ABSTRACT
Insoluble aluminum salts such as aluminum oxyhydroxide have been used for decades as adjuvants in human vaccines, and many vaccines contain aluminum salts as adjuvants. Aluminum salt-adjuvanted vaccines must be managed in cold-chain (2–8° C) during transport and storage, as vaccine antigens in general are too fragile to be stable in ambient temperatures, and unintentional slowing freezing causes irreversible aggregation and permanent damage to the vaccines. Previously, we reported that thin-film freeze-drying can be used to convert vaccines adjuvanted with an aluminum salt from liquid suspension into dry powder without causing particle aggregation or decreasing in immunogenicity following reconstitution. In the present study, using ovalbumin (OVA)-adsorbed Alhydrogel® (i.e. aluminum oxyhydroxide, 2% w/v) as a model vaccine, we showed that the immunogenicity of thin-film freeze-dried OVA-adsorbed Alhydrogel® vaccine powder was not significantly changed after it was exposed for an extended period of time in temperatures as high as 40° C or subjected to repeated slow freezing-and-thawing. It is expected that immunization programs can potentially benefit by integrating thin-film freeze-drying into vaccine preparations.
KEYWORDS: aluminum oxyhydroxide, antibody responses, antigen desorption, particle aggregation, thin-film freeze-drying
Introduction
Some insoluble aluminum salts, e.g., aluminum oxyhydroxide and aluminum hydroxyphosphate, have been widely used as human vaccine adjuvants for decades, and many currently licensed and commercially available vaccines, including those for diphtheria-tetanus-pertussis, hepatitis A and B, pneumococcal disease, anthrax, and rabies, contain aluminum salts as adjuvants.1-3 The primary particles of aluminum oxyhydroxide and aluminum hydroxyphosphate are in the nanometer range. However, when dispersed in an aqueous solution, these particles aggregate to form large microparticles (e.g., 1–20 µm).3,4 Thus, a vaccine that is prepared by binding an antigen onto an aluminum salt is physically a liquid suspension of aluminum salt particles with antigens adsorbed on them.
Protein antigens adsorbed on aluminum salt adjuvants in liquid suspension are generally too fragile to be stable in ambient temperatures. Also, exposing the liquid suspension of aluminum salt-adjuvanted vaccines to slow freezing causes irreversible aggregation of the aluminum salt particles that damages the vaccines (e.g., permanent loss in potency and/or effectiveness).5-10 Because of these reasons, aluminum salt-adjuvanted vaccines must be maintained in cold-chain (i.e., 2–8° C) during production, handling, transport, storage, and use.11 Unfortunately, published reports and field evidence demonstrate that inadvertent freezing of vaccines during transport and storage in cold-chain is a commonplace, and not resource limited.9,12-17 This in effect causes the widespread delivery of vaccines with compromised potency, which could result in unintentional administration of suboptimal vaccines to patients,8,18-22 and/or costly waste of global vaccine supplies.14,23 Meanwhile, as the costs and/or logistical constraints of vaccine delivery associated with the cold-chain requirements significantly obstructs global vaccine access, there is increasing interest in novel approaches to vaccine stability management such as controlled temperature chain (CTC) storage.24,25 CTC allows vaccines to be managed in temperatures outside of the traditional cold-chain for a limited period of time, typically a single excursion into ambient temperature not exceeding 40° C for the duration of a specific number of days before administration.26 For example, in 2012, MenAfriVac™ became the first to be prequalified by the World Health Organization (WHO) to receive regulatory approval during mass vaccination that allowed vaccine storage at or below 40° C for up to 4 d.23,24,27 It is estimated that by using CTC, the costs of using cold-chain and the associated logistics can be reduced by 50%.23
Thin-film freeze-drying (TFFD) is a rapid freezing technology originally studied to enhance the solubility of poorly water soluble compounds, and was recently used to prepare stable submicron protein particles.28,29 In the process of TFFD, droplets of drug formulation are rapidly frozen upon impact with a cryogenically-cooled substrate to form thin films in less than a second. These thin films are then lyophilized to remove solvent in the formulation.29 Previously, we reported that vaccines adjuvanted with aluminum salts can be successfully converted from liquid suspension to dry powder by TFFD, without causing particle aggregation or decreasing in immunogenicity following reconstitution.30 In an effort to test whether converting vaccines adjuvanted with aluminum salts from liquid suspension to solid dry powder can afford managing the vaccines in CTC, here we tested the stability of a thin-film freeze-dried, aluminum oxyhydroxide-adjuvanted vaccine powder. The stability of the dry vaccine powder was evaluated in temperatures as high as 40° C for up to 6 months by measuring the particle size distribution and the immunogenicity of the vaccine powder upon reconstitution. Moreover, as the risk of unintentional exposure of vaccines to freezing temperatures remains regardless whether the vaccines are managed in cold-chain or CTC, or by any other approaches, we also tested the immunogenicity of a thin-film freeze-dried, aluminum oxyhydroxide-adjuvanted vaccine powder after it was subjected to repeated slow freezing-and-thawing cycles (and then reconstitution) in a mouse model. Data from our previous study showed that subjecting thin-film freeze-dried, aluminum salt-adjuvanted vaccines to repeated slow freezing-and-thawing cycles does not cause particle aggregation upon reconstitution.30
Commercial vaccines usually contain additional excipients such as buffer, stabilizer(s), and preservative(s) that are unique and often proprietary to each vaccine and could potentially protect the vaccines from freezing or heat stress to a certain degree. Therefore, in the present study, we prepared a model vaccine by adsorbing ovalbumin (OVA) as a model antigen onto Alhydrogel®, i.e., aluminum oxyhydroxide (2%, w/v), and used it for the immunogenicity evaluations. It is known that freezing or heating OVA denatures the protein,31,32 and denatured OVA is less immunogenic than native OVA and has different immunogenic epitopes.33,34 There are also reports that certain excipients such as high concentrations of phosphate (e.g., 40 or 100 mM) and histidine can help improve the thermal stability of vaccine, and polyols such as propylene glycol, polyethylene glycol 300, and glycerol can help make vaccine insensitive to freezing.35-37 In this study, the OVA-adsorbed Alhydrogel® liquid vaccine was prepared in the absence of those excipients, making it ideal, in our opinion, for the present study. The OVA-adsorbed Alhydrogel® dry powder was prepared by TFFD using OVA-adsorbed Alhydrogel® liquid vaccine containing 2% (w/v) trehalose.30
Results and discussion
Previously, we reported that vaccines adjuvanted with an insoluble aluminum salt, e.g., aluminum oxyhydroxide, aluminum hydroxyphosphate, or amorphous aluminum hydroxyphosphate sulfate, can be converted from liquid suspension to dry powder without causing particle aggregation or decreasing in immunogenicity following reconstitution.30 In the present study, we tested whether an aluminum salt-adjuvanted vaccine powder prepared by TFFD could be a potential candidate for CTC. CTC allows a single excursion into ambient temperature not exceeding 40° C for the duration of a specific number of days before administration. Therefore, we evaluated the immunogenicity of a model vaccine prepared by adsorbing OVA as a model antigen onto Alhydrogel® after it was converted to a dry powder by TFFD in the presence of 2% (w/v) of trehalose and then stored in temperatures as high of 40° C for up to 6 months. OVA was adsorbed onto Alhydrogel® at an OVA to aluminum ratio of 1:10 (w/w, with ∼100% antigen adsorption, data not shown).
