Abstract
Enrichment of fat grafts with adipose-derived stem cells (ASCs) has gained popularity due to promising preliminary results. Herein, we present two patients who were treated with ASC-enriched fat grafts following mastectomy and breast reconstruction with implants. Both exhibited favorable outcomes achieving a significant improvement of breast defects and asymmetries.
Keywords: Adipose-derived stem cells, fat transfer, breast surgery
Introduction
Breast cancer is the most common cancer among female population with an estimated lifetime risk of 12.32% in the USA [1]. Surgical options such as complete or partial mastectomy and lumpectomy have been used for the treatment of breast cancer for many years but recently oncoplastic procedures and breast reconstruction have gained ground mainly due to their tremendous impact on the psychological status of the patients [2]. Breast reconstruction can be performed in one or two stages, either immediately after the primary intervention or after a particular time period by using mostly two methods; prosthetic implants with or without acellular dermal matrix and autologous tissue.
Autologous fat grafting refers to the transplantation of fat tissue from one part of the body to another and is an FDA-approved option for breast reconstruction in the USA [3]. However, complications such as low graft survival, formation of cysts, microcalcifications and fat necrosis have necessitated the improvement of this approach [4,5].
Due to their inherent properties, adipose-derived stem cells (ASCs) are believed to exhibit improved grafting results, thus holding a great promise for future translation into clinical practice [6]. Their multipotent nature and particularly the fact that they promote angiogenesis are considered responsible for their favorable outcomes [7]. Nevertheless, the use of ASCs is not yet FDA-approved [6].
Herein, we report our first cases of breast reconstruction that was treated with fat grafting enriched with ASCs. Informed consent from both patients as well as approval from our Institutional Review Board was obtained. The study protocol was in accordance with the World Medical Association Declaration of Helsinki.
Patient 1
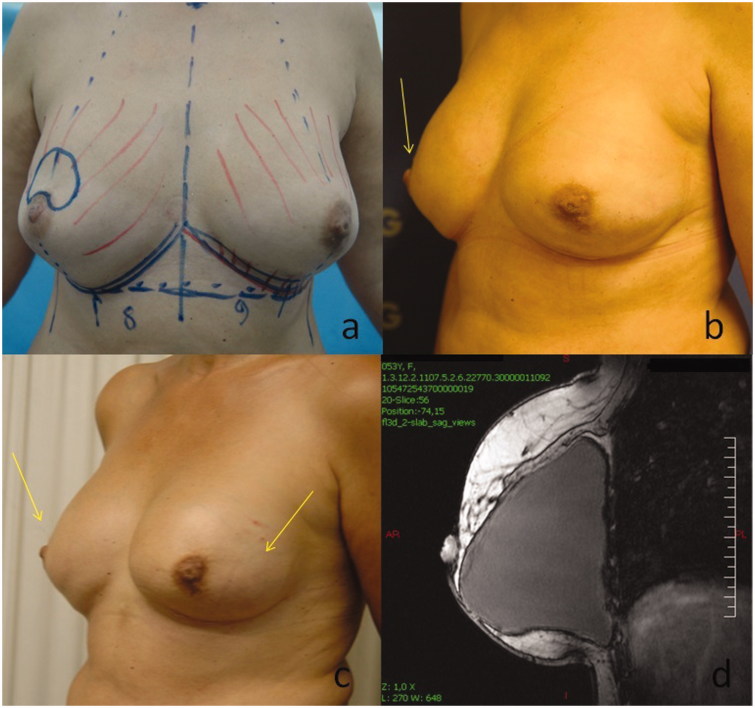
A 51-year-old woman with a history of severe fibrocystic disease and a family history of breast cancer-attributable deaths (three maternal aunts died of breast cancer) underwent bilateral prophylactic nipple-areola-complex (NAC) sparing mastectomies, as requested by the patient regardless the genetic counseling. A two-staged reconstruction started immediately after the initial operation. Initially, Allergan 133MV14 500 cm3 expanders (Allergan, Inc., Irvine, CA) were used bilaterally and placed in a complete submuscular pocket (below the pectoralis major, serratus anterior and the insertion of rectus abdominus muscles) without acellular dermal matrix; 5 months later were replaced by Allergan 410LX 455gr implants. However, hollowness over the NAC had remained as well as contour irregularities (Figure 1(a)–(d)) and, therefore, cell-enriched fat grafting near the NAC was considered an option to achieve a more satisfying result. Tulip low-pressure syringe lipoaspiration system was used to obtain 520 cm3 of lipoaspirate from the hypogastrium and the thighs. Half of this amount was processed with the Celution System (Cytori Therapeutics, San Diego, CA) yielding about 6 cm3 suspension of ASCs. The