Abstract
Chronic pain is a disease that encompasses both sensory and emotional elements. Opioids are highly effective analgesics because they target both of these elements, by inhibiting pain pathways and alleviating negative affect (including depression) by engaging reward or hedonic pathways. Unfortunately, chronic opioid use is limited by the development of unwanted side effects, such as tolerance, hyperalgesia, and abuse liability. Thus, the challenge of providing effective pain treatment while minimizing these unwanted side effects is an ongoing issue with significant clinical and societal impact. In this review, we posit that neuroinflammation within the central nervous system is a shared phenomenon between chronic pain and opioids that contributes to pain sensitization and negative affect. The implications for pain progression, addiction liability, and alternative treatment strategies are discussed.
Pain is a multidimensional experience encompassing both sensory and emotional elements. The emotional component, or how much the pain is ‘bothersome’, significantly impacts the quality of life of the sufferer and has been argued to be the greatest metric for quality of life. Chronic pain may be considered an epidemic in our society affecting 25% of Americans, and quality of life of chronic pain patients is reported to be lower than other disorders such as heart failure, renal failure, and depression [1]. Opioid analgesics are highly effective and the most commonly prescribed class of medications in the United States, with a significant portion of these prescriptions dedicated to long-term opioid therapy. However, the conundrum of providing effective pain treatment while minimizing prescription opioid abuse has become a major challenge. There are alarming statistics on therapeutic opioid misuse, although the abuse of therapeutic opioids is largely a result of diversion (obtaining opioids from family and friends) [2]. In the United States in 2013, over 37% of all drug overdose deaths could be attributed to prescription opioids. The United States Center for Disease Control and Prevention (CDC) reported, for the first time, that drugs surpassed the leading cause of death in 16 states over car accidents. However, not everyone exposed to opioid analgesics becomes addicted to their medication. The question that needs to be addressed is what makes patients susceptible to opioid misuse. We will argue that a commonality of chronic pain and opioid misuse is dysphoria and the occurrence of neuroinflammation. This immune response contributes to dysphoria and drives negative affect by disrupting mesocorticolimbic dopaminergic circuitry responsible for reward and motivation. Dampened reward circuitry is a causative factor in many psychiatric illnesses including depression, and neuroinflammation also exacerbates depressive symptoms in these illnesses. We posit that both chronic pain and chronic opioids induce neuroinflammation in limbic brain structures leading to the genesis of negative affective states (anxiety and depression). Thus, chronic opioid regimens may exacerbate the negative affect associated with chronic pain, and patients taking opioids to treat pain may become reliant on opioids to alleviate this negative affect, making them susceptible to opioid misuse and consequently the transition to addictive-like behaviors.
Are chronic pain patients at risk for opioid addiction?
Opioid prescription abuse occurs in both chronic pain populations and in individuals that obtain opioids from friends and/or family, where misusers may migrate to heroin (as a cheaper source). It is therefore disturbing that physicians prescribe opioids for patients with chronic pain with little (if any) assessment of the level of risk for medication abuse [3], although new CDC guidelines are enforcing change.
The high abuse rates of therapeutic opioids have fueled a strong debate on the treatment practices of using prescription opioids and whether pain is a major factor in addiction susceptibility. Epidemiological reports and case studies have suggested that pain is ‘protective’ against the development of opioid dependence and addiction where rates of abuse are low at 3.75% even when considering patients with prior illicit drug use [4]. This argument is validated by preclinical studies showing that opioids are self-administered only at doses that reverse mechanical hypersensitivity in a model of neuropathic pain and that anti-inflammatory drugs reduce opioid consumption in a model of chronic inflammatory pain [5][for review, 5]. However, others argue that opioid misuse and aberrant drug related behavior in chronic pain patients range from 21 to 29% [6], and iatrogenic (physician-induced) addiction may be as high as 26% [2]. Relevant to this argument are data showing that chronic pain patients that are opioid tolerant (defined as >60 mg morphine equivalent) or exhibit high catastrophizing, distress intolerance and/or impulsivity scores have greater likelihood of abusing their opioid medication [7,8]. The claim that chronic pain increases opioid addiction-like behaviors is supported by preclinical research where persistent inflammatory pain (complete Freund’s adjuvant, CFA) caused animals to self-administer more heroin than non-pain animals [9] or reduced the number of conditioning sessions required to produce drug-conditioned place preference [10]. However, these studies cannot differentiate between the negative reinforcement associated with pain relief from the hedonic reward produced by the opioid, which obfuscates conclusions regarding motivated behavior within the context of chronic pain. Similarly, although animals experiencing CFA-induced inflammatory pain maintained intravenous self-administration of morphine (0.2 mg/kg/infusion, but not 1 mg/kg/infusion) at the same levels as control animals, rats in pain demonstrated significantly greater morphine-seeking behavior during withdrawal [11]. Thus, it remains unclear if chronic pain patients have higher susceptibility to prescription opioid misuse and addiction. However, chronic pain patients with comorbid depression are more likely to develop opioid abuse than non-depressed patients, which is correlated with higher opioid doses and longer duration of use [12]. Thus, as with non-chronic pain populations, there are recognized susceptibilities for developing addictive behaviors. Identifying the endophenotypes that lead to opioid misuse susceptibilities, and the neurobiology that encodes them, should be a current focus of addiction medicine (Figure 1a). The research reviewed above indicates that underlying affect or motivational states have significant influence on abuse liability, and thus function within emotional or affective circuits should be considered when contemplating the impact of pain on addictive behaviors.