Stability of OVA/Alhydrogel® liquid vaccine
Initially, we evaluated the immunogenicity of the OVA/Alhydrogel® vaccine in liquid suspension (with 2% trehalose, w/v, to keep the composition identical to that in the dry vaccine powder). The liquid suspension was stored at 4° C, room temperature, 30° C, and 40° C for 3 months, and the particle size and size distribution of the vaccine were determined 1 and 3 months later. Shown in Figs. 1A-B are representative particle size distribution curves of the OVA/Alhydrogel® vaccine liquid suspension after 1 month (Fig. 1A) or 3 months (Fig. 1B) of storage in various temperatures. The representative particle size distributions, expressed as X10, X50, and X90 of the OVA/Alhydrogel® vaccine liquid suspension after 1 month or 3 months of storage in various temperatures, are shown in Table 1. X10, X50, and X90 denote particle dimensions corresponding to 10%, 50%, and 90% of the cumulative undersize distribution. Aggregation was minimal after 1 month of storage, but became apparent after 3 months, as can be seen by the increase in X90 values (Fig. 1B, and Table 1). For example, the X90 values of the liquid vaccine suspension stored at 30° C increased from ∼10 µm to ∼42 µm (Table 1). For the liquid vaccine suspension stored at 30° C, there is a slight decrease in the X10 and X50 values from 1 month to 3 months of storage, but the X90 values are increased, indicating that smaller size particles may have aggregated to form bigger particles. This increase in X90 values skews the particle size distribution to the right. However, it is not clear why there was a slight decrease in the particle size for the OVA/Alhydrogel® liquid vaccine stored at 40° C (Table 1).
Figure 1.
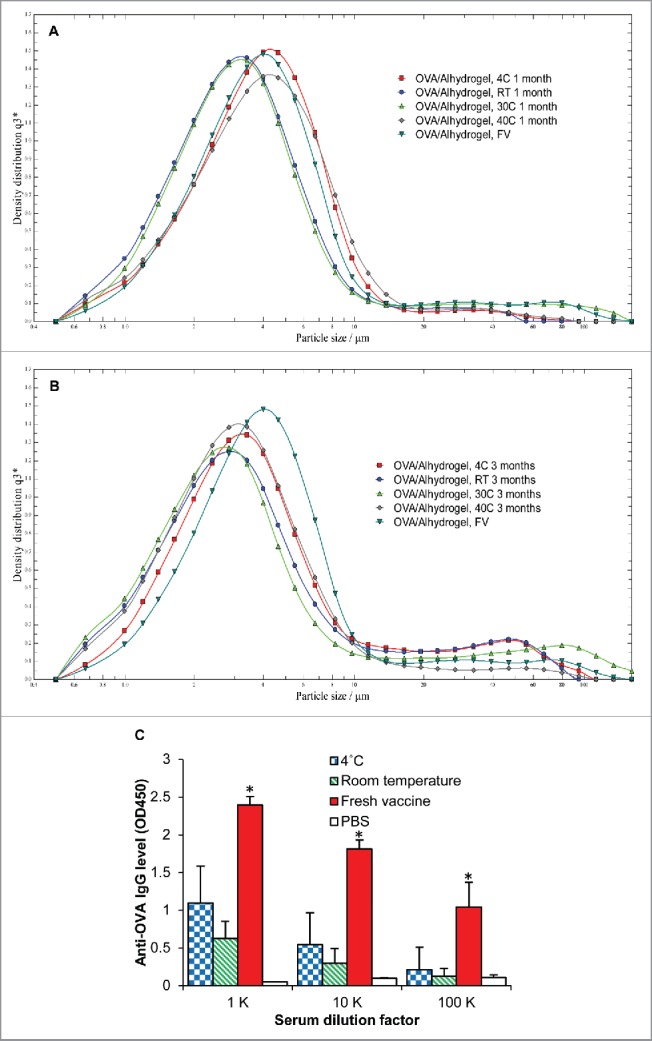
Representative particle size distribution curves of OVA/Alhydrogel® vaccine liquid suspension after 1 month (A) or 3 months (B) of storage in various temperatures. The experiment was performed with 3 replicates with similar results (RT, room temperature; FV, fresh vaccine). (C) Serum anti-OVA IgG levels in mice immunized with OVA/Alhydrogel® liquid vaccine that was stored in 4° C or room temperature for 3 months. As controls, mice were injected with sterile PBS or freshly prepared OVA/Alhydrogel® vaccine (i.e., Fresh vaccine). Female BALB/c mice (n = 5) were injected (s.c.) on days 0, 14, and 28 with 5 μg of OVA per mouse. Total anti-OVA IgG levels in serum samples were measured about 3 weeks after the third dose. Data are mean ± SD (*p < 0.05, Fresh vaccine vs. others).
Table 1.
Effect of storage temperatures on the particle size and size distribution of OVA/Alhydrogel® liquid vaccine.
| X10 (µm) | X50 (µm) | X90 (µm) | |
|---|---|---|---|
| 1 month | |||
| OVA/Alhydrogel®, FV | 1.56 ± 0.07 | 4.29 ± 0.68 | 10.77 ± 3.46 |
| OVA/Alhydrogel®, RT | 1.30 ± 0.04 | 3.03 ± 0.09 | 8.59 ± 1.57 |
| OVA/Alhydrogel®, 4°C | 1.65 ± 0.08 | 4.50 ± 0.66 | 10.35 ± 2.98 |
| OVA/Alhydrogel®, 30°C | 1.41 ± 0.04 | 3.23 ± 0.17 | 9.25 ± 1.56 |
| OVA/Alhydrogel®, 40°C | 1.50 ± 0.04 | 3.86 ± 0.55 | 9.25 ± 2.07 |
| 3 months | |||
| OVA/Alhydrogel®, FV | 1.56 ± 0.07 | 4.29 ± 0.68 | 10.77 ± 3.46 |
| OVA/Alhydrogel®, RT | 1.23 ± 0.05 | 3.19 ± 0.15 | 24.81 ± 15.07 |
| OVA/Alhydrogel®, 4°C | 1.35 ± 0.05 | 3.23 ± 0.16 | 17.38 ± 8.35 |
| OVA/Alhydrogel®, 30°C | 1.20 ± 0.06 | 3.07 ± 0.24 | 41.56 ± 23.29 |
| OVA/Alhydrogel®, 40°C | 1.04 ± 0.25 | 2.37 ± 0.51 | 6.38 ± 0.72 |
X10, X50, and X90 denote particle dimensions corresponding to 10%, 50%, and 90% of the cumulative undersize distribution (FV, fresh vaccine; RT, room temperature). Data are mean ± SD (n ≥ 3).
