 found reduced 18F-DOPA uptake in humans after chronic IFNα administration. Chronic treatment with IFNα was also observed to reduce striatal dopamine release in primates as measured by microdialysis.26 In fact, the same monkeys studied by Felger et al.25 that experienced cytokine-enhanced DA release after 2 weeks showed a reversal of this effect when IFNα administration was extended to 4 weeks.
Imaging studies in humans have demonstrated a link between neuroinflammation and activity in the striatum. However, there appear to be differences in the effects on the ventral versus dorsal striatum. Using fMRI, Felger et al.20 showed a decrease in ventral striatal connectivity (during resting state) in depressed individuals with neuroinflammation. In non-depressed populations, ventral striatal BOLD activity in response to reward was blunted by both an acute dose of LPS12, 13 and chronic IFNα administration.52 The findings of these fMRI studies were localized to ventral striatum and indicate a reduction in reward reactivity during immune activation. In contrast, the present study found a synergistic effect of immune activity on DA elevation only in the dorsal striatum (caudate and putamen). DA in the dorsal (as opposed to ventral) striatum has been linked to response inhibition, another important behavioral characteristic of substance abuse.53, 54, 55 Low DA receptor availability in the dorsal striatum (as measured by PET imaging in humans) has been associated with impaired response inhibition.56, 57, 58, 59 The results of the present study are consistent with this body of work and represent a novel contribution to it, namely, that DA activity in response to a stressor is enhanced in the dorsal striatum.
The interaction between an immune response and DA signaling may carry unappreciated risks. If in the presence of inflammation, a dopaminergic medication or other stimulus with addiction potential produces higher DA elevation in the dorsal striatum than without, then it is reasonable to expect that the addiction liability of the stimulus may be increased through impaired response inhibition. Consider an individual experimenting with illicit stimulants or an adolescent prescribed Ritalin (MP), either of whom are experiencing short-term neuroinflammation. Both could be at increased risk for addiction due to reduced inhibitory control caused by a supraphysiologic DA elevation. We believe our findings call for further investigation of patient populations who may be suffering from neuroinflammation and are using or abusing stimulants.
Conclusion
MP-induced DA elevation in the striatum was increased in the presence of LPS versus placebo (seven of eight subjects, P<0.01). Co-administration of MP and LPS elevated subjects' inflammatory cytokine concentrations but did not elicit negative behavioral effects typically observed during systemic inflammation. The synergistic effect between immune activation and DA stimulants could have important implications in the treatment of neuroimmune compromised and DA dysfunctional populations.
Acknowledgments
We thank Dr Lesley Devine and the staff of the Yale PET Center. Research support was provided by the National Institute of Health grant R21 AG043702-01 to ED Morris. JR Petrulli is supported by the National Science Foundation Graduate Research Fellowship under grant DGE-1122492.
Disclaimer
Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Kostic M, Stojanovic I, Marjanovic G, Zivkovic N, Cvetanovic A. Deleterious versus protective autoimmunity in multiple sclerosis. Cell Immunol 2015; 296: 122–132. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005; 46: 1724–1743. [DOI] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 2011; 25: S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav Immun 2011; 25: S13–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci 2015; 18: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, Whittle S, Allen NB. The role of brain structure and function in the association between inflammation and depressive symptoms: a systematic review. Psychosom Med 2016; 78: 389–400. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2015; 2: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 42: 115–121. [DOI] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 2015; 16: 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage 2009; 47: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 2010; 68: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cercignani M, Voon V, Critchley HD. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology 2015; 40: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B et al. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med 2012; 53: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Devine L, Pittman B, Hannestad J. Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun 2013; 31: 197–204. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun 2011; 25: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty GD, Kivimaki M. Inflammation and specific symptoms of depression. JAMA Psychiatry 2016; 73: 87–88. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neurosci Biobehav Rev 2016; 64: 148–166. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 2015; 40: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 2016; 21: 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA 2015; 112: 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Scott J, Ellison-Wright I, Mechelli A. Neuroimaging distinction between neurological and psychiatric disorders. Br J Psychiatry 2015; 207: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther 1992; 261: 964–969. [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 2013; 38: 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Hernandez CR, Miller AH. Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int J Neuropsychopharmacol 2015; 18: pyu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Pappas NR, Wong CT et al. Reproducibility of repeated measures of endogenous dopamine competition with [11C]raclopride in the human brain in response to methylphenidate. J Nucl Med 1999; 40: 1285–1291. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 1994; 16: 255–262. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse 2002; 43: 181–187. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001; 21: RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Aalto S, Lumme V, Nagren K, Kajander J, Vilkman H et al. Measurement of striatal and thalamic dopamine D2 receptor binding with 11C-raclopride. Nucl Med Commun 2003; 24: 1207–1214. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H et al. Sex differences in the brain's dopamine signature of cigarette smoking. J Neurosci 2014; 34: 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 2012; 71: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann K, Jenewein J, Sprott H, Hasler G, Schnyder U, Warnock G et al. Relation of dopamine receptor 2 binding to pain perception in female fibromyalgia patients with and without depression - A [(11)C] raclopride PET-study. Eur Neuropsychopharmacol 2016; 26: 320–330. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Mamo DC, Mulsant BH, Chung JK, Plitman E et al. Dopamine D(2)/(3) receptor availability in the striatum of antipsychotic-free older patients with schizophrenia-A [(1)(1)C]-raclopride PET study. Schizophr Res 2015; 164: 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry 2011; 16: 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer O, Nagren K, Dolle F, Lundkvist C, Sandell J, Swahn CG et al. Precursor synthesis and radiolabelling of the dopamine D-2 receptor ligand [C-11]raclopride from [C-11]methyl triflate. J Labelled Compd Rad 1999; 42: 1183–1193. [Google Scholar]
- van Velden FH, Kloet RW, van Berckel BN, Buijs FL, Luurtsema G, Lammertsma AA et al. HRRT versus HR+ human brain PET studies: an interscanner test-retest study. J Nucl Med 2009; 50: 693–702. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Services: San Diego, CA, 1971. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski MP, Rajeevan N, DiStasio M, Okuda H, Constable RT et al. BioImage suite: an integrated medical image analysis suite: an update. Insight J 2006; 2006: 209. [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging 1998; 17: 463–468. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4(3 Pt 1): 153–158. [DOI] [PubMed] [Google Scholar]
- Chen DL, Rosenbluth DB, Mintun MA, Schuster DP. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol 2006; 100: 1602–1609. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001; 58: 445–452. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 2010; 68: 854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Welfeld K, Leyton M, Dagher A, Boileau I, Sibon I et al. Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl Psychiatry 2016; 6: e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Ho LB, Tolliver BK, Wolkowitz OM, Berger SP. Partial reversal of stress-induced behavioral sensitization to amphetamine following metyrapone treatment. Brain Res 1998; 783: 133–142. [DOI] [PubMed] [Google Scholar]
- Tortorelli LS, Engelke DS, Lunardi P, Mello EST, Santos-Junior JG, Goncalves CA. Cocaine counteracts LPS-induced hypolocomotion and triggers locomotor sensitization expression. Behav Brain Res 2015; 287: 226–229. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 2012; 69: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology 2013; 230: 89–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS ONE 2007; 2: e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend 2005; 79: 273–277. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F et al. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci 2015; 35: 5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 2012; 32: 7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 2009; 29: 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.