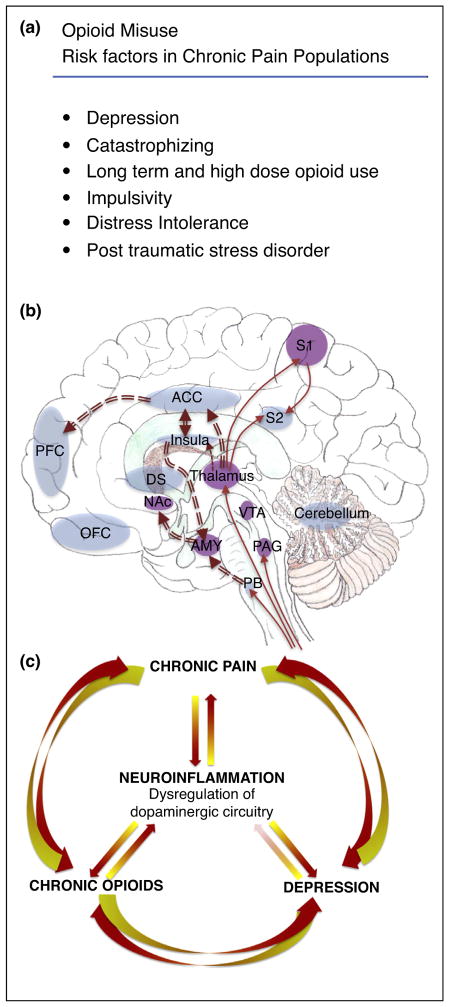
Figure 1.
Overlapping neuroanatomical and functional circuitry involved in chronic pain and opioid misuse. Neuroinflammation occurs in mesolimbic circuitry important for reward and motivation. A. Risk factors identified for opioid misuse in chronic pain populations. B. Chronic pain and chronic opioids induce neuroinflammation in brain regions associated with sensory and affective components. Red solid line arrows indicate sensory pain pathways. Red double line arrows indicate affective component of pain pathways. Purple brain regions denote where changes in neuroinflammation was detected by protein or mRNA of Iba-1 (a marker of microglia), cd11b (marker of microglia) or pro-inflammatory cytokines. Many structures overlap with opioid reward/reinforcement and negative affect/aversion such as the anterior cingulate cortex (ACC), prefrontal cortex (PFC), insula, thalamus, nucleus accumbens (NAc), and amygdala (AMY). C. Schematic cartoon of the central role neuroinflammation has in producing dysregulation of dopaminergic circuitry in many psychiatric disorders including chronic opioid use, chronic pain and depression. Note in panels B and C, the absence of purple shading does not reflect that neuroinflammation is absent in these structures, but rather many of these regions have not been examined. DS: dorsal striatum, OFC: orbitofrontal cortex, PAG: periaqueductal grey, PB: parabrachial nucleus, S1: primary somatosensory cortex, S2: secondary somatosensory cortex, VTA: ventral tegmental area.