remaining fat (260 cm3) was washed in the Celution System to remove blood and waste and then enhanced with the concentrated ASCs. The resulting cell-enriched fat graft was injected into and around the defect area in multiple planes through blunt-tipped 17-gauge cannulae. As for the right breast, 50 cm3 of cell-enriched fat grafting were injected into the hollowness over the NAC and another 100 cm3 in the upper pole, while 90 and 20 cm3 were used in the upper and the lower pole of the left breast, respectively (Figure 2(a)). There was significant contour improvement in both breasts that remained stable at 3 and 22 months of follow-up (Figure 2(b)–(d)). Two minor complications occurred in the left breast; one episode of cellulitis 4 months post grafting that resolved with IV antibiotics uneventfully, and the development of a slightly painful lump 10 months post grafting that turned out to be a liponecrotic cyst after excision biopsy. Both had no impact on the cosmetic effect.
Figure 1.
(a) Before mastectomy. (b–d) Images showing the remaining hollowness over the NAC (arrows) and contour irregularities of the breasts after mastectomy and two-staged breast reconstruction.
Figure 2.
(a) Image showing the injection sites of ASC-enriched fat grafts. Significant contour improvement after cell-enriched fat grafting at 3 (b) and 22 months (c) of follow-up (d) MRI scan of the right breast showing no shape irregularities after 22 months.
Patient 2
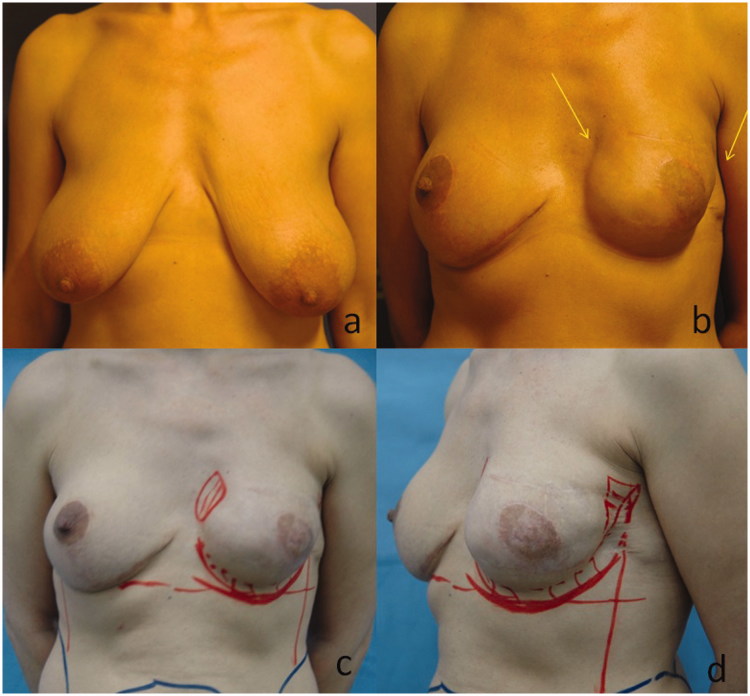
A 43-year-old female presented with a suspicious area of microcalcifications on the left breast. After the stereotactic biopsy turned out to be positive for adenocarcinoma, she underwent wired guided local excision using an oncoplastic approach. An inferior pedicle breast reduction was performed combined with mastopexy to the opposite breast in order to achieve symmetry. Histology report, however, revealed multifocal disease with foci of lobular carcinoma in situ as well as infiltrative ductal carcinoma. Thus, a left simple mastectomy with sentinel lymph node biopsy was implemented. Sentinel lymph node was negative on frozen section and we proceeded with a two-stage immediate breast reconstruction; at first, Allergan 133MV 400 cm3 expander was used in a complete submuscular pocket without acellular dermal matrix that was replaced by Allergan 410LX 365gr anatomic implant after 6 months. During the second stage, submammary augmentation of the right breast with Allergan CML 170gr implant was also implemented. After 4 months, left NAC reconstruction was attempted using a skate flap and full-thickness skin graft from the inguinal area. Patient was lost to follow-up for 1 year and presented again with marked asymmetry due to shape distortion of the left breast that clinically resembled – though not actually – a capsular contracture (Figure 3(a,b)). Using the same method as described above, we obtained 580 cm3 of lipoaspirate from the hypogastrium and the thighs, 260 cm3 of which were processed and yielded 7 cm3 of ASCs that subsequently enriched the remaining fat. Skin adherence to the lateral aspect of the left breast was initially released. Overall, 320 cm3 of enriched fat was grafted in the periphery of the reconstructed breast in order to improve the contour and correct the tethering of the skin (Figure 3(c,d)). No complications occurred and a satisfying cosmetic result was retained at 3 and 19 months follow-up (Figure 4(a–h)).