To evaluate the immunogenicity of the OVA/Alhydrogel® vaccine liquid suspension after 3 months of storage in room temperature or 4° C, mice were immunized (s.c.) with it, and the serum anti-OVA IgG levels induced were compared with that induced by freshly prepared OVA/Alhydrogel® vaccine liquid suspension. As shown in Fig. 1C, storing the OVA/Alhydrogel® vaccine liquid suspension in room temperature for 3 months led to significantly reduced anti-OVA IgG response, as compared with freshly prepared OVA/Alhydrogel® vaccine liquid suspension (Fig. 1C). We did not test the immunogenicity of the OVA/Alhydrogel® liquid vaccine stored at 30° C and 40° C because the vaccine does not contain any excipient that is known to improve the thermal stability of vaccines, which could explain the decrease in immunogenicity even when the liquid vaccine was stored at 4° C (Fig. 1C). This is critical as there are aluminum salt-adjuvanted vaccines in liquid suspension that were reported to be stable at elevated temperatures (e.g., Havrix™ is stable at 37° C for 3 weeks; NeisVac-C™ is stable at 40° C for 1 month).38-41 Therefore, the OVA/Alhydrogel® vaccine is a good candidate for us to test the immunogenicity of thin-film freeze-dried, aluminum salt-adjuvanted vaccine dry powder when exposed to temperatures outside the cold-chain. Data from a previous study reported that high concentrations of phosphate ion (≥ 40 mM) increase the thermal stability of aluminum oxyhydroxide-adjuvanted hepatitis B vaccine.36 When preparing the OVA-adsorbed Alhydrogel® vaccine, we dissolved the OVA in a low concentration of PBS, and the final PBS concentration in the OVA/Alhydogel® liquid vaccine was 5 mM. As expected, the low concentration phosphate ion did not render the liquid vaccine thermostable.36
Stability of OVA/Alhydrogel® vaccine dry powder
We then stored the thin-film freeze-dried OVA/Alhydrogel® vaccine powder in room temperature, 30° C, and 40° C. The dry powder was reconstituted 1, 3, and 6 months later to evaluate the particle size and size distributions, and/or the immunogenicity of the vaccine. Shown in Fig. 2 are representative particle size distribution curves of the OVA/Alhydrogel® vaccine reconstituted from thin-film freeze-dried powder that was stored in room temperature, 30° C, or 40° C for 1 month (Fig. 2A), 3 months (Fig. 2B), or 6 months (Fig. 2C), respectively. Particle aggregation was not detected after 1 month of storage in all 3 temperatures (Fig. 2A, Table 2). Slight particle aggregation was detected after 3 months of storage at 40° C, as can be seen by the increase of X90 values from 20 µm to 51 µm (Fig. 2B, Table 2). After 6 months of storage, particle aggregation became noticeable in all 3 temperatures, especially 40° C (Fig. 2C, Table 2). Similar to the particle size distribution in the liquid vaccine after storage, the increase in the particle size in the dry powder after storage can be seen in the X90 values only. This is likely because as the particles aggregate, there is a slight increase in the X90 value. An increase in the X50 value would indicate the aggregation is more severe, as will be showed in the case of subjecting the vaccine to repeated freezing-and-thawing cycles in a later section.
Figure 2.
Representative particle size distribution curves of OVA/Alhydrogel® vaccine dry powder after stored in various temperatures for 1 month (A), 3 months (B), or 6 months (C) and then reconstituted (TFFD, thin-film freeze-dried vaccine powder; RT, room temperature; FV, fresh vaccine). The experiment was performed with 3 replicates with similar results.
Table 2.
Effect of storage temperatures on the particle size and size distribution of OVA/Alhydrogel® dry vaccine powder.
| X10 (µm) | X50 (µm) | X90 (µm) | |
|---|---|---|---|
| 1 month | |||
| OVA/Alhydrogel®, FV | 2.36 ± 0.005 | 6.37 ± 0.02 | 13.79 ± 0.11 |
| OVA/Alhydrogel®, RT | 2.14 ± 0.02 | 5.96 ± 0.46 | 15.39 ± 2.54 |
| OVA/Alhydrogel®, 30°C | 1.97 ± 0.21 | 5.44 ± 0.87 | 13.89 ± 3.42 |
| OVA/Alhydrogel®, 40°C | 2.13 ± 0.33 | 6.25 ± 1.49 | 20.37 ± 4.41 |
| 3 months | |||
| OVA/Alhydrogel®, FV | 2.36 ± 0.005 | 6.37 ± 0.02 | 13.79 ± 0.11 |
| OVA/Alhydrogel®, RT | 1.73 ± 0.05 | 4.76 ± 0.06 | 15.28 ± 0.55 |
| OVA/Alhydrogel®, 30°C | 1.95 ± 0.04 | 6.29 ± 0.31 | 28.24 ± 15.39 |
| OVA/Alhydrogel®, 40°C | 1.88 ± 0.01 | 6.17 ± 0.20 | 51.17 ± 28.14 |
| 6 months | |||
| OVA/Alhydrogel®, FV | 2.36 ± 0.005 | 6.37 ± 0.02 | 13.79 ± 0.11 |
| OVA/Alhydrogel®, RT | 1.10 ± 0.01 | 3.78 ± 0.36 | 16.44 ± 5.01 |
| OVA/Alhydrogel®, 30°C | 2.19 ± 0.03 | 6.86 ± 0.18 | 30.04 ± 5.49 |
| OVA/Alhydrogel®, 40°C | 2.20 ± 0.32 | 7.53 ± 1.75 | 47.03 ± 44.14 |
X10, X50, and X90 denote particle dimensions corresponding to 10%, 50%, and 90% of the cumulative undersize distribution (FV, fresh vaccine; RT, room temperature). Data are mean ± SD (n ≥ 3).
The chemical stability of the OVA antigen in the OVA/Alhydrogel® vaccine dry powder was also monitored. SDS-PAGE data show that after the OVA/Alhydrogel® vaccine dry powder was stored for 1 month in different storage temperatures, the OVA desorbed from the OVA/Alhydrogel® dry powder was similar to the OVA desorbed from freshly prepared OVA/Alhydrogel® liquid suspension (data not shown). However, after 6 months of storage, especially at 40° C, the band intensity of the OVA desorbed from the OVA/Alhydrogel® dry powder became much weaker than that desorbed from freshly prepared OVA/Alhydrogel® liquid suspension (data not shown), which may be attributed to protein chemical degradation and/or decreased desorption of OVA from Alhydrogel® due to tight binding. There are reports that storing vaccines for an extended period of time may increase the tightness of the binding of the antigens to Alhydrogel®.42-44
Shown in Fig. 3 are the anti-OVA IgG levels in mice that were immunized with OVA/Alhydrogel® vaccine reconstituted from dry powder that was stored in various temperatures for 1 month (Fig. 3A) or 3 months (Fig. 3B). Clearly, after 3 months of storage, the serum anti-OVA IgG levels in mice immunized with OVA/Alhydrogel® reconstituted from the thin-film freeze-dried powder were not significantly different from that induced by freshly prepared OVA/Alhydrogel® vaccine (Fig. 3). Also, the serum anti-OVA IgG levels for the dry vaccine powders stored for 1 month and 3 months are not different. Due to the physical and chemical instability (e.g., particle aggregation, potential antigen degradation) detected after the OVA/Alhydrogel® dry powder was stored in various temperatures, especially at 40° C, it became meaningless to test the immunogenicity of the vaccine dry powder after 6 months of storage.
Figure 3.
Serum anti-OVA IgG levels in mice immunized with OVA/Alhydrogel® vaccine dry powder that was stored at 40° C, 30° C, or room temperature for 1 month (A) or 3 months (B) and then reconstituted. As controls, mice were injected with sterile PBS or freshly prepared OVA/Alhydrogel® vaccine (i.e., Fresh vaccine). Female BALB/c mice (n = 5) were injected (s.c.) on days 0, 14, and 28 with 5 μg of OVA per mouse. Total anti-OVA IgG levels in serum samples were measured about 3 weeks after the third dose. Data are mean ± SD (n.s., not significant).
It was previously reported that converting vaccines from liquid to dry powder can render the otherwise fragile vaccine stable in ambient temperatures.45-51 The OVA/Alhydrogel® particles in the thin-film freeze-dried powder are embedded in a glass of trehalose with a glass transition temperature (i.e., Tg) of 120° C. The Tg is significantly higher than highest temperature (i.e., 40° C) in which the vaccine dry powder was stored, allowing the vaccine to remain in a low-mobility, glassy state. However, it is worth pointing out that in the present study, while the temperatures in which the OVA/Alhydrogel® vaccine was stored was controlled, the moisture content in the powder increased with the extended period of storage, and was higher in higher temperature. For example, after 3.5 months of storage, the moisture content in the powders stored at 40° C, 30° C, and room temperature increased from the initial value of 1–3% to 7.7 ± 1.9%, 6.0 ± 0.9%, 5.4 ± 0.5%, respectively (n = 6–9, p < 0.05, values at 40° C vs. in room temperature). It is thus expected that the vaccine dry powder may potentially be stored in room temperature or above for a longer period of time, if the moisture content of the dry powder is better controlled during storage.48 Alternatively, an excipient that is less hygroscopic than trehalose and has a higher Tg value, e.g., dextran, could be potentially used to improve the storage stability.47 For instance, recently Kunda et al. showed that modified packaging system (inert N2 gas, oxygen scavengers, and desiccant sachet) can be used to control the moisture content to increase the stability of the dry powder vaccines in elevated temperatures.52 Nonetheless, data in Fig. 3 show that thin-film freeze-drying offers a potentially viable approach to manage aluminum salt-adjuvanted vaccines in CTC, because exposing the thin-film freeze-dried vaccine powder to temperatures as high as 40° C for up to 3 months did not cause significant decrease in the immunogenicity of the vaccine upon reconstitution.
Stability of OVA/Alhydrogel® dry powder vaccine to freezing
The risk of exposing a vaccine to freezing temperatures during transport and/or storage exists regardless whether the vaccine is managed in cold-chain, CTC, or in ambient temperatures. Converting aluminum salt-adjuvanted vaccines from a liquid suspension to dry powder by thin-film freeze-drying is expected to render the vaccine insensitive to freezing. The OVA/Alhydrogel® particles in the thin-film freeze-dried powder are embedded in a glass of trehalose with a Tg value of 120° C.30 Trehalose limits the mobility of the aluminum salt particles by forming glass during freezing,53 and we have previously shown using transmission electron microscopy that the OVA-adsorbed aluminum hydroxide particles are embedded in the bulk structure of trehalose, which likely prevents the particles from interacting with one another during freeze-drying process.30 Similarly, the trehalose glass is expected to make it difficult, if possible, for any subsequent slow freezing process to break the lattice that binds the antigen (i.e., OVA) to the Alhydrogel® and/or for the separated Alhydrogel® particles, if any, to aggregate to form larger and heavier particles. This explains our previous finding that subjecting the thin-film freeze-dried powder of aluminum salt-adjuvanted vaccines, e.g., GSK's Engerix-B™, to repeated slow freezing-and-thawing cycles does not cause particle aggregation upon reconstitution.30
In the present study, we further tested whether the immunogenicity of thin-film freeze-dried OVA/Alhydrogel® vaccine is affected after it was subjected to 3 cycles of repeated slow freezing-and-thawing and reconstitution. Changes (or lack of them) in the immunogenicity of a vaccine are difficult to predict. Any slight change in the physical and chemical characteristics of the antigen (i.e., OVA) and/or the vaccine if inadvertently subjected to freezing can potentially lead to a decrease in the immunogenicity of the vaccine. Shown in Table 3 are the particle size distributions of the OVA/Alhydrogel® vaccine in liquid suspension or as dry powder, before and after being subjected to 3 cycles of slow freezing-and-thawing (i.e., F/T stress). As expected,30 after the OVA/Alhydrogel® vaccine in liquid suspension was subjected to 3 cycles of slow freezing-and-thawing, the X50 value increased by 8.6-fold (i.e., from 7.5 to ∼64 μm) (Table 3), but subjecting the thin-film freeze-dried OVA/Alhydrogel® powder to the same 3 cycles of freezing-and-thawing did not cause any significant change in the particle size and size distribution (Table 3). Data in Fig. 4 show that the serum anti-OVA IgG levels in mice immunized with OVA/Alhydrogel® dry powder that was subjected to the slow freezing-and-thawing cycles are not significantly different from that in mice that were immunized with OVA/Alhydrogel® dry powder that was not subjected to the freeze-and-thaw cycles, or in mice that were immunized with freshly prepared OVA/Alhydrogel® liquid vaccine (Fig. 4). On the other hand, the serum anti-OVA IgG levels in mice that were immunized with OVA/Alhydrogel® liquid vaccine that was subjected to 3 cycles of slow freezing-and-thawing are significantly lower than that in mice immunized with the freshly prepared OVA/Alhydrogel® liquid vaccine (Fig. 4). It is clear that the OVA/Alhydrogel® liquid vaccine is sensitive to freezing, because freezing caused significant particle aggregation (Table 3) and significantly reduced its immunogenicity (Fig. 4). It was reported previously that subjecting OVA protein to 5 repeated freezing-and-thawing cycles induces OVA denaturation in a concentration dependent manner.31 However, it is unclear to what extent the reduction in the immunogenicity (i.e., anti-OVA IgG levels) of the OVA/Alhydrogel® liquid vaccine after it was subjected to repeated freezing-and-thawing cycles can be attributed to OVA denaturation in the present study. It is certain though that converting the OVA/Alhydrogel® vaccine from liquid suspension to dry powder by thin-film freeze-drying can protect the vaccine from damages induced by slow freezing (and thawing) (Fig. 4).
Table 3.
Effect of repeated freezing-and-thawing on the particle size and size distribution of OVA/Alhydrogel® vaccine dry powder.
| X10 (µm) | X50 (µm) | X90 (µm) | |
|---|---|---|---|
| OVA/Alhydrogel® FV | 2.13 ± 0.35 | 7.51 ± 1.92 | 26.66 ± 11.35 |
| OVA/Alhydrogel® TFFD | 2.83 ± 0.06 | 7.58 ± 0.21 | 22.27 ± 2.15 |
| OVA/Alhydrogel® FV, F/T | 12.35 ± 0.48 | 64.31 ± 1.86 | 137.39 ± 3.31 |
| OVA/Alhydrogel® TFFD, F/T | 1.36 ± 0.05 | 5.01 ± 0.29 | 16.15 ± 2.19 |
The particle size distributions of freshly prepared OVA/Alhydrogel® liquid vaccine (i.e., OVA/Alhydrogel® FV) or thin-film freeze-dried OVA/Alhydrogel® vaccine powder after reconstitution (i.e., OVA/Alhydrogel® TFFD) were measured before or after they were subjected to 3 cycles of freeze-and-thaw (F/T). X10, X50, and X90 denote particle dimensions corresponding to 10%, 50%, and 90% of the cumulative undersize distribution. Data are mean ± SD (n ≥ 3).
Figure. 4.
Serum anti-OVA IgG levels in mice immunized with dry powder that was subjected to 3 cycles of freezing-and-thawing (F/T) and then reconstitution or the same OVA/Alhydrogel® liquid vaccine subjected to the same 3 cycles of freezing-and-thawing. As controls, mice were immunized with freshly prepared OVA/Alhydrogel® liquid vaccine or dry powder upon reconstitution. Female BALB/c mice (n = 5) were injected (s.c.) on days 0, 14, and 28 with 5 μg of OVA per mouse. Total anti-OVA IgG levels in serum samples were measured about 3 weeks after the third immunization. Data are mean ± SD (*p < 0.05, Fresh vaccine, after F/T vs. other immunized groups).
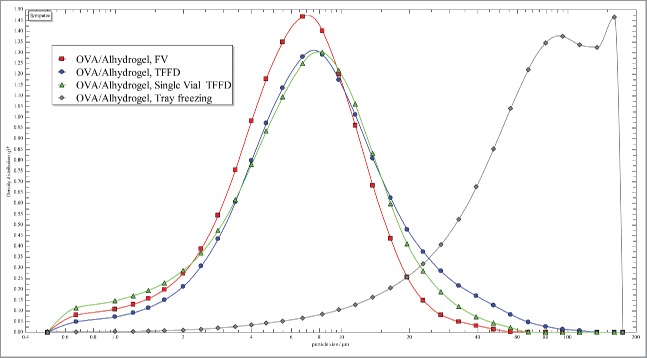
The development of aluminum salt-adjuvanted vaccines that can be managed in CTC or potentially in ambient temperatures and are not sensitive to freezing will not only help decrease the loss of global vaccine supplies due to breaches in cold-chain, but also avoid the unintentional administration of damaged, suboptimal vaccines to patients. Data in the present study confirm that TFFD is a technology that can potentially enable the development of such vaccines. The traditional TFFD process involves dropping droplets of liquid (e.g., a liquid suspension of vaccine) over a cryogenically-cooled substrate, e.g., the surface of a rotating cooled metal drum, to form frozen thin-film upon impact.28 We also showed that the inner surface of silanized glass vials can be used as a cryogenically-cooled substrate as well to drop liquid vaccine droplets onto to form frozen thin-films upon impact. Subsequently, the glass vials with the frozen vaccine thin films can be subjected to standard lyophilization to remove water. As shown in Fig. 5, the particle size distribution of OVA/Alhydrogel® reconstituted from the dry powder prepared by single vial TFFD is similar to that of the freshly prepared OVA/Alhydrogel® liquid vaccine or that reconstituted from the dry powder prepared by the traditional TFFD. As expected,5-10 slow tray-freezing followed by lyophilization led to significant aggregation of the OVA/Alhydrogel® vaccine (Fig. 5). The feasibility of TFFD in single vials is expected to allow the TFFD technology to be readily adopted in existing freeze-drying facilities with minimal modifications.
Figure 5.
Representative particle size distribution curves of OVA/Alhydrogel® vaccine before (i.e., OVA/Alhydrogel®, FV, □) and after it was subjected to thin-film freezing on a pre-cooled rotating metal drum (OVA/Alhydrogel®, TFFD, ∘), thin-film freeze-drying on the inner surface of a silalized glass vial (OVA/Alhydrogel®, single vial TFFD, Δ), or conventional tray-freezing (OVA/Alhydrogel®, Tray freezing, ◊), and then lyophilization and reconstitution (FV, fresh vaccine; TFFD, thin-film freeze-drying). The experiment was performed with 3 replicates with similar results.
It is worth mentioning that besides TFFD, other freezing and/or drying techniques are also explored to convert protein products into dry powders.54-57 Spray freeze-drying has been particularly studied to convert aluminum salt-adjuvanted vaccines into dry powder using various excipients (e.g., mixture of glycine, mannitol, and dextran up to 10% w/v).58,59 Besides the approach of converting aluminum salt-adjuvanted vaccine from liquid suspension to dry powder, there are also reports that certain excipients such as high concentrations of phosphate anions and histidine can help improve the thermal stability of vaccine. In addition, polyols such as propylene glycol and glycerol can help make vaccine insensitive to freezing.21,35-37 Neither one of those approaches alone will likely be universally applicable to all vaccines, but it is certain, as demonstrated in the present study, that technologies are available to make aluminum salt-adjuvanted vaccines thermostable.
In conclusion, using a model vaccine prepared by adsorbing OVA onto Alhydrogel®, we confirmed that the vaccine dry powder prepared using TFFD is stable in temperatures as high as 40° C for 3 months and no longer sensitive to slow freezing. Global immunization program may potentially benefit by integrating TFFD into vaccine preparations.
Materials and methods
Preparation of OVA-adsorbed Alhydrogel® vaccine dry powder
The OVA-Alhydrogel® vaccine was prepared by adding 25 mL of Alhydrogel® (2% w/v, or 10 mg/mL aluminum, manufactured by Brenntag, and supplied by InvivoGen, San Diego, CA) into a 50 mL tube followed by the addition of 25 mL of an OVA solution (1 mg/mL in phosphate-buffered saline (PBS), pH 7.4, 10 mM, Sigma-Aldrich, St. Louis, MO) and 1 g of trehalose (as a cryoprotectant) (Sigma-Aldrich) to obtain a final formulation with 2% (w/v) of trehalose, ∼1% (w/v) of Alhydrogel®, and 0.5 mg/mL of OVA, with a low 5 mM concentration of PBS. The size and size distribution of the OVA-adsorbed Alhydrogel® in suspension was determined using a Sympatec Helos laser diffraction instrument equipped with an R3 lens (Sympatec GmbH, Germany). The vaccine suspension was converted into a dry powder using our previously reported thin-film freeze-drying method.28-30 The powder was dried using a VirTis AdVantage Bench Top Lyophilizer (The VirTis Company, Inc. Gardiner, NY). Lyophilization was performed over 72 h at pressures less than 200 mTorr, while the shelf temperature was gradually ramped up from −40° C to 26° C. After lyophilization, the solid dry vaccine powder was transferred into a sealed container and stored in a desiccator.60 The moisture content in the dried powder was determined using a Karl Fisher Titrator Aquapal III from CSC Scientific Company (Fairfax, VA).
To test the feasibility of preparing dry vaccine powder by thin-film freeze-drying in a single vial, silanized glass vials were used to provide the cryogenic surface for rapidly freezing the liquid vaccine. Glass vials were submerged into liquid nitrogen to make the cryogenic surface, with the mouth and neck of the vials remaining in the air (not submerged). Using a pipette or syringe, the OVA/Alhydrogel® vaccine liquid suspension with 2% of trehalose (w/v) (0.25–0.5 mL) was added drop wise into the glass vials. Thin films of vaccine were quickly formed by ultra-rapid freezing on the inner surface of the vial, which were then lyophilized as mentioned above. After lyophilization, the glass vials were quickly crimped, transferred to a sealed container, and stored in a desiccator in room temperature before further use. As a control, OVA/Alhydrogel® vaccine liquid suspension in a glass vial was placed on the shelf on the lyophilizer. The shelf temperature was gradually decreased from room temperature to −40° C, and then ramped from −40° C to 26° C over 72 h at pressures of less than 200 mTorr. To test whether the above mentioned freeze-drying method significantly affected the particle size distribution of the vaccine, the lyophilized dry vaccine powder was reconstituted using water. The particle size and particle size distribution were determined using a Sympatec Helos laser diffraction instrument. As controls, the particle size distribution profiles of freshly prepared OVA/Alhyrogel® vaccine and the same OVA/Alhydrogel® vaccine that was subjected to TFFD using the previously reported method and then reconstitution were also evaluated.30
Stability study
The OVA/Alhydrogel® dry powder was crimp-sealed with aluminum seals over rubber lids in silanized glass vials, which were then stored in desiccators placed in room temperature (i.e., 22–24° C) or in incubators (30° C or 40° C). As a control, OVA/Alhydrogel® liquid suspension that was crimp-sealed with aluminum seals over rubber lids in silanized glass vials was also stored at 4°C, room temperature, 30° C, and 40° C. At various time points (i.e., 1, 3, or 6 months later), the particle size and size distribution of the vaccine, in liquid suspension or reconstituted from dry powder, were determined using a Sympatec Helos laser diffraction instrument. The immunogenicity of the vaccine was evaluated in a mouse model.
Chemical stability of the OVA protein desorbed from OVA/Alhydrogel®
SDS-PAGE electrophoresis was used to evaluate the chemical integrity of the OVA desorbed from Alhydrogel® after storage as mentioned above. Briefly, OVA/Alhydrogel® dry vaccine powder containing 1 mg of OVA was reconstituted and incubated in the presence of sodium citrate (Sigma-Aldrich) to a final concentration of 10% (w/v) at 37° C overnight. The samples were then centrifuged to collect the supernatant. Control samples included reconstituted OVA-adsorbed Alhydrogel® incubated similarly but in the absence of sodium citrate, freshly prepared OVA/Alhydrogel®, and OVA alone. Samples were mixed with a Laemmli sample buffer (Sigma-Aldrich) before applied to 7.5% Mini-PROTEAN® TGX™ precast polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Precision plus™ protein standards (Bio-Rad) were also run along with the samples at 130 V for 1 h. The gels were then stained in a Bio-Safe™ Coomassie blue staining solution and scanned using a Kodak Image Station 440CF (Rochester, NY).
Repeated freezing-and-thawing studies
Thin-film freeze-dried OVA/Alhydrogel® powder was placed at −20° C for 8 h and then thawed at 4° C for 16 h for 3 cycles. After the third cycle, the dry powder was reconstituted to (i) measure its particle size and size distribution using a Sympatec Helos laser diffraction instrument and (ii) evaluate its immunogenicity in a mouse model. As a control, OVA/Alhydrogel® vaccine in liquid suspension was also subjected to 3 cycles of freezing-and-thawing, and its particle size distribution and immunogenicity were evaluated.
Animal studies
All animal studies were conducted following the US. National Research Council Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at The University of Texas at Austin approved the animal protocol. Female BALB/c mice, 6–8 weeks of age, were from Charles River Laboratories, Inc. (Wilmington, MA). Mice (n = 5) were subcutaneously (s.c.) injected with OVA/Alhydrogel®, freshly prepared, after storage, or after freezing-and-thawing cycles. Mice were immunized on days 0, 14, and 28, and the dose of OVA was 5 μg per mouse. As controls, mice were s.c. injected with sterile PBS or OVA alone in PBS. Sixteen days after the third dose, mice were bled, and the anti-OVA IgG levels in serum samples were determined using enzyme-linked immunosorbent assay (ELISA).61 Briefly, EIA/RIA flat bottom, NUNC Maxisorp, 96-well plate (Thermo Fisher) were coated with 1 ng/μl of OVA solution in carbonate buffer (0.1 M, pH 9.0) overnight at 4° C. Plates were blocked with horse serum for one hour before adding the blood serum. Horse radish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin (IgG, 5000-fold dilution, Southern Biotechnology Inc., Birmingham, AL) was added into the plates, and the presence of bound antibody was detected in the presence of 3,3′,5,5′-tetramethylbenzidine solution (TMB, Sigma–Aldrich). The absorbance was read at 450 nm.
Statistics
Statistical analyses were conducted using analysis of variance followed by Fischer's protected least significant difference procedure. A p-value of < 0.05 (2-tail) was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Hugh Smyth at UT Austin for kindly allowing us to use the Sympatec Helos laser diffraction instrument available in his laboratory.
Funding
This work was supported in part by a grant from the US. National Institute of Allergy and Infectious Diseases (AI105789 to ZC), a University of Texas Transform Seed Grant (to ZC and ROW), and the Alfred and Dorothy Mannino Fellowship in Pharmacy at UT Austin (to ZC).
References
- [1].USFDA Vaccines Licensed for Immunization and Distribution in the US with Supporting Documents. 2013. Available from http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm (accessed October 28, 2016). [Google Scholar]
- [2].Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol 1999; 17:1075-81; PMID:10545912; http://dx.doi.org/ 10.1038/15058 [DOI] [PubMed] [Google Scholar]
- [3].O'Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng 2001; 18:69-85; http://dx.doi.org/ 10.1016/S1389-0344(01)00101-0 [DOI] [PubMed] [Google Scholar]
- [4].Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine 2007; 25:825-33; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.039 [DOI] [PubMed] [Google Scholar]
- [5].Kristensen D, Chen D, Cummings R. Vaccine stabilization: Research, commercialization, and potential impact. Vaccine 2011; 29:7122-4; PMID:21651941; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.070 [DOI] [PubMed] [Google Scholar]
- [6].Zapata MI, Feldkamp JR, Peck GE, White JL, Hem SL. Mechanism of freeze-thaw instability of aluminum hydroxycarbonate and magnesium hydroxide gels. J Pharm Sci 1984; 73:3-8; PMID:6694078; http://dx.doi.org/ 10.1002/jps.2600730103 [DOI] [PubMed] [Google Scholar]
- [7].Organization WH. The effects of freezing on the appearance, potency, and toxicity of adsorbed and unadsorbed DTP vaccines. Weekly Epidemiological Record 1980; 55:385-92 [Google Scholar]
- [8].Diminsky D, Moav N, Gorecki M, Barenholz Y. Physical, chemical and immunological stability of CHO-derived hepatitis B surface antigen (HBsAg) particles. Vaccine 1999; 18:3-17; PMID:10501230; http://dx.doi.org/ 10.1016/S0264-410X(99)00149-8 [DOI] [PubMed] [Google Scholar]
- [9].Milhomme P. Cold chain study: danger of freezing vaccines. Can Commun Dis Rep 1993; 19:33. [PubMed] [Google Scholar]
- [10].Boros CA, Hanlon M, Gold M, Roberton D. Storage at− 3 C for 24 h alters the immunogenicity of pertussis vaccines. Vaccine 2001; 19:3537-42; PMID:11348721; http://dx.doi.org/ 10.1016/S0264-410X(01)00063-9 [DOI] [PubMed] [Google Scholar]
- [11].Milstien JB, Um Kartoglu, Zaffran M, Galazka AM. Temperature sensitivity of vaccines. World Health Organization 2006. Available from http://apps.who.int/iris/bitstream/10665/69387/1/WHO_IVB_06.10_eng.pdf [Google Scholar]
- [12].Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine 2007; 25:3980-6; PMID:17382434; http://dx.doi.org/ 10.1016/j.vaccine.2007.02.052 [DOI] [PubMed] [Google Scholar]
- [13].Nelson CM, Wibisono H, Purwanto H, Mansyur I, Moniaga V, Widjaya A. Hepatitis B vaccine freezing in the Indonesian cold chain: evidence and solutions. Bulletin World Health Organization 2004; 82:99-105; PMID:15042231 [PMC free article] [PubMed] [Google Scholar]
- [14].Theo W, Steven T, Nan M, Chris M, John CC. A vaccine cold chain freezing study in PNG highlights technology needs for hot climate countries. Vaccine 2007; 25:691-7; PMID:16968657; http://dx.doi.org/ 10.1016/j.vaccine.2006.08.028 [DOI] [PubMed] [Google Scholar]
- [15].Miller N, Harris M. Are childhood immunization programmes in Australia at risk? Investigation of the cold chain in the Northern Territory. Bulletin World Health Organization 1994; 72:401; PMID:8062398 [PMC free article] [PubMed] [Google Scholar]
- [16].Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine 2007; 25:1328-33; PMID:17157419; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.092 [DOI] [PubMed] [Google Scholar]
- [17].Matthias D, Robertson J, Garrison M, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine 2007; 25:3980-6; PMID:17382434; http://dx.doi.org/ 10.1016/j.vaccine.2007.02.052 [DOI] [PubMed] [Google Scholar]
- [18].Nygaard UC, Samuelsen M, Aase A, Løvik M. The capacity of particles to increase allergic sensitization is predicted by particle number and surface area, not by particle mass. Toxicological Sci 2004; 82:515-24; PMID:15456925; http://dx.doi.org/ 10.1093/toxsci/kfh287 [DOI] [PubMed] [Google Scholar]
- [19].Davaalkham D, Ojima T, Wiersma S, Lkhagvasuren T, Nymadawa P, Uehara R, Watanabe M, Oki I, Nakamura Y. Administration of hepatitis B vaccine in winter as a significant predictor of the poor effectiveness of vaccination in rural Mongolia: evidence from a nationwide survey. J Epidemiol Community Health 2007; 61:578-84; PMID:17568048; http://dx.doi.org/ 10.1136/jech.2006.051375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mansoor O, Pillans P. Vaccine adverse events reported in New Zealand 1990-5. N Z Med J 1997; 110:270-2; PMID:9269289 [PubMed] [Google Scholar]
- [21].Frankel AE, Kuo SR, Dostal D, Watson L, Duesbery NS, Cheng CP, Cheng HJ, Leppla SH. Pathophysiology of anthrax. Front Biosci 2009; 14:4516-24; http://dx.doi.org/ 10.2741/3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Menon P, Sahai G, Joshi V, Murthy R, Boparai M, Thomas A. Field trial on frozen and thawed tetanus toxoid. Indian J Med Res 1976; 64:25-32; PMID:1270098 [PubMed] [Google Scholar]
- [23].Patrick L, Simona Z, Carole T-B, Harouna DM, Placide G, Oumar YB, Michel Z. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull WHO 2014; 92:86-92; PMID:24623901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zipursky S, Djingarey MH, Lodjo J-C, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine 2014; 32:1431-5; PMID:24559895; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lydon P, Zipursky S, Tevi-Benissan C, Djingarey MH, Gbedonou P, Youssouf BO, Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Organ 2014; 92:86-92; PMID:24623901; http://dx.doi.org/ 10.2471/BLT.13.123471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].WHO Controlled temperature chain. World Health Organization. Available from http://www.who.int/immunization/programmes_systems/supply_chain/resources/Controlled-Temperature-Chain-FAQ.pdf (accessed December 21, 2016). [Google Scholar]
- [27].WHO. Revolutionary meningitis vaccine breaks another barrier; first to gain approval to travel outside cold chain. World Health Organization 2012. Available from http://www.who.int/immunization/newsroom/menafrivac_20121114/en/ (accessed August 22, 2016). [Google Scholar]
- [28].Engstrom JD, Lai ES, Ludher BS, Chen B, Milner TE, Williams RO 3rd, Kitto GB, Johnston KP. Formation of stable submicron protein particles by thin film freezing. Pharm Res 2008; 25:1334-46; PMID:18286357; http://dx.doi.org/ 10.1007/s11095-008-9540-4 [DOI] [PubMed] [Google Scholar]
- [29].Zhang M, Li H, Lang B, O'Donnell K, Zhang H, Wang Z, Dong Y, Wu C, Williams RO 3rd. Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur J Pharm Biopharm 2012; 82:534-44; PMID:22974985; http://dx.doi.org/ 10.1016/j.ejpb.2012.06.016 [DOI] [PubMed] [Google Scholar]
- [30].Li X, Thakkar SG, Ruwona TB, Williams RO, Cui Z. A method of lyophilizing vaccines containing aluminum salts into a dry powder without causing particle aggregation or decreasing the immunogenicity following reconstitution. J Control Release 2015; 204:38-50; PMID:25735896; http://dx.doi.org/ 10.1016/j.jconrel.2015.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Watanabe T, Kitabatake N, Doi E. Method for the Accurate Measurement of Freezing-induced Denaturation of Ovalbumin with 5, 5′-Dithiobis-(2-nitrobenzoic acid). Biosci Biotechnol Biochem 1994; 58:359-62; http://dx.doi.org/ 10.1271/bbb.58.359 [DOI] [Google Scholar]
- [32].Photchanachai S, Mehta A, Kitabatake N. Heating of an ovalbumin solution at neutral pH and high temperature. Biosci Biotechnol Biochem 2002; 66:1635-40; PMID:12353621; http://dx.doi.org/ 10.1271/bbb.66.1635 [DOI] [PubMed] [Google Scholar]
- [33].Weijers M, Barneveld PA, Cohen Stuart MA, Visschers RW. Heat-induced denaturation and aggregation of ovalbumin at neutral pH described by irreversible first-order kinetics. Protein Sci 2003; 12:2693-703; PMID:14627731; http://dx.doi.org/ 10.1110/ps.03242803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koch C, Jensen SS, Oster A, Houen G. A comparison of the immunogenicity of the native and denatured forms of a protein. APMIS 1996; 104:115-25; PMID:8619913; http://dx.doi.org/ 10.1111/j.1699-0463.1996.tb00696.x [DOI] [PubMed] [Google Scholar]
- [35].Braun LJ, Tyagi A, Perkins S, Carpenter J, Sylvester D, Guy M, Kristensen D, Chen D. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine 2009; 27:72-9; PMID:18973782; http://dx.doi.org/ 10.1016/j.vaccine.2008.10.027 [DOI] [PubMed] [Google Scholar]
- [36].Jezek J, Chen D, Watson L, Crawford J, Perkins S, Tyagi A, Jones Braun L. A heat-stable hepatitis B vaccine formulation. Hum Vaccin 2009; 5:529-35; PMID:19556877; http://dx.doi.org/ 10.4161/hv.5.8.8600 [DOI] [PubMed] [Google Scholar]
- [37].Xue H, Yang B, Kristensen DD, Chen D. A freeze-stable formulation for DTwP and DTaP vaccines. Hum Vaccin Immunother 2014; 10:3607-10; PMID:25668668; http://dx.doi.org/ 10.4161/21645515.2014.980195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peetermans J. Production, quality control and characterization of an inactivated hepatitis A vaccine. Vaccine 1992; 10:S99-S101; PMID:1335671; http://dx.doi.org/ 10.1016/0264-410X(92)90557-Z [DOI] [PubMed] [Google Scholar]
- [39].Wiedermann G, Ambrosch F, André F, Delem A, D'hondt E, Safary A. Thermostability of an inactivated hepatitis A vaccine stored at 37 C for one week. J Medical Virol 1994; 44:442-; PMID:7897377; http://dx.doi.org/ 10.1002/jmv.1890440423 [DOI] [PubMed] [Google Scholar]
- [40].Wiedermann G, Ambrosch F. Immunogenicity of an inactivated hepatitis A vaccine after exposure at 37 C for 1 week. Vaccine 1994; 12:401-2; PMID:8023546; http://dx.doi.org/ 10.1016/0264-410X(94)90113-9 [DOI] [PubMed] [Google Scholar]
- [41].Lee S-M, Petermann R, Porte Q, Berezuk G, Crowe B, Shirtz J. Long-term thermal stability of group C meningococcal polysaccharide-tetanus toxoid conjugate vaccine. Hum Vaccin 2007; 3:27-32; PMID:17264684; http://dx.doi.org/ 10.4161/hv.3.1.3749 [DOI] [PubMed] [Google Scholar]
- [42].Hansen B, Sokolovska A, HogenEsch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 2007; 25:6618-24; PMID:17681647; http://dx.doi.org/ 10.1016/j.vaccine.2007.06.049 [DOI] [PubMed] [Google Scholar]
- [43].Hansen B, Soung G, Song L, Egan PM, Capen R, HogenEsch H, Mancinelli R, Hem SL. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine 2009; 27:888-92; PMID:19071182; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.078 [DOI] [PubMed] [Google Scholar]
- [44].Vessely C, Estey T, Randolph TW, Henderson I, Cooper J, Nayar R, Braun LJ, Carpenter JF. Stability of a trivalent recombinant protein vaccine formulation against botulinum neurotoxin during storage in aqueous solution. J Pharmaceutical Sci 2009; 98:2970-93; PMID:18680175; http://dx.doi.org/ 10.1002/jps.21498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Geeraedts F, Saluja V, ter Veer W, Amorij J-P, Frijlink HW, Wilschut J, Hinrichs WL, Huckriede A. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J 2010; 12:215-22; PMID:20195930; http://dx.doi.org/ 10.1208/s12248-010-9179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Amorij J, Meulenaar J, Hinrichs W, Stegmann T, Huckriede A, Coenen F, Frijlink H. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine 2007; 25:6447-57; PMID:17673338; http://dx.doi.org/ 10.1016/j.vaccine.2007.06.054 [DOI] [PubMed] [Google Scholar]
- [47].Murugappan S, Patil HP, Kanojia G, ter Veer W, Meijerhof T, Frijlink HW, Huckriede A, Hinrichs WL. Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. Eur J Pharm Biopharm 2013; 85:716-25; ; http://dx.doi.org/ 10.1016/j.ejpb.2013.07.018 [DOI] [PubMed] [Google Scholar]
- [48].Hassett KJ, Cousins MC, Rabia LA, Chadwick CM, O'Hara JM, Nandi P, Brey RN, Mantis NJ, Carpenter JF, Randolph TW. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm 2013; 85:279-86; PMID:23583494; http://dx.doi.org/ 10.1016/j.ejpb.2013.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hassett KJ, Meinerz NM, Semmelmann F, Cousins MC, Garcea RL, Randolph TW. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur J Pharm Biopharm 2015; 94:220-8; PMID:25998700; http://dx.doi.org/ 10.1016/j.ejpb.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Saboo S, Tumban E, Peabody J, Wafula D, Peabody DS, Chackerian B, Muttil P. Optimized Formulation of a Thermostable Spray-Dried Virus-Like Particle Vaccine against Human Papillomavirus. Mol Pharm 2016; 13:1646-55; PMID:27019231; http://dx.doi.org/ 10.1021/acs.molpharmaceut.6b00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mensink MA, Nethercott MJ, Hinrichs WL, van der Voort Maarschalk K, Frijlink HW, Munson EJ, Pikal MJ. Influence of Miscibility of Protein-sugar lyophilizates on their storage stability. AAPS J 2016; 18(5):1225-32:1-8; PMID:26377333 [DOI] [PubMed] [Google Scholar]
- [52].Kunda NK, Wafula D, Tram M, Wu TH, Muttil P. A stable live bacterial vaccine. Eur J Pharm Biopharm 2016; 103:109–117; PMID:27020530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm 2000; 203:1-60; PMID:10967427; http://dx.doi.org/ 10.1016/S0378-5173(00)00423-3 [DOI] [PubMed] [Google Scholar]
- [54].Pisal S, Wawde G, Salvankar S, Lade S, Kadam S. Vacuum foam drying for preservation of LaSota virus: effect of additives. AAPS PharmSciTech 2006; 7:60; PMID:17025241; http://dx.doi.org/ 10.1208/pt070360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen D, Kapre S, Goel A, Suresh K, Beri S, Hickling J, Jensen J, Lal M, Preaud JM, Laforce M, et al.. Thermostable formulations of a hepatitis B vaccine and a meningitis A polysaccharide conjugate vaccine produced by a spray drying method. Vaccine 2010; 28:5093-9; ; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.112 [DOI] [PubMed] [Google Scholar]
- [56].Maa YF, Zhao L, Payne LG, Chen D. Stabilization of alum-adjuvanted vaccine dry powder formulations: mechanism and application. J Pharm Sci 2003; 92:319-32; PMID:12532382; http://dx.doi.org/ 10.1002/jps.10294 [DOI] [PubMed] [Google Scholar]
- [57].Yu Z, Johnston KP, Williams RO 3rd. Spray freezing into liquid versus spray-freeze drying: influence of atomization on protein aggregation and biological activity. Eur J Pharm Sci 2006; 27:9-18; PMID:16188431; http://dx.doi.org/ 10.1016/j.ejps.2005.08.010 [DOI] [PubMed] [Google Scholar]
- [58].Clausi A, Cummiskey J, Merkley S, Carpenter JF, Braun LJ, Randolph TW. Influence of particle size and antigen binding on effectiveness of aluminum salt adjuvants in a model lysozyme vaccine. J Pharm Sci 2008; 97:5252-62; PMID:18398901; http://dx.doi.org/ 10.1002/jps.21390 [DOI] [PubMed] [Google Scholar]
- [59].Clausi AL, Morin A, Carpenter JF, Randolph TW. Influence of protein conformation and adjuvant aggregation on the effectiveness of aluminum hydroxide adjuvant in a model alkaline phosphatase vaccine. J Pharm Sci 2009; 98:114-21; PMID:18506831; http://dx.doi.org/ 10.1002/jps.21433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Watts AB, Wang YB, Johnston KP, Williams RO 3rd. Respirable low-density microparticles formed in situ from aerosolized brittle matrices. Pharm Res 2013; 30:813-25; PMID:23229856; http://dx.doi.org/ 10.1007/s11095-012-0922-2 [DOI] [PubMed] [Google Scholar]
- [61].Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release 2010; 141:93-100; PMID:19729045; http://dx.doi.org/ 10.1016/j.jconrel.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]