Overlapping and interacting brain regions contribute to opioid addiction and the subjective experience of pain
Seeking pain relief and seeking reward are two motivational states that engage overlapping brain structures and circuitry involved in affect and salience (e.g. anterior cingulate cortex, dorsal and ventral striatum, habenula, and amygdala (Figure 1b)). The mesocorticolimbic circuit plays a central role in motivational processes, and incorporates dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc). Dopamine release within this circuit acts as an incentive salience signal underlying reinforcement learning produced by environmental (e.g. sex, food), rewarding drugs, as well as aversive stimuli. VTA dopamine neurons respond to salient, motivational stimuli by burst firing, which is suppressed by tonic GABA input. VTA GABAergic neurons and other GABAergic input from the rostromedial tegmental nucleus (RMTg), NAc, and ventral pallidum, tonically inhibit dopamine neuronal activity. Opioids produce reinforcing effects, in part, by inhibiting GABAergic tone on VTA dopamine neurons (disinhibition). Indeed, rodents will self-administer opioids directly into the VTA [13] and opioids locally injected into the VTA will produce a conditioned place preference [14,15]. Pain relief is also reinforcing and engages dopaminergic circuitry. For example, local administration of a peripheral nerve block in a rodent model of persistent pain stimulates dopamine release in the NAc and produces a place preference [16]. Thus, untangling the motivational salience of opioids, which are both analgesic and rewarding, in the context of pain is a challenging task. Moreover, the analgesic and rewarding effects of opioids engage overlapping circuits. Above we discussed the evidence that supports a role for dopamine release in the reinforcing properties of opioids. Next we present evidence that demonstrates engagement of the mesocorticolimbic dopamine system contributes to opioid analgesic efficacy, as well.
The pain experience is comprised of three components: a sensory component that relays information regarding location and intensity, a cognitive component, and an affective component influenced by context, culture, and experience. Opioids are effective in diminishing both the sensory and affective components of pain. For example, post-operative patients receiving opioid analgesics will describe being satisfied with their level of pain because it does not bother them although they often still report ‘feeling’ pain. The ability of opioids to dissociate the emotional component of pain is partly caused by their ability to release dopamine in the NAc, hence alleviating the affective dimension of pain. Preclinical models have demonstrated this concept directly where morphine suppression of formalin-induced (but not tail flick) pain was attenuated by destruction of VTA dopaminergic neurons [17] and morphine injected directly into the VTA or NAc produced analgesia in this same pain model [18], suggesting that on-going or persistent pain states engage dopaminergic circuitry, and activation of the system is sufficient to produce analgesia. However, dopamine release in the NAc has also been proposed to facilitate pain [19]. This discrepancy may be caused by the topographical organization of heterogeneous dopaminergic inputs within the NAc and differential activation of specific medium spiny projection neuronal populations. Indeed, optogenetic stimulation of dynorphin-expressing cells in the ventral NAc shell elicits aversive behaviors via kappa opioid receptor activation, and in contrast photo-stimulation of dorsal NAc shell dynorphin cells induces a kappa opioid receptor-mediated place preference [20••]. How VTA dopamine neurons projecting to the NAc encodes aversion or reward remains opaque, but there is evidence that plasticity within the NAc is contributive to both chronic pain states and chronic opioids [21,22].
Hypodopaminergia driven by neuroinflammation is a shared phenomenon between chronic pain and opioid addiction
Depression and mood disorders are co-morbid conditions common in both chronic pain and opioid addiction patient groups. The prevalence of depression in chronic pain patients ranges from 30 to 80%, depending on the pain etiology. The relevance of this co-morbidity is highlighted in that chronic pain is second only to bipolar disorder as the major cause of suicide among all medical illnesses [23,24]. Similarly, opioid dependent patients have a high incidence of depression. Indeed, opioid use in the absence of pain has been shown to induce a protracted abstinence syndrome [25,26], and can increase the likelihood of major depressive episodes [27]. There is also recent evidence that long-term prescription opioid use is associated with both new onset and recurrence of depression [28], as well as enhances the risk of developing treatment resistant depression [29•]. This is particularly concerning as the short-term benefits of opioids that alleviate the sensory and affective symptoms of pain may lead to longer duration of opioid use, which theoretically could increase risk of adverse outcomes, including depression. The interface of pain and opioid addiction is complex given the high comorbidity of pain with negative affect and that opioids provide relief of both negative affect and pain. One, therefore, cannot rule out the possibility that reported opioid misuse in the chronic pain population may be due, in part, to pain patients taking opioids to relieve the negative affect associated with chronic pain that may be exacerbated by opioid withdrawal, and where the relief of dysphoric states becomes an important driver of opioid seeking behavior. In support of this theory, clinical studies investigating subjective reporting in electronic diaries obtained from heroin addicts identified feeling sad as a driver of opioid craving [30], whereas subjects on buprenorphine or methadone maintenance continued therapy because they want to feel ‘normal’ [31,32]. We have recently argued that the opioid abstinence syndrome and the anxiogenic and negative affective states that evolve following abstinence [25,33] are likely salient factors for learning that opioids relieve negative affect, which drives subsequent addictive behaviors [34•].
The mechanisms underlying the high incidence of mood disorder co-morbidity are poorly understood. However, one commonality to chronic pain, depression, and opioid addiction is diminished or perturbed function of mesolimbic dopaminergic circuitry. A common endophenotype in both chronic pain and opioid addicts is anhedonia with a lack of appreciation of natural rewards such as sex and food. An elegant review recently argued that reward deficiency and anti-reward systems contributes to the vulnerability of suicidal acts and behaviors in chronic pain populations [24](8). There exists convincing evidence that dysfunction of mesolimbic circuitry (including dopamine neurotransmission) precipitates mood disorders and impairs motivated behavior [35,36]. Opioid dependent states induce hypo-function of the VTA-NAc mesolimbic dopaminergic projections [37]. This is evidenced by the loss of conditioned place preference produced by intra-VTA administration of a selective mu opioid agonist DAMGO [38], an effect also evident in (opioid naïve) chronic pain states [14]. Preclinical and human subject research have identified strong correlations between dysfunction of mesolimbic circuitry and chronic pain [39](13). We have shown that drug (morphine and cocaine)-evoked release of dopamine is blunted in chronic pain states [14]. This decrease in release was correlated with a blunting of reward assessed by conditioned place preference to both cocaine and opioids that we show may be caused by an increase in GABAergic neuronal excitability in the VTA. We proposed that GABA tone on dopaminergic neurons was increased by the occurrence of chronic pain leading to a blunting effect of dopaminergic transmission. Subsequently, others have reported that chronic pain causes a decrease in basal dopamine release within the NAc, and this decrease in release was positively correlated with a decrease in VTA dopaminergic neuronal firing [40••]. However, a blunting of dopaminergic transmission is not always observed with reports that pain causes an increase in dopamine neuronal burst firing that is associated with an increase in basal release [41]. Differences in outcomes may be caused by the topographical organization of heterogeneous dopamine neurons, where cells in the dorsal and ventral VTA are inhibited or excited by noxious electrical stimuli, respectively [42]. Topographical organization of the VTA also exists in the rostral caudal axis and likely dictates projection target and function [43]. Moreover, opioid agonists can have bidirectional effects in the VTA, whereby locally applied opioid agonists can activate or inhibit different populations of VTA dopamine neurons with unique projection targets [44,45], and may contribute to the diversity of behavioral responses recorded following manipulation of VTA dopamine output. Nevertheless, imaging studies on human volunteers and chronic pain patients suggest that perturbation of the mesolimbic-cortical dopaminergic reward circuitry contributes to chronic pain syndromes, including altered activity within the VTA [46]. Moreover, a high prevalence of chronic pain is common in disorders linked with deficits in the dopamine system, including disorders of mood and affect, substance abuse, and Parkinson disease.
The mechanism underlying the hypofunction of dopaminergic circuitry in either opioid dependent states or chronic pain remains elusive; however, one commonality that may link these disorders is an increase in brain-derived neurotrophic factor (BDNF). In models of depression, including social defeat, BDNF is up-regulated in the NAc [47]. Additionally, phasic, but not tonic, activation of VTA dopamine neurons projecting to the NAc, but not the prefrontal cortex (PFC), induced susceptibility for a depressive phenotype, which was associated with increased BDNF in the NAc [48]. Selective deletion of BDNF from the VTA-NAc circuitry prevented the development of social defeat stress [47]. It must be emphasized that an increase in BDNF in the NAc contributing to depression is a site-specific effect. In the hippocampus, many pharmacotherapies that alleviate depressive symptoms elevate BDNF, and social defeat stress model of depression decreases BDNF in the hippocampus [49]. Several clinical studies have implicated the BDNF Val66Met polymorphism as associated with severity of depressive symptoms [50] and risk of bipolar disorder [51]. However, a recent genome-wide association study (GWAS) did not identify the BDNF locus as associated with depression in a large Chinese cohort [52]. BDNF is also a critical modulator of VTA dopamine function in opioid dependence. Intra-VTA administration of BDNF was shown to be a negative modulator of opioid reward and blunted morphine-evoked dopamine release [53]. Opioid withdrawal elevated BDNF mRNA and protein levels in the VTA [54]. Interestingly, BDNF serum levels were altered in heroin-dependent patients after 26 weeks of abstinence, which correlated with protracted withdrawal symptoms (appearance of anxiety and depressive symptoms) [33]. Similarly, serum BDNF was proposed as a biomarker or determinant of bipolar disorder or depressive symptoms [55,56]. BDNF is the most abundant growth factor in the brain and while it is primarily derived from neurons, microglia, the resident immune cells in the brain, also have the capacity to release BDNF [57]. In the spinal cord, microglial-derived BDNF was responsible for the pain sensitization associated with chronic opioid exposure [58]. We posit that a commonality in both chronic pain and opioid misuse is occurrence of neuroinflammation in the basal ganglia and other limbic structures that contributes to the hypofunction of the mesolimbic system that may be a common pathophysiology triggering the incidence of depression in both disorders (Figure 1c).
The central innate immune system, comprised of microglia and astrocytes, is “activated” in response to a number of events associated with chronic pain, including trauma to peripheral or spinal nerves. Indeed, spinal cord glia are intrinsically involved in cell-to-cell signaling pathways producing chronic pain [57], at least in males [59•]. While much of what we know about glial contribution to chronic pain emanates from studies in the spinal cord, more recent reports have found evidence for widespread glial activation throughout pain and affective brain circuits [60,61]. Thus, activated glia are poised to contribute to both the sensory, cognitive, and emotional aspects of chronic pain. The innate immune system is also reported to modulate opioid-induced effects within the central nervous system and may account for the reduced efficacy of opioids in the treatment of neuropathic pain. Critical experiments in support of this hypothesis observed that drugs disrupting glial functioning prevented and/or reversed pain behavior in a number of chronic pain models and improved opioid effectiveness [62]. Chronic opioids, similar to chronic pain, induces neuroinflammation and increases the production of pro-inflammatory cytokines such as IL-1b and TNFa throughout the neuroaxis. Cytokines can affect glutamatergic homeostasis (17) that leads to morphine tolerance and opioid-induced hyperalgesia. Blocking glial activation interferes with the development of many of the unwanted side effects associated with chronic opioid use such as hyperalgesia, without interfering with analgesia [58]. We hypothesize that the mechanism by which immune cells increase pain and impair reward circuitry is shared between chronic pain and chronic opioid conditions. Pertinent to this thesis, BDNF released from spinal microglia and subsequent down-regulation of the K+/Cl− co-transporter KCC2 is at least partially responsible for pain hypersensitivities in a chronic pain model [63]. Moreover, both chronic pain and chronic opioids activate microglia and increase BDNF expression in the VTA, a phenomenon that impairs mesolimbic dopamine function and reward behavior [14,54]. Inhibiting neuroinflammation recovered opioid-evoked DA release and reward behavior in chronic pain animals [14]. Similarly, the antibiotic ceftriaxone, which inhibits microglial activation, normalized the effect of morphine on NAc shell D1 and D2 neurons [64]. Thus, glial activation in the mesocorticolimbic system is a common pathophysiology triggering the genesis of depression in both chronic pain and opioid dependence. Indeed, inhibitors of neuroinflammation (including the tetracycline microglial inhibitor minocycline) were shown to be effective adjunct therapy in major depressive disorders [65] and improved the affective dimension of chronic neuropathic pain without altering its intensity [66•].
Conclusion
Despite significant advances in our understanding of the mechanisms underlying chronic pain, the basis for its persistence and resistance to most medical treatments are not known. One of the mechanisms proposed to underlie synaptic plasticity pertinent to the development and persistence of chronic pain includes neuronal–glia interactions, particularly products released from activated microglia. Opioids are often prescribed to manage chronic pain, though their side effect profile, including hyperalgesia, opponent processes and addiction liability, raise questions regarding their clinical utility over the long term. In this review, we explore the evidence that both chronic pain and chronic opioids engage similar neuroimmune mechanisms in the brain and spinal cord that contribute to pain sensitization and negative affect. This suggests that the use of opioids may, paradoxically, exacerbate the progression of chronic pain by enhancing the immune responses, an idea that is supported by both preclinical and clinical reports. Moreover, both chronic pain and chronic opioids promote neuroinflammation in limbic brain structures leading to the genesis of negative affective states. This negative affect may increase the likelihood of opioid misuse and addictive-like behaviors in the chronic pain population. Therapies that restore function in reward circuitry, such as inhibitors of neuroinflammation, may be effective co-therapies to enhance the analgesic efficacy of opioids and restore affect to mitigate addiction liability in the chronic pain population.
