ABSTRACT
Xianyang city is one of the main hemorrhagic fever with renal syndrome (HFRS) epidemic areas in northwest China. Although the HFRS immunity program has been provided in this city, HFRS is still occurred every year. In order to implement the vaccination program effectively and to control HFRS, the analysis of antibody responses specific to Hantaan virus (HTNV) in individuals after vaccination is essential. In this study, a total of 100 subjects were divided into 5 groups: unvaccinated, 1, 3, 29 and 33 months after boost vaccination. The levels and the positive rates of HTNV-NP-specific IgM and IgG antibodies as well as HTNV neutralizing antibodies were significantly increased in the serum of the vaccinated individuals. The positive rates and levels of HTNV-NP-specific IgG and HTNV neutralizing antibody reached their highest values at 3 months respectively and could be sustained up to 33 months after vaccination. Moreover, the titres of HTNV-NP-specific IgM or IgG antibody and the titres of HTNV neutralizing antibody at 1 month after vaccination have a positive correlation. The level of HTNV-NP-specific IgG antibody was much higher than that of HTNV-NP-specific IgM antibody or HTNV neutralizing antibody. In addition, the strongest responses of antibody-secreting cells were observed at 3 months after vaccination, which was consistent with the serum results. Therefore, the HFRS immunization program is effective to induce humoral immunity in the population of northwest China.
KEYWORDS: antibody response, humoral immunity, Hantaan virus, hemorrhagic fever with renal syndrome, vaccination
Hemorrhagic fever with renal syndrome (HFRS) has become an important threat to human health worldwide. The HFRS cases in China primarily infected with Hantaan virus (HTNV) strain account for 90% of the total global cases.1 The morbidity of HFRS has gradually declined in China in recent years since the HFRS vaccination program was implemented.2 For example, the vaccination compliance ranged from 4.55% in 1994 to 83.67% in 2010 in Hu County of China. Meanwhile, the HFRS incidence ranged from 300.57/100,000 in 1984 to 9.53/100,000 in 2005.2 However, because of both natural and human factors, novel variations of hantaviruses have appeared.3 Hantaviruses carrying rates and infection rates in rodents increased rapidly in 2012.4 For the epidemiological profiles of HTNV and the immune state of the population were inconsistent in different regions, the effect of HFRS vaccination may be limited.
Shaanxi province is one of the main HFRS epidemic areas in China.5 From the year 2010 to 2012, the mortality of HFRS in Shaanxi has surpassed Heilongjiang and became the province with the highest HFRS incidence in China.6 Xianyang City in Shaanxi province always has a high incidence of HFRS during recent years. Therefore, an inactive bivalent vaccine was provided from 1994 and expanded from 2008 in Xianyang City.7 However, the effect of vaccination on the population remained unclear in this region.
Hantaviruses have a tripartite genome encoding a nucleocapsid protein (NP), 2 envelope glycoproteins (GP) and a viral RNA polymerase.8 HTNV-NP has been demonstrated to be highly immunogenic and conservative, which could induce vigorous cellular and humoral immune responses in humans.9 HTNV glycoprotein is considered to be the main source of neutralizing antibody (NAb).10 To evaluate the effect of vaccines, the calculation of protective rate and antibody seroconversion rate needs to be performed on vaccinated persons.11 In our study, we assessed the HTNV-specific antibody responses of 80 subjects from Xianyang city who had received HTNV vaccination. We also explored the effect of the HFRS vaccine program by analyzing the influence of vaccination on the positivity rates and levels of HTNV-NP-specific IgM, IgG antibody and HTNV neutralizing antibody at different time points after vaccination. The results may provide a scientific basis for the evaluation and improvement of HFRS vaccination planning.
The HFRS inactivated vaccine used in this study was commercially obtained from Zhejiang Weixin Bio-Pharmaceutical Co., Ltd., China. The purified vaccine was provided as a bivalent and derived from a mixture of HTNV (type I) and Seoul virus (SEOV) (type II). People aged from 16 to 60 y were vaccinated free of charge according to the Pharmacopeia of the People's Republic of China (2005)12,13 in Xianyang city. The vaccination program is consisted of 3 doses administration at 0 d, 15 d and one year respectively. One vaccine dose was 1.0 ml with aluminum hydroxide adjuvant. The regimen was carried out according to the commercial vaccine instruction.
The Xianyang Qian County and Qindu District were selected randomly to be the study area. A total of 80 vaccinated healthy persons were enrolled in this study. Considering the effect and duration of the HFRS vaccine, the serum samples were collected at 4 time points: 1 month, 3 months, 29 months and 33 months after vaccination. A vaccination and sampling scheme is shown in Fig. S1. Another 20 unvaccinated healthy persons aged 16 to 60 y old were enrolled as the control group. Demographic characteristics of the enrolled subjects are given in Table S1. The study was approved by the Ethics Committee at the Fourth Military Medical University. The informed consent was obtained from each trial participant.
HTNV-NP-specific IgG and IgM antibodies were detected in the serum of study subjects, negative and positive controls with Hantavirus Hantaan IgM/IgG kits (Westang Bio-Tech Co., Ltd, China) according to the manufacturer's instructions. The OD450nm of each well was detected with a microplate reader (Bio-Rad) and duplicates of each sample were analyzed. The titer ≥40 is considered to be positive. The geometric mean titer (GMT) was calculated.
The cell microculture neutralization test was performed as described previously.14 The sera were diluted serially 2-fold and combined with an equal volume of 100 TCID50 HTNV (76-118 strain). The mixture was then transferred to monolayer of Vero E6 cells and incubated for 9 to 11 d. Thereafter, HTNV antigen in the cell lysate was detected by sandwich ELISA. The neutralizing antibody titer was defined as the maximum dilution of serum that inhibited HTNV infection in 50% of the cells. The titer that can inhibit HTNV infection in 50% of the cells is considered a positive take for neutralization. HTNV strain 76-118 stock, Vero E6 cells and the mouse anti-HTNV-NP monoclonal antibodies (mAb) were provided by the Department of Microbiology in the Fourth Military Medical University.
All data were analyzed using GraphPad prism 5.0 software (GraphPad Software, Inc.; La Jolla, CA). Student's t-test was used to determine significant differences between the experimental and control groups. One-way ANOVA was used to determine statistically significant differences among the experimental groups. The Chi-square test was used to analyze the enumeration data. Pearson correlation statistic was used to identify correlations between the 2 groups. P < 0.05 was considered statistically significant.
The positive rates and levels of serum HTNV-NP-specific IgM, IgG and HTNV neutralizing antibodies after vaccination were evaluated. The positive rates of HTNV-NP-specific IgM antibody in the vaccinated population at 1 month, 3 months, 29 months and 33 months after vaccination were 90.0%, 80.0%, 80.0% and 80.0%, respectively (Fig. 1a), and were higher than that of the unvaccinated group in which the positive rate is only 10%. The positive rate reached the highest at 1 month after vaccination. No statistically significant differences were observed among the vaccinated groups (χ2 = 0.5195, P > 0.05). Indirect ELISA was carried out to test the levels of serum HTNV-NP-specific IgM antibodies. The GMT in the unvaccinated group was 1.45, whereas the GMT in the groups 1, 3, 29 and 33 months after vaccination could reach 27.66, 28.59, 27.97 and 19.13, respectively. The level of serum HTNV-NP-specific IgM antibodies reached a peak 3 months after vaccination and then gradually declined (Fig. 1a). Compared with the levels of IgG antibodies, the levels of IgM antibodies was not high. There was significant difference of the IgM antibody levels between the unvaccinated and vaccinated groups (P<0.01). To evaluate IgM levels accurately, it may require further investigation with more serum samples. The positive rate of HTNV-NP-specific IgG antibody in the unvaccinated was 10.0%. However, the positive rates at 1 month, 3 months, 29 months and 33 months after vaccination were 60%, 100%, 80% and 90%, respectively. The positive rates in the vaccinated groups were significantly higher than that of the unvaccinated group (χ2 = 30.09, P < 0.01) (Fig. 1b). The positive rate reached a peak at 3 months after vaccination. Importantly, the high positive rate was maintained until 33 months after vaccination. The GMT of HTNV-NP-specific IgG antibody was 21.0, 1689.0, 497.2 and 272.4 at 1, 3, 29 and 33 months after vaccination, respectively. The GMT at 3 months was the highest among all of the groups and was approximately 80-fold higher than that at 1 month, 3-fold higher than that at 29 months and 6-fold higher than that at 33 months after vaccination. After 3 months, the GMT of HTNV-NP-specific IgG antibody declined gradually (Fig. 1b). The IgG antibody levels showed obviously difference between the unvaccinated and vaccinated groups (P < 0.0001). The positive rate of HTNV neutralizing antibody in the unvaccinated group was 10.0%, whereas the positive rates at 1, 3, 29 and 33 months after vaccination were 80.0%, 90.0%, 50.0% and 90.0%, respectively. The positive rate of neutralizing antibody increased obviously at 1 month after vaccination. In the vaccinated groups, the positive rates of neutralizing antibody were significantly higher than that in the unvaccinated group (χ2 = 33.97, P < 0.01) (Fig. 1c). The positive rate of neutralizing antibody reached a peak at 3 months after vaccination, which was maintained until 33 months after vaccination. The GMTs of HTNV neutralizing antibody were 1.1, 5.0, 9.2, 5.3 and 5.3 in the unvaccinated group and vaccinated groups at 1, 3, 29 and 33 months after vaccination, respectively (Fig. 1c). The GMT of HTNV neutralizing antibody was elevated at 1 month after vaccination and reached a peak at 3 months after vaccination.
Figure 1.
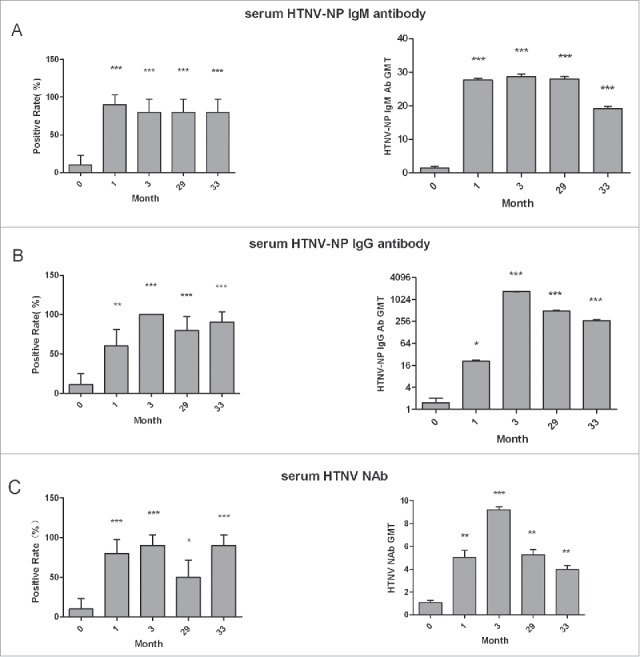
The evaluation of serum HTNV-NP-specific IgM, IgG antibodies and HTNV neutralizing antibodies after vaccination. The positive rates and levels of serum HTNV-NP-specific IgM (A), IgG (B) antibodies and HTNV neutralizing antibodies (C) in the groups after vaccination. Groups 0, 1, 3, 29 and 33: no vaccine, and 1, 3, 29 and 33 months after vaccination, respectively; 20 people in each group. The titre ≥ 40 is considered a positive take for HTNV-NP-specific IgM and IgG antibodies. The titer that can inhibit HTNV infection in 50% of the cells is considered a positive take for neutralization. P values relative to month 0 are identified by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001).Vertical lines indicate 95% confidence interval (CI) for positive rate and Standard Deviation (SD) for GMT.
The positive rates or the serum levels of HTNV-NP-specific IgM or IgG antibodies and HTNV neutralizing antibodies after vaccination were compared. The positive rates of HTNV-NP-specific IgM and IgG antibodies reached peaks at 1 months and 3 months, after vaccination respectively. The higher positive rates of HTNV-NP-specific IgG and HTNV neutralizing antibody were maintained 33 months after vaccination. Similar changes in the positive rates of HTNV-NP-specific IgG and HTNV neutralizing antibody were observed (Fig. 2a). The levels of HTNV-NP-specific IgM or IgG antibody and HTNV neutralizing antibody all reached the peak at 3 months after vaccination and then decreased slowly. The level of HTNV-NP-specific IgG antibody was obviously higher than that of HTNV-NP-specific IgM and HTNV neutralizing antibody at 3, 29, and 33 months after vaccination (Fig. 2b, c).
Figure 2.
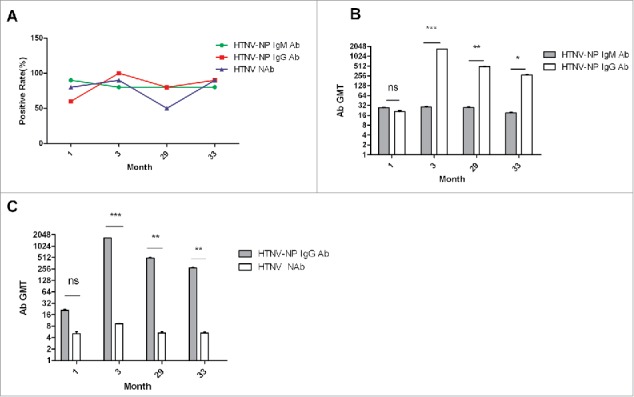
The comparison between HTNV-NP-specific IgM, IgG and HTNV neutralizing antibodies after vaccination. (A) The positive rates of HTNV-NP-specific IgM, IgG and HTNV neutralizing antibodies in the groups at 1, 3, 29 and 33 months after vaccination. (B) The levels of HTNV-NP-specific IgM and IgG antibodies in the groups at 1, 3, 29 and 33 months after vaccination. (C) The levels of HTNV-NP-specific IgG and HTNV neutralizing antibody in the groups at 1, 3, 29 and 33 months after vaccination. Vertical lines indicate Standard Deviation (SD). 1, 3, 29 and 33: 1, 3, 29 and 33 months after vaccination, respectively; 20 people in each group. P values are identified by asterisks (*P < 0.05, ** P < 0.01, *** P < 0.001).
The correlations of the HTNV-NP-specific IgG, IgM and HTNV neutralizing antibody were analyzed. The titres of HTNV-NP-specific IgM and IgG antibodies were positive correlation. Meanwhile, the titres of HTNV-NP-specific IgM or IgG and the titres of the neutralizing antibody at 1 month after vaccination were positive correlation too (Fig. 3a). Moreover, there was a significant association between the HTNV-NP-specific IgM and IgG titres at 3 months after vaccination (Fig. 3b). Positive correlations were also found between the titres of HTNV-NP-specific IgG and HTNV neutralizing antibody at 29 and 33 months after vaccination respectively (Fig. 3c, d).
Figure 3.
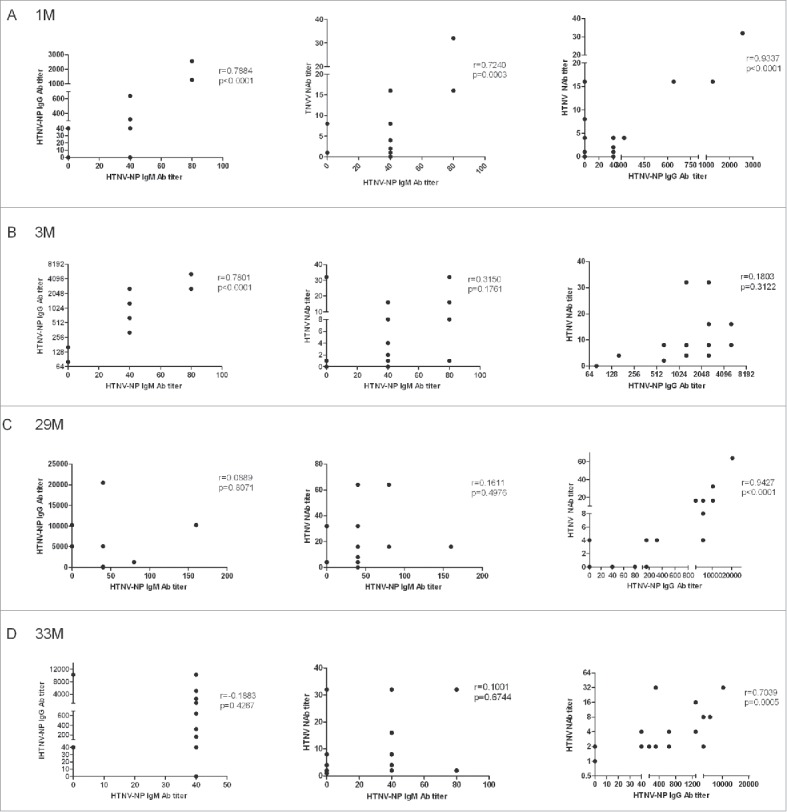
The correlation analysis between the titres of the HTNV-NP-specific IgG, IgM and HTNV neutralizing antibody in the groups (A) 1 month, (B) 3 months, (C) 29 months and (D) 33 months after vaccination. Some dots overlap. 1M, 3M, 29M and 33M: 1, 3, 29 and 33 months after vaccination, respectively; 20 people in each group.
After EBV-transformed B lymphoblastoid cell lines (BLCLs) for each vaccinated person were established successfully, the HTNV antibodies produced by BLCLs were detected at 3 months and 29 months after vaccination. From Fig. S2, we can see the cell volume increased dramatically and clumps of cells were scattered in the culture solution. The highest positive rate of HTNV-NP-specific IgG antibody in the culture supernatants was found at 3 months after vaccination, which was consistent with the results from the serum samples. The HTNV neutralizing antibody in the BLCLs culture supernatants was detected in all groups, whereas the IgG antibody was not detected at 1 month and 33 months after vaccination, and the IgM antibody was not detected at 1 month after vaccination (Fig. 4a). Importantly, the total positive rate of the IgG was higher than that of the IgM in all BLCLs culture supernatants (Fig. 4b).
Figure 4.
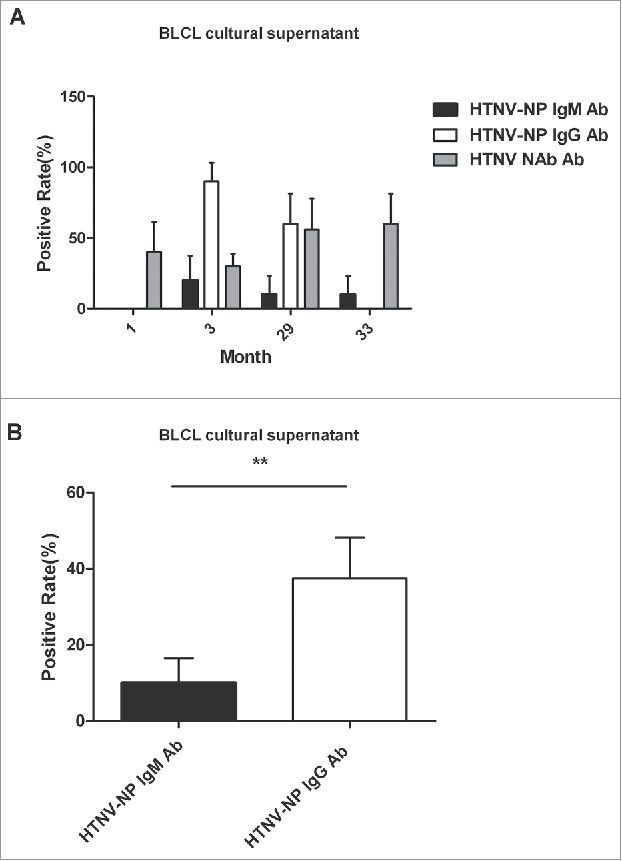
The HTNV antibody responses of BLCLs. (A) The positive rates of HTNV-NP-specific IgG, IgM and HTNV neutralizing antibody were determined at 1, 3, 29, and 33 months after vaccination in the culture supernatants of BLCLs. (B) The total positive rate of HTNV-NP-specific lgG or lgM antibodies in all BLCL culture supernatants. Vertical lines indicate 95% confidence interval (CI). P values are identified by asterisks (*P< 0.05, **P < 0.01, ***P < 0.001). BLCLs: EBV-transferred B lymphoblastoid cell lines.
In this study, we found that the positive rates and the levels of HTNV-NP-specific IgM, IgG and HTNV neutralizing antibody were significantly increased and sustained for a long time in the serum of individuals who received HTNV vaccination in Xianyang City. This indicates that the vaccination scheme can induce effective humoral immunity in northwest China.
Vaccination has become the most effective medical intervention to reduce morbidity and mortality caused by infectious diseases.15 To monitor the impact of the vaccine, the detection of immunization coverage and effectiveness in protecting against disease were necessary.16 The HFRS vaccination should cover most of the population in China. It has significance to test and verify the effect of vaccine at the population level.2 Therefore in this study we used a cross-sectional study and selected randomly 4 groups of vaccinated persons with different vaccination time as study subjects to evaluate the vaccination effect of HFRS vaccine at the population level.
It exists the positive rates of HTNV-NP-specific IgM, IgG and neutralizing antibodies in the unvaccinated group indicated that there were high unapparent infection rates in the population. It has been reported that the unapparent infection rate was approximately 3.41%-21.29%.16 Our results showed that HTNV-NP-specific IgM and IgG were negative, while the neutralizing antibody was positive in some people. These results were consistent with the finding that the neutralizing antibody response was sustained for a longer period than that of the HTNV-NP-specific IgM and IgG antibody.17 The level of IgM or IgG antibody specific to HTNV-NP was correlated with the titres of neutralizing antibody respectively, suggesting that these 2 kinds of anti-NP antibodies all might inhibit HTNV infection.9 It has been reported that Hantavirus GP and NP all contributed to protective neutralizing activity.18 Furthermore, if the stable HTNV-GP antigen available, the antibody response specific to HTNV-GP needs to be further studied in our next step.
Moreover, in our study, the positive rate of HTNV-NP-specific IgM antibody rose initially after vaccination and reached the highest at 1 month after vaccination, whereas the antibody level and positive rate of HTNV-NP-specific IgG antibody reached peaks at 3 months after vaccination. These results were corresponded to the rule that IgM antibody always appears earlier than IgG antibody.19 The HTNV-NP-specific IgM antibodies appeared earlier at lower levels, whereas the HTNV-NP-specific IgG antibodies appeared later at higher levels and were maintained longer after vaccination. This indicated that the HTNV-NP-specific IgG antibody may be the main anti-HTNV infection component. The changes in the positive rates of HTNV-NP-specific IgG antibody and HTNV neutralizing antibody were similar. However, the positive rates of HTNV-NP-specific IgG and HTNV neutralizing antibody at 29 months were lower than that at 33 months. This may be due to the samples collected in the study is only 20 every group and more samples needs to be analyzed further. The effect of humoral immunity depends on the end production of various antibodies induced by vaccine. The positive correlations between the titres of HTNV-NP-specific IgM or IgG antibody and HTNV neutralizing antibody at 1 month after vaccination were observed. These suggested that the nearer the last vaccination, the better immune effect could be produced. However, the correlation was not significant at 3, 29, and 33 months after vaccination, which required further investigation with much more serum samples.
The robust serological responses indicated the substantial formation of antibody-secreting cells (ASCs) after vaccination. EBV is a lymphotropic herpesvirus that converts normal human B lymphocytes into established lines. This ‘immortalisation’ preserves the characteristics of the original B cell with surface immunoglobulin and secretory immunoglobulin.20 Therefore, the BLCLs of vaccinated persons can be used to assess the response of antibody secreting cells. The positive rates of HTNV-specific antibodies were lower or undetected in the culture supernatants of BLCLs than that in the sera, which was probably due to the insufficient concentration of specific antibodies in the culture supernatants of BLCLs. The results showed that the antibody responses in the supernatant of BLCLs reached an effective level at 3 months after vaccination, which was consistent with that in the sera. All of these results demonstrated that the rapid and vigorous B cell response occured after vaccination. In addition, according to some reports,20,21 the type of antibody secreted by BLCL was mainly IgM. However, in our study, the primary antibody after vaccination was IgG. The positive rate of HTNV-NP IgG antibody was higher than that of the HTNV-NP IgM antibody in the culture supernatant of all BLCL cells. This may be because that the BLCLs in our study were obtained from persons who had been vaccinated 3 times and the vaccinated persons may have more memory B lymphocytes. The BLCLs secreting HTNV neutralizing antibody can be used to build a phage antibody library to produce human neutralizing antibody. The HTNV neutralizing mAb, HTNV-NP or GP-specific mAb will be isolated and be used to diagnose and cure HTNV infection in the next step.
In conclusion, firstly, our study showed that HFRS vaccination might play an important role in the control and prevention of HFRS in the northwest region of China and novel vaccines capable of eliciting a potent neutralizing antibody still need to be designed further. Secondly, more samples should be used to evaluate the response of antibodies after vaccination and the cellular immunity induced by the vaccine should also be studied in the next step. For an overall evaluation of the effect of a vaccine, it is necessary to examine SEOV specific antibody response. Thirdly, for the HFRS incidence in people younger than 16 or older than 60 y has increased in Shaanxi province in recent years,22 we recommend expanding the scope of HFRS vaccination to people younger than 16 and older than 60 y. Meanwhile, the relationship between the fluctuation of HFRS epidemic and vaccination rates in northwest China needs to be analyzed.
Supplementary Material
Abbreviations
- HFRS
hemorrhagic fever with renal syndrome
- HTNV
Hantaan virus
- NP
nucleocapsid protein
- SEOV
Seoul virus
- GP
glycoprotein
- NAb
neutralizing antibody
- OD
optical density
- GMT
geometric mean titer
- BLCLs
EBV-transformed B lymphoblastoid cell lines
- ASCs
antibody-secreting cells
- EBV
Epstein-Barr virus
- mAb
monoclonal antibody
- ELISA
enzyme-linked immunosorbent assay
- PBMCs
peripheral blood mononuclear cells
Disclosure of potential conflicts of interest
The authors have declared no conflict of interest exists. All authors have read this manuscript and approved its publication.
Acknowledgments
The authors would like to thank Liu Meining (Centers for Disease Control and Prevention, Xianyang, China) for providing the blood specimens.
Funding
This study was supported by National major special projects (grant no. 2013ZX10004609) and National Natural Science Foundation (grant no. 81401297) of China.
References
- [1].Zhang WY, Wang LY, Liu YX, Yin WW, Hu WB, Magalhaes RJ, Ding F, Sun HL, Zhou H, Li SL, et al.. Spatiotemporal transmission dynamics of hemorrhagic fever with renal syndrome in China, 2005-2012. PLoS Negl Trop Dis 2014; 8:e3344; PMID:25412324; http://dx.doi.org/ 10.1371/journal.pntd.0003344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xiao D, Wu K, Tan X, Yan T, Li H, Yan Y. The impact of the vaccination program for hemorrhagic fever with renal syndrome in Hu County, China. Vaccine 2014; 32:740-5; PMID:24252696; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.024 [DOI] [PubMed] [Google Scholar]
- [3].Zhang YZ, Zou Y, Yan YZ, Hu GW, Yao LS, Du ZS, Jin LZ, Liu YY, Li MH, Chen HX. Detection of phylogenetically distinct Puumala-like viruses from red-greyvole Clethrionomys rufocanus in China. J Med Virol 2007; 79:1208-18. [DOI] [PubMed] [Google Scholar]
- [4].Zhang S, Wang S, Yin WW, Liang MF, Li JD, Zhang QF, Feng ZJ, Li DX. Epidemic characteristics of hemorrhagic fever with renal syndrome in China, 2006-2012. BMC Infect Dis 2014; 14:384; PMID:25012160; http://dx.doi.org/ 10.1186/1471-2334-14-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang LY, Zhou H, Yin WW, Wang Q, Sun H, Ding F, Man TF, Li Q, Feng ZJ. The current epidemic situation and surveillance regarding hemorrhagic fever with renal syndrome in China, 2010. Zhonghua Liu Xing Bing Xue Za Zhi 2012; 33:685-91; [PubMed] [Google Scholar]
- [6].Huang X, Yin H, Yan L, Wang X, Wang S. Epidemiologic characteristics of haemorrhagic fever with renal syndrome in Mainland China from 2006 to 2010. West Pac Surveill Response J 2012; 3:12-8; http://dx.doi.org/ 10.5365/wpsar.2012.3.4.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ministry of Health of the People's Republic of China Implement Program of National Expanded Program on Immunization. 2007. [Google Scholar]
- [8].Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. J Gen Virol 1996; 77:2677-87; ; http://dx.doi.org/ 10.1099/0022-1317-77-11-2677 [DOI] [PubMed] [Google Scholar]
- [9].Zeier M, Handermann M, Bahr U, Rensch B, Muller S, Kehm R, Muranyi W, Darai G. New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention-a review. Virus Genes 2005; 30:157-80; PMID:15744574; http://dx.doi.org/ 10.1007/s11262-004-5625-2 [DOI] [PubMed] [Google Scholar]
- [10].Yan G, Zhang Y, Ma Y, Yi J, Liu B, Xu Z, Zhang Y, Zhang C, Zhang F, Xu Z, et al.. Identification of a novel B-cell epitope of Hantaan virus glycoprotein recognized by neutralizing 3D8 monoclonal antibody. J Gen Virol 2012; 93:2595-600; PMID:22933664; http://dx.doi.org/ 10.1099/vir.0.045302-0 [DOI] [PubMed] [Google Scholar]
- [11].Leval A, Herweijer E, Ploner A, Eloranta S, Fridman SJ, Dillner J, Young C, Netterlid E, Sparén P, Arnheim-Dahlström L. Quadri-valent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst 2013; 105:469-74; PMID:23486550; http://dx.doi.org/ 10.1093/jnci/djt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].National Pharmacopoeia Committee Pharmacopoeia of the People's Republic of China, the third division of 2005 edition. Beijing: China Chemical Industry Press; 2005; pp 80-88. [Google Scholar]
- [13].Luo CW, Chen HX. Epidemiological characteristics and the strategy of vaccination on hemorrhagic fever with renal syndrome in the last 10 years, in China. Zhonghua Liu Xing Bing Xue Za Zhi 2008; 29:1017-9; PMID:19173887 [PubMed] [Google Scholar]
- [14].Papa A. Dobrava-Belgrade virus: phylogeny, epidemiology, disease. Antiviral Res 2012; 95:104-17; PMID:22659378; http://dx.doi.org/ 10.1016/j.antiviral.2012.05.011 [DOI] [PubMed] [Google Scholar]
- [15].Delany I, Rappuoli R, Gregorio ED. Vaccines for the 21st century. EMBO Mol Med 2014; 6:708-20; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hardt K, SchmidtOtt R, Glismann S, Adegbola RA, Meurice1 FP. Sustaining vaccine confidence in the 21st Century. Vaccines (Basel) 2013; 1:204-24; PMID:26344109; http://dx.doi.org/ 10.3390/vaccines1030204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao XF, Zhang LY, Na DY, Shao WA. The application of micro-cytopathic effect neutralization test (MCPENT) in the detection of antibody of epidemic hemorrhagic fever. Zhong Guo Wei Sheng Jian Yan Za Zhi 2001; 11:153-6. [Google Scholar]
- [18].Jiang DB, Sun YJ, Cheng LF, Zhang GF, Dong C, Jin BQ, Song CJ, Ma Y, Zhang FL, Yang K. Construction and evaluation of DNA vaccine encoding Hantavirus glycoprotein N-terminal fused with lysosome-associated membrane protein. Vaccine 2015; 33:3367-76; PMID:26027907; http://dx.doi.org/ 10.1016/j.vaccine.2015.05.007 [DOI] [PubMed] [Google Scholar]
- [19].Tess LK, William HK. Ontogeny of adaptive antibody response to a model antigen in captive altricial zebra finches. PLOS One 2012; 7:e47294; ; http://dx.doi.org/ 10.1371/journal.pone.0047294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steinitz M, Klein G, Koskimies S, Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature 1977; 269:420-2; PMID:198669; http://dx.doi.org/ 10.1038/269420a0 [DOI] [PubMed] [Google Scholar]
- [21].Sundararajan A, Sangster MY, Frey S, Atmar RL, Chen WH, Ferreira J, Bargatze Robert , Mendelman PM, Treanor JJ, Topham DJ. Robust mucosal-homing antibody-secreting B cell responses induced by intramuscular administration of adjuvanted bivalent human norovirus-like particle vaccine. Vaccine 2015; 33:568-76; PMID:25444793; http://dx.doi.org/ 10.1016/j.vaccine.2014.09.073 [DOI] [PubMed] [Google Scholar]
- [22].He XZ, Wang SW, H XX, Wang XF. Changes in age distribution of hemorrhagic fever with renal syndrome: an implication of China's expanded program of immunization. BMC Public Health 2013; 13:394; PMID:23622420; http://dx.doi.org/ 10.1186/1471-2458-13-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