Figure 3.
(a) View of the breasts at presentation. (b) Image showing a shape distortion of the left breast 16 months after mastectomy and two-staged breast reconstruction (c), (d) injection sites of ASC-enriched fat grafts.
Figure 4.
(a, d) Before fat transfer, (b, e) after 3 months and (c, f) after 19 months. MRI scan of the breasts before fat grafting revealing no evidence of capsular contracture (g). A second MRI, 19 months following fat grafting revealing favorable and durable outcomes (h).
Discussion
Enrichment of autologous fat grafting with ASCs during breast reconstruction following breast surgery seemed to be a feasible option with favorable and durable outcomes in selected patients. In both cases, the implemented method achieved a substantial cosmetic improvement of breast defects and asymmetries with minimal and non-significant complications.
ASCs are derived from the stromal component of adipose tissues, and have the potential to proliferate extensively as well as differentiate into various cell lineages such as adipogenic, osteogenic, myogenic, chondrogenic and vascular endothelial cell types [6,8–10]. In addition, easy harvest of ASCs, low donor site morbidity [10] and the fact that adipose tissue aspirates are considered to contain much higher stem cell concentration compared to bone marrow ones [11], render these cells an attractive supplemental tool in improving long term fat grafting survival. Preclinical studies have also suggested that ASCs may contribute to the regeneration of adipose tissue and sustain graft survival by promoting angiogenesis [7].
As a result, cell-assisted lipotransfer (CAL) technique has been introduced that refers to the ASC enhancement of lipoinjections. The main idea is the isolation of the stromal vascular fraction (SVF) cells from one half of the aspirated fat and the combination of these primitive cells with the other half, thus resulting in ASC-rich fat [7]. In enriching fat grafts, a variety of commercially available devices have been proposed, including the Celution System.
Celution device is an FDA-approved system that uses a single-use sterile disposable set for tissue processing and the Celase® processing enzyme reagent for isolation of cells from aspirated adipose tissue. After performing a wet test to ensure the integrity of the closed system, the tissue is introduced into the processing canister where it is weighed and then washed with the lactated Ringers solution to remove the residual wetting solution and extravasated blood. Based on the tissue weight, the device calculates the amount of Celase reagent to be used [12]. The tissue is continuously agitated during enzymatic digestion of the connective tissue. After completion of the digestion, the ASC fraction is pumped into a centrifuge chamber where it is washed and concentrated. The final cell product can then be aspirated from the chamber and combined with the remaining aspirated fat [12].
Compared to other non-enzymatic processing methods, Celution System seems to provide significant advantages to the lipoaspirates processed by this device; higher number of isolated cells per lipoaspirate, higher concentration of putative stromal cell-containing population, increased number of primitive cells and finally, increased expression of stem cell markers, such as Nanog, Sox-2 and c-Kit in ASC-enriched samples [13].
There is currently increasing evidence that ASCs may significantly confer better cosmetic outcomes and therefore, several clinical trials are still ongoing. Yoshimura et al. first described the CAL technique in 40 women for cosmetic purposes achieving satisfactory results. After mean fat injection of 270 ml, breast volumes showed an increase of 100–200 ml, while no major complications occurred apart from minimal postoperative atrophy and cyst formation or microcalcification in four patients [5]. The same author suggested CAL as a feasible method for replacing complicated breast implants with favorable outcomes [14]. Overall, the largest cohort of patients undergoing CAL has been described by Yoshimura et al. demonstrating encouraging preliminary outcomes. Amongst them, 269 have been performed on breasts; 177 cosmetic breast augmentations, 52 breast implant replacements, 40 postmastectomy breast reconstructions and the rest on the face, hip and hands of selected patients [15].
Of note, a large prospective, multicenter clinical trial enrolled 67 patients who were treated with ASC-enriched fat grafting for breast deficits following breast conservation therapy. Based on MRI sequence assessment, 54 of 65 patients yielded a significant improvement in breast contours at 12 months follow-up compared to baseline. No local cancer recurrences were reported and no major complications occurred apart from the development of injection site cysts in ten patients [16].
Recently, Dos Anjos et al. reported 77 cell-enhanced fat grafting procedures, of which 21 contained a low dose of SVF cells, while the rest were enriched with a 10-fold higher concentration [17]. Interestingly, while the breast volume increase was lower in the high dose-SVF group compared with the low dose group during the immediate postoperative period, the former group exhibited better long-term results, as suggested by a higher volume retention index after 1.5 years (75% vs. 50%, p < .05) [17]. Additionally, Kamakura and Ito used cell-enriched fat grafting for breast reconstruction in 20 women reporting a mean increase in breast circumference of 3.3 cm 9 months after surgery without major complications [18].
Although the aforementioned studies endorse the use of ASCs, their efficacy and superiority over existing techniques has already been questioned. A prospective controlled clinical trial compared stem cell enriched fat grafts (stem cell group) with pure ones (control group) in secondary breast reconstruction with the former showing better volumetric persistence; nevertheless statistical significance was not achieved [19]. Similarly, another prospective study compared patients undergoing water-assisted lipotransfer with or without stem cell enrichment. At 6 months’ follow-up, MRI volumetry of the whole graft did not show significant differences between the two groups [20].
Furthermore, two more concerns have been raised regarding the implementation of stem cell-based techniques in breast reconstruction surgeries [21]. Possible contamination of the graft during the extracorporeal procedure of stem cell enrichment necessitates special attention to the sterilization process [21]. Another controversial issue is the putative crosstalk between stem cells and breast carcinogenesis. It has been suggested that stem cells may induce molecular cascades and changes in the breast microenvironment that may promote de novo carcinogenesis, proliferation of residual cancer cells and metastasis [22]. The available data from preclinical and clinical studies have been rather contradictory, although results from preclinical models support the aforementioned theories, clinical studies have not yet reported an increased risk of recurrence [22–24]. The number of transplanted stem cells is also believed to play an important role in the observed discordances. However, the crucial cut-off value cannot be determined with the existing evidence [23].
Nowadays, advances in technical aspects of autologous fat grafting have limited the incidence of complications. As noted by Groen et al., total complication rate was lower (8.4%, 95% CI 7.6–9.1) than other reconstructive breast procedures [4]. Particularly, fewer cysts (6.9%) and calcifications (5.2%) were seen, whereas fat necrosis (9%) was slightly increased [4]. Of note, our first patient appeared with a liponecrotic cyst on excision biopsy. Although this incident did not affect the cosmetic outcome, it highlights the need for careful manipulation and precise technique when applying the graft. Fat needs to be harvested with minimal trauma to adjacent tissues, using low negative pressure and the least possible exposure to the air prior to grafting. Blunt-tipped small caliber infiltration cannulae (17–18G; maximum diameter 1.5 mm) may be used for fat grafting, always taking into consideration that injection of particles larger than 3 mm is associated with increased risk of fat necrosis, calcification and oil cyst formation [4].
Overall, the implementation of ASCs for breast reconstruction following breast surgery seems a promising approach. Future research should be oriented to both preclinical and clinical studies in order to elucidate all the contradictory points in this field [23]. Another interesting perspective would be the simultaneous use of ASCs as carriers of chemotherapeutic drugs with locoregional action [22]. Ideally, results from multicenter randomized controlled trials could provide a basis for formulating specific guidelines for a personalized approach in the clinical milieu [25,26]. In that context, we should currently act in accordance with the ‘ASAPS/ASPS Position Statement on Stem Cells and Fat Grafting’, which suggests stem cell therapies be conducted ‘within clinical studies under Institutional Review Board approval, including compliance with all guidelines for human medical studies’ [27].
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- Kaya B, Serel S. Breast reconstruction. Exp Oncol. 2013;35:280–286. [PubMed] [Google Scholar]
- Schmauss D, Machens HG, Harder Y. Breast reconstruction after mastectomy. Front Surg. 2015;2:71. doi: 10.3389/fsurg.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen JW, Negenborn VL, Twisk DJ, et al. Autologous fat grafting in onco-plastic breast reconstruction: a systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthet Surg. 2016;69:742–764. doi: 10.1016/j.bjps.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips BJ, Marra KG, Rubin JP. Healing of grafted adipose tissue: current clinical applications of adipose-derived stem cells for breast and face reconstruction. Wound Repair Regen. 2014;22(Suppl1):11–13. doi: 10.1111/wrr.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs (Print) 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Pikula M, Marek-Trzonkowska N, Wardowska A, et al. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther. 2013;13:1357–1370. doi: 10.1517/14712598.2013.823153. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Wulur I, Alfonso Z, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Hicok KC, Shanahan R, et al. The Celution(R) System: automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care (New Rochelle) 2014;3:38–45. doi: 10.1089/wound.2012.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenis R, Lazzaro L, Calabrese S, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther. 2015;6:2. doi: 10.1186/scrt536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Asano Y, Aoi N, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–175. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273. doi: 10.2217/17460751.4.2.265. [DOI] [PubMed] [Google Scholar]
- Perez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- Dos Anjos S, Matas-Palau A, Mercader J, et al. Reproducible volume restoration and efficient long-term volume retention after point-of-care standardized cell-enhanced fat grafting in breast surgery. Plast Reconstr Surg Glob Open. 2015;3:e547. doi: 10.1097/GOX.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthetic Plast Surg. 2011;35:1022–1030. doi: 10.1007/s00266-011-9727-7. [DOI] [PubMed] [Google Scholar]
- Tissiani LA, Alonso N. A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int. 2016;2016:2636454. doi: 10.1155/2016/2636454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- McArdle A, Senarath-Yapa K, Walmsley GG, et al. The role of stem cells in aesthetic surgery: fact or fiction? Plast Reconstr Surg. 2014;134:193–200. doi: 10.1097/PRS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli A, Scioli MG, Gentile P, et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3:345. doi: 10.1186/2193-1801-3-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet HJ, Orbay H, Wong MS, et al. The oncologic safety of breast fat grafting and contradictions between basic science and clinical studies: a systematic review of the recent literature. Ann Plast Surg. 2015;75:471–479. doi: 10.1097/SAP.0000000000000604. [DOI] [PubMed] [Google Scholar]
- Schweizer R, Tsuji W, Gorantla VS, et al. The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem Cells Int. 2015;2015:120949. doi: 10.1155/2015/120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Zhao W, Wang Z, et al. Potential drawbacks in cell-assisted lipotransfer: a systematic review of existing reports (review). Mol Med Rep. 2016;13:1063–1069. doi: 10.3892/mmr.2015.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumboeck A, Giovanoli P, Plock JA. Fat grafting and stem cell enhanced fat grafting to the breast under oncological aspects – recommendations for patient selection. Breast. 2013;22:579–584. doi: 10.1016/j.breast.2013.05.006. [DOI] [PubMed] [Google Scholar]
- ASAPS/ASPS position statement on stem cells and fat grafting. Aesthet Surg J. 2011;31:716–717. doi: 10.1177/1090820X11416919. [DOI] [PubMed] [Google Scholar]