Acknowledgments
Funding was provided by the National Institutes of Health (Grant K99DA040016, to A.M.W.T), the Shirley and Stefan Hatos Center (A.M.W.T), a gift from Shirley Hatos (C.M.C.) and the Department of Defense (C.M.C).
Footnotes
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan AT. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med (Malden, Mass) 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 5.Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(Pt A):60–64. doi: 10.1016/j.neulet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- 7.McHugh RK, Weiss RD, Cornelius M, Martel MO, Jamison RN, Edwards RR. Distress intolerance and prescription opioid misuse among patients with chronic pain. J Pain. 2016;17:806–814. doi: 10.1016/j.jpain.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vest N, Reynolds CJ, Tragesser SL. Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav. 2016;60:184–190. doi: 10.1016/j.addbeh.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Hipolito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, et al. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area mu opioid receptors. J Neurosci. 2015;35:12217–12231. doi: 10.1523/JNEUROSCI.1053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Tao W, Hou YY, Wang W, Lu YG, Pan ZZ. Persistent pain facilitates response to morphine reward by downregulation of central amygdala gabaergic function. Neuropsychopharmacology. 2014;39:2263–2271. doi: 10.1038/npp.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou YY, Cai YQ, Pan ZZ. Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced mecp2 repression of glua1 in rat central amygdala. J Neurosci. 2015;35:3689–3700. doi: 10.1523/JNEUROSCI.3453-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 13.Devine DP, Wise RA. Self-administration of morphine, damgo, and dpdpe into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor AM, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci. 2015;35:8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980;12:965–968. doi: 10.1016/0091-3057(80)90460-8. [DOI] [PubMed] [Google Scholar]
- 16.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan MJ, Franklin KB. 6-hydroxydopamine lesions of the ventral tegmentum abolish d-amphetamine and morphine analgesia in the formalin test but not the tail flick test. Brain Res. 1990;519:144–149. doi: 10.1016/0006-8993(90)90072-j. [DOI] [PubMed] [Google Scholar]
- 18.Manning BH, Morgan MJ, Franklin KB. Morphine analgesia in the formalin test: evidence for forebrain and midbrain sites of action. Neuroscience. 1994;63:289–294. doi: 10.1016/0306-4522(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 19.Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Exp Neurol. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–1077. doi: 10.1016/j.neuron.2015.08.019. First paper showing regional specificity of dynorphinergic neurons in the nucleus accumbens that drive aversion and reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–222. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 24.Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz PE, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, Befort K, Ouagazzal AM, Kieffer BL. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology. 2014;39:2694–2705. doi: 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker JA, Kieffer BL, Le Merrer J. Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, thc and alcohol. Addict Biol. 2016 doi: 10.1111/adb.12405. http://dx.doi.org/10.1111/adb.12405Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 27.Scherrer JF, Salas J, Copeland LA, Stock EM, Schneider FD, Sullivan M, Bucholz KK, Burroughs T, Lustman PJ. Increased risk of depression recurrence after initiation of prescription opioids in noncancer pain patients. J Pain. 2016;17:473–482. doi: 10.1016/j.jpain.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherrer JF, Salas J, Copeland LA, Stock EM, Ahmedani BK, Sullivan MD, Burroughs T, Schneider FD, Bucholz KK, Lustman PJ. Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann Fam Med. 2016;14:54–62. doi: 10.1370/afm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Scherrer JF, Salas J, Sullivan MD, Schneider FD, Bucholz KK, Burroughs T, Copeland L, Ahmedani B, Lustman PJ. The influence of prescription opioid use duration and dose on development of treatment resistant depression. Prev Med. 2016;91:110–116. doi: 10.1016/j.ypmed.2016.08.003. This study demonstrates an association between opioid use and recurrence of depression in at risk populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rossler A, Smieskova R, Lang UE, Walter M. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Winstock AR, Lintzeