ABSTRACT
Background: The high coverage for ≥3 pertussis vaccine doses among Taiwanese children might not imply timely vaccination. Recently, resurgence of pertussis and challenges with availability of DTaP-IPV-Hib prompted this study.
Methods: In the 1996–2012 national birth cohort, we calculated the prevalence and days of undervaccination against pertussis by age 36 months. We also compared the odds of undervaccination in each laboratory-confirmed pertussis patient at ages 3–35 months with sex-, residence-, and age-matched controls from the general population, using conditional logistic regression.
Results: The prevalence of undervaccination was 60.6% (median 16 days) and decreasing (p < 0.0001). Among 145 cases and 2,900 controls, 58 (40.0%) and 721 (24.9%) were undervaccinated (OR 2.28, 95% CI 1.57–3.31). The attributable risk percent was 22.5% (95% CI 14.5–27.9).
Conclusions: Undervaccination was decreasing. Approximately up to one-fifth pertussis cases in children aged 3–35 months could have been prevented with on-time vaccination.
KEYWORDS: Immunization schedules, pertussis, Taiwan, timeliness, undervaccination, vaccine shortage
Introduction
Age-appropriate vaccination reduces a child's risk of pertussis and prevents disease outbreaks.1-3 In Taiwan, control of pertussis was achieved through widespread use of whole-cell pertussis vaccine, combined with diphtheria and tetanus toxoids (as DTwP) since 1954.4,5 In 1996, the country started the transition from DTwP to acellular pertussis vaccines (DTaP) and by March 2010, a pentavalent vaccine (combined DTaP, inactivated polio [IPV], and Haemophilus influenzae type b [Hib] vaccine) (DTaP-IPV-Hib) was being used for all 4 doses at ages 2, 4, 6, and 18 months.5 Vaccination coverage among children aged 12–23 months was 97.4% for at least 3 doses.6
This high vaccination coverage, however, did not necessarily imply timely vaccination.7,8 In January 2014, Taiwan Centers for Disease Control issued a recommendation to defer the fourth dose of DTaP-IPV-Hib from age 18 months to 27 months because of a global shortage.9 The challenges with availability of DTaP-IPV-Hib and recent resurgence of pertussis in infants and young children10 prompted us to (1) describe national prevalence and trends of undervaccination with pertussis vaccines during the first 36 months of life; and (2) examine the association between undervaccination and risk of pertussis among children 3–35 months of age.
Materials and methods
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board at Taiwan Centers for Disease Control (#105203).
Study population
The study was conducted within a cohort of Taiwanese children registered in the National Immunization Information System (NIIS), a central database that periodically consolidates distributed immunization records from clinics, hospitals, and local health authorities.11 For each child born since 1995, details of vaccines administered mainly at preschool ages are recorded in an NIIS account under his/her unique personal identification number, and compiled through secure data exchange between local and central databases. This mechanism allows children to be vaccinated anywhere in the country without being missed or repeated, and ensures overall high (>98%) data completeness.11
The study observation period was January 1, 1996 through December 31, 2015. We used the NIIS database to create a retrospective cohort of children born between 1996 and 2012 and their vaccination records; each child was followed up until the age of 36 months or death, whichever earlier.
Children undervaccinated
For each child, date(s) of DTwP or DTaP vaccination and date of birth were used to determine age at vaccination. Days undervaccinated for specific doses were calculated based on a modified metric7,12,13 that measures the difference between when the vaccine dose was administered and when the vaccine dose should have been administered (Table S1). The magnitude of undervaccination accumulated until the child was vaccinated or at age 36 months.
Cases and controls
Pertussis is a notifiable disease in Taiwan.4 Physicians are required to report suspect cases to corresponding local health authorities within 7 days and submit nasopharyngeal and/or serum specimens for culture, polymerase chain reaction (PCR), and/or serologic tests for Bordetella pertussis. By linking NIIS to the National Notifiable Disease Surveillance System, we defined case patients as children who had onset of illness during January 1, 1996–December 31, 2015, and were positive for B. pertussis on culture or PCR tests at ages 3–35 months. Each case patient was matched to 20 controls by sex (male or female), age at the illness onset date (the index date) (±7 days), and county of residence. The control group consisted of the 1996–2012 nationwide birth cohort; children were excluded as controls if they had a record of pertussis prior to the index date.
Analysis
The data were processed and analyzed using SAS® 9.4 (SAS Institute Inc., Cary, North Carolina).
Timeliness of vaccination
We calculated the prevalence of DTwP or DTaP undervaccinated children, and mean and median days undervaccinated within each birth cohort from 1996 through 2012.13 Time trends were analyzed with linear regression and Cochran-Armitage trend tests. Trends in undervaccination of dose 1, 2, or 3 were compared with dose 4 across the birth cohorts.
Risk of pertussis
Case patients and controls were linked to the NIIS database using their personal identification number, and classified as either age-appropriately vaccinated (0 doses/days missing or delayed) or undervaccinated (by 1, 2, 3, or 4 doses; or for 1–30, 31–90, or 91–1,003 days) of DTwP or DTaP at the index date. We conditioned the logistic regression model on all matching variables to compare the odds of undervaccination in the cases with the control group. Age-appropriately vaccinated children represented the referent group.
Results
Among 4,104,526 children born between 1996 and 2012, 2,487,831 (60.6%) were undervaccinated with DTwP or DTaP (median 16 days, interquartile range 0–109 days). Of those with any delay, 755,306 (30.4%) were undervaccinated for more than 6 months while other children had shorter periods of delay (28.5% for 1–30 days, 25.6% for 1–3 months, and 15.6% for 4–6 months). The prevalence of undervaccinated children and mean and median days undervaccinated decreased significantly (p < 0.0001), but rebounded for those born in 2012 (Fig. 1). This rebound effect was only observed for the fourth dose.
Figure 1.
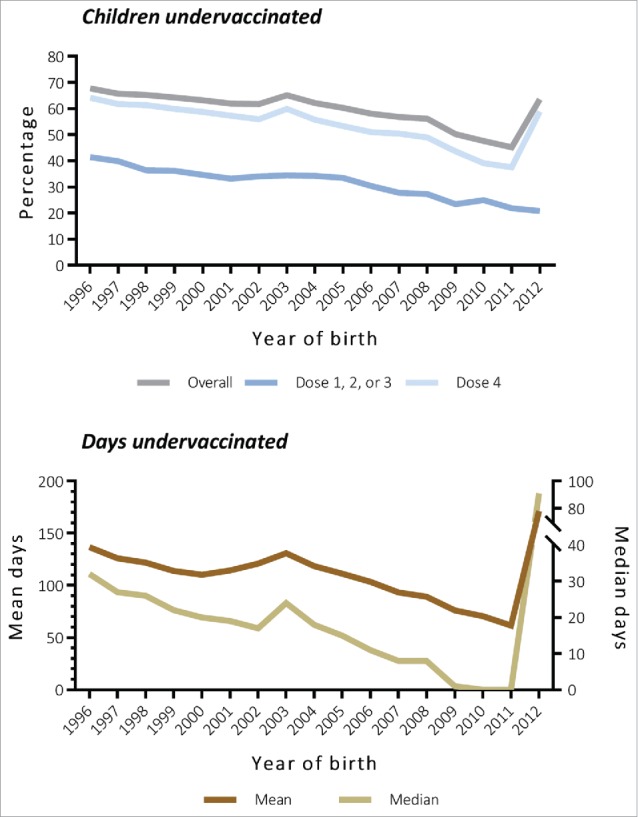
Trends in prevalence (top) and mean and median days (bottom) of undervaccination with DTwP or DTaP by birth cohort. All trends were significant using the Cochran-Armitage trend test (p < 0.0001) and linear regression (p < 0.0001). Numbers were listed in Table S2. DTwP, diphtheria, tetanus and whole-cell pertussis vaccine; DTaP, diphtheria, tetanus, and acellular pertussis vaccine.
Of 145 case patients (mean age 9.77 months), 73 (50.3%) were hospitalized. Characteristics of cases and controls (n = 2,900) were similar (Table 1). At the index date, 58 (40.0%) of the cases and 721 (24.9%) of the controls were undervaccinated of DTwP or DTaP (odds ratio [OR] 2.28, 95% confidence interval [CI] 1.57–3.31). A dose-response relationship was observed between risk of pertussis and the magnitude of undervaccination, but only with delays in the first 3 vaccination doses (Table 2). Overall, 22.5% (95% CI 14.5–27.9) of all B. pertussis patients were attributed to undervaccination.
Table 1.
Characteristics of Bordetella pertussis positive children (cases) and matched controls.
| Cases (n = 145) | Controls (n = 2,900) | p-value | |
|---|---|---|---|
| Sex | 1.0000 | ||
| Male | 81 (55.9) | 1,620 (55.9) | |
| Female | 64 (44.1) | 1,280 (44.1) | |
| Age at index date, mean (SD), months | 9.77 (8.57) | 9.77 (8.55) | 1.0000 |
| Age group, months | 0.9775 | ||
| <12 | 106 (73.1) | 2,116 (73.0) | |
| 12–23 | 26 (17.9) | 529 (18.2) | |
| 24–35 | 13 (9.0) | 255 (8.8) | |
| Region of residencea | 1.0000 | ||
| Northern | 62 (42.8) | 1,240 (42.8) | |
| Central | 15 (10.3) | 300 (10.3) | |
| Southern | 13 (9.0) | 260 (9.0) | |
| Others | 55 (37.9) | 1,100 (37.9) | |
| Year of index date | 1.0000 | ||
| 1996–2007 | 100 (69.0) | 2,000 (69.0) | |
| 2008–2011 | 29 (20.0) | 580 (20.0) | |
| 2012–2015 | 16 (11.0) | 320 (11.0) |
SD, standard deviation.
Northern (from Keelung to Miaoli), Central (Taichung, Changhua, and Nantou), and Southern (from Yunlin to Pingtung) Taiwan are broad geographic regions along the west coast. Other regions included Eastern Taiwan (Hualien and Taitung) and offshore islands (Penghu, Kinmen, and Lienchiang).
Table 2.
Matched OR and 95% CI for Bordetella pertussis infection, under- versus age-appropriately vaccinated of DTwP or DTaPa.
| Cases(n = 145) | Controls(n = 2,900) | Matched ORb | (95% CI)b | p-valueb | |
|---|---|---|---|---|---|
| Number of doses undervaccinated | |||||
| 0 | 87 (60.0) | 2,179 (75.1) | 1.0 | ||
| 1, 2, 3, or 4 | 58 (40.0) | 721 (24.9) | 2.28 | (1.57–3.31) | <0.0001 |
| 1 | 29 (20.0) | 439 (15.1) | 1.84 | (1.17–2.89) | 0.0080 |
| 2 | 12 (8.3) | 129 (4.4) | 2.80 | (1.44–5.44) | 0.0023 |
| 3 | 13 (9.0) | 113 (3.9) | 3.85 | (1.93–7.67) | 0.0001 |
| 4 | 4 (2.8) | 40 (1.4) | 3.79 | (1.19–12.07) | 0.0243 |
| Days undervaccinated | |||||
| 0 | 87 (60.0) | 2,179 (75.1) | 1.0 | ||
| 1–30 | 26 (17.9) | 370 (12.8) | 1.93 | (1.21–3.06) | 0.0057 |
| 31–90 | 13 (9.0) | 193 (6.7) | 2.06 | (1.09–3.88) | 0.0255 |
| 91–1,003 | 19 (13.1) | 158 (5.4) | 4.16 | (2.26–7.67) | <0.0001 |
| Dose number undervaccinated | |||||
| None | 87 (60.0) | 2,179 (75.1) | 1.0 | ||
| Dose 1, 2, or 3 only | 47 (32.4) | 500 (17.2) | 2.52 | (1.71–3.71) | <0.0001 |
| Dose 4 only | 5 (3.4) | 115 (4.0) | 1.02 | (0.33–3.15) | 0.9784 |
| Dose 1, 2, or 3; plus dose 4 | 6 (4.1) | 106 (3.7) | 1.35 | (0.49–3.72) | 0.5657 |
OR, odds ratio; CI, confidence interval; DTwP, diphtheria, tetanus and whole-cell pertussis vaccine; DTaP, diphtheria, tetanus, and acellular pertussis vaccine.
Children undervaccinated by 0 doses or 0 days, or none of the dose number of DTwP or DTaP were considered as age-appropriately vaccinated.
The ORs and 95% CIs were estimated by conditioning on sex, county of residence, and age at index date.
Discussion
Our analysis found that more than half of the children were delayed for at least one DTwP or DTaP vaccine doses before age 36 months; among them, approximately one-third were undervaccinated for more than 6 months. Contrary to the rising trend in the US,13,14 this analysis also suggested that undervaccination in Taiwan decreased over time. The decreasing prevalence and magnitude of undervaccination against pertussis highlighted public health successes in improving access to healthcare, vaccine delivery, and systems to identify and catch up children behind on immunizations over the past decade. Although timeliness of vaccination for children born in 2012 was hindered by the limited supply of DTaP-IPV-Hib,9 54.8% of those born in the previous year (2011) received all 4 doses of DTaP-IPV-Hib at age-appropriate times, a rate that was comparable to US estimates of 52.1% and 55.9% for DTaP from the National Immunization Survey.7,15
In the case-control analysis we found significant associations between undervaccination and laboratory-confirmed pertussis in children aged 3–35 months. Had these children received vaccination with DTwP or DTaP on time, approximately up to 1 in 5 B. pertussis infections in this age group could have been prevented, as the vaccines could fail to prevent infection, but still protect children against severe disease. We also observed a dose-effect relationship that had been reported for US children of the same ages by Glanz et al.1 Within cohorts of children born during 2004–2008, Glanz argued that children who missed 3 or 4 doses of DTaP vaccine were nearly 19 and 28 times more likely to develop pertussis than children who were age-appropriately vaccinated.1 Our data, however, showed that this increased risk of acquiring pertussis was only associated with delays in the 3-dose primary series, and comparing risk between children undervaccinated by 3 and 4 doses of DTwP- or DTaP-containing vaccine showed very similar results (OR 3.85 and 3.79, respectively). Given the lack of association of pertussis with delaying receipt of the fourth vaccination by age 36 months, our findings provide additional support for the government's policy that has been deferring this routine booster dose of DTaP-IPV-Hib to age 27 months since 2014, particularly when this vaccine is still in short supply.
This study has limitations. First, the validity of NIIS data compared with actual vaccine receipt is unknown. Misclassification of vaccination status can occur if the vaccines are not administered in Taiwan. Second, prior studies estimate that overall 10%–25% of children were undervaccinated because of vaccine-hesitant parents.13,16,17 Our study, however, was unable to provide reasons for undervaccination as this information was not captured in the NIIS or assessed through reviews of medical records. Third, pertussis is highly underreported. While we applied a matched analysis, controls from the general population might have contracted pertussis, but not been tested and notified. Also, physicians' awareness differed and could be higher among under- compared with age-appropriately vaccinated children. This misclassification of disease status and diagnostic bias could have resulted in an overestimate of the true association between undervaccination and pertussis. Finally, use of laboratory tests and availability of whole-cell or acellular pertussis vaccines changed over the study period. Also, the variable patterns of undervaccination contributed to exposure heterogeneity in vaccination histories.12 We were unable to perform stratified analyses for a subset of these variants due to low numbers of exposed cases in each stratum.
Despite these limitations, linking national immunization with notifiable disease datasets had allowed us to efficiently ascertain vaccination status among pertussis patients. Our study indicated that in Taiwan, undervaccination was decreasing but had placed young children at risk for pertussis. By pooling additional data from multiple sources (e.g., hospital/laboratory, cancer, and mortality registries), this model can be further used to study other rare outcomes following the childhood immunization schedules.
Supplementary Material
Abbreviations
- CI
confidence interval
- DTaP
diphtheria, tetanus, and acellular pertussis vaccine
- DTaP-IPV-Hib
diphtheria, tetanus, and acellular pertussis, inactivated polio, and Haemophilus influenzae type b vaccine
- DTwP
diphtheria, tetanus, and whole-cell pertussis vaccine
- NIIS
National Immunization Information System
- OR
odds ratio
- PCR
polymerase chain reaction
Disclosure of potential conflicts of interest
The authors have declared that no competing interests exist.
Acknowledgments
The authors would like to thank staff from the National Immunization Information System and Office of Information Technology, Taiwan Centers for Disease Control, for their support in data coordination.
Funding
This work was supported by Taiwan Centers for Disease Control (http://www.cdc.gov.tw). The funder had no role in study design, data collection and analysis, or preparation of the draft manuscript; but participated in review, approval, and decision to submit the manuscript for publication.
Author contributions
Conceived and design the experiments: WTH. Performed the experiment: WTH. Analyzed the data: WTH. Contributed reagents/materials/analysis tools: WTH CHY. Wrote the first draft of the manuscript: WTH. Contributed to the writing of the manuscript: WTH HCL CHY. Agree with manuscript results and conclusions: WTH HCL CHY.
References
- [1].Glanz JM, Narwaney KJ, Newcomer SR, Daley MF, Hambidge SJ, Rowhani-Rahbar A, Lee GM, Nelson JC, Naleway AL, Nordin JD, et al.. Association between undervaccination with diphtheria, tetanus toxoids, and acellular pertussis (DTaP) vaccine and risk of pertussis infection in children 3 to 36 months of age. JAMA Pediatr 2013; 167: 1060-4; PMID:24019039; http://dx.doi.org/ 10.1001/jamapediatrics.2013.2353 [DOI] [PubMed] [Google Scholar]
- [2].Grant CC, Roberts M, Scragg R, Stewart J, Lennon D, Kivell D, Ford R, Menzies R. Delayed immunization and risk of pertussis in infants: unmatched case-control study. BMJ 2003; 326: 852-3; PMID:12702617; http://dx.doi.org/ 10.1136/bmj.326.7394.852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kolos V, Menzies R, McIntyre P. Higher pertussis hospitalization rates in indigenous Australian infants, and delayed vaccination. Vaccine 2007; 25: 588-90; PMID:16971026; http://dx.doi.org/ 10.1016/j.vaccine.2006.08.022 [DOI] [PubMed] [Google Scholar]
- [4].Lin YC, Yao SM, Yan JJ, Chen YY, Chiang CS, Wu HS, Li SY. Epidemiological shift in the prevalence of pertussis in Taiwan: implications for pertussis vaccination. J Med Microbiol 2007; 56: 533-7; PMID:17374896; http://dx.doi.org/ 10.1099/jmm.0.46741-0 [DOI] [PubMed] [Google Scholar]
- [5].Taiwan Centers for Disease Control History of immunization schedules in Taiwan [Chinese]. Available: http://www.cdc.gov.tw/professional/page.aspx?treeid=5B0231BEB94EDFFC&nowtreeid=F97AFF690D98DA47. Accessed 19August2016. [Google Scholar]
- [6].Taiwan Centers for Disease Control Vaccination coverage in Taiwan [Chinese]. Available: http://www.cdc.gov.tw/professional/page.aspx?treeid=5B0231BEB94EDFFC&nowtreeid=3D1CDC342B28E123. Accessed 19August2016. [Google Scholar]
- [7].Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States. JAMA 2005; 93: 1204-11; http://dx.doi.org/ 10.1001/jama.293.10.1204 [DOI] [PubMed] [Google Scholar]
- [8].Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373: 1543-9; PMID:19303633; http://dx.doi.org/ 10.1016/S0140-6736(09)60317-2 [DOI] [PubMed] [Google Scholar]
- [9].Taiwan Centers for Disease Control Limited supply of a pentavalent vaccine (DTaP-IPV-Hib, Pentacel®): the fourth dose should be delayed to the age of 27 months [Chinese]. Available: http://www.cdc.gov.tw/professional/info.aspx?treeid=cf7f90dcbcd5718d&nowtreeid=f94e6af8daa9fc01&tid=9B891ED0438F4FE2. Accessed 19August2016. [Google Scholar]
- [10].Yeh NC, Chen CM, Kuo HW, Liu DP. Pertussis in Taiwan, 2001–2014. Epidemiol Bull 2016; 32: 23-7 [Google Scholar]
- [11].Liu DP, Wang ET, Pan YH, Cheng SH. Innovative applications of immunization registration information systems: example of improved measles control in Taiwan. Euro Surveill. 2014; 19: 20994; PMID:25597542; http://dx.doi.org/ 10.2807/1560-7917.ES2014.19.50.20994 [DOI] [PubMed] [Google Scholar]
- [12].Glanz JM, Newcomer SR, Jackson ML, Omer SB, Bednarczyk RA, Shoup JA, DeStefano F, Daley MF. White paper on studying the safety of the childhood immunization schedule in the Vaccine Safety Datalink. Vaccine 2016; 34S: A1-29; http://dx.doi.org/ 10.1016/j.vaccine.2015.10.082 [DOI] [PubMed] [Google Scholar]
- [13].Glanz JM, Newcomer SR, Narwaney KJ, Hambidge SJ, Daley MF, Wagner NM, McClure DL, Xu S, Rowhani-Rahbar A, Lee GM, et al.. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr 2013; 167: 274-81; PMID:23338829; http://dx.doi.org/ 10.1001/jamapediatrics.2013.502 [DOI] [PubMed] [Google Scholar]
- [14].Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics 2012; 130: 32-8; PMID:22711719; http://dx.doi.org/ 10.1542/peds.2011-3154 [DOI] [PubMed] [Google Scholar]
- [15].Kurosky SK, Davis KL, Krishnarajah G. Completion and compliance of childhood vaccinations in the United States. Vaccine 2016; 34: 387-94; PMID:26597149; http://dx.doi.org/ 10.1016/j.vaccine.2015.11.011 [DOI] [PubMed] [Google Scholar]
- [16].Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics 2011; 128: 848-56; PMID:21969290; http://dx.doi.org/ 10.1542/peds.2011-0400 [DOI] [PubMed] [Google Scholar]
- [17].Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics 2008; 122: 718-25; PMID:18829793; http://dx.doi.org/ 10.1542/peds.2007-0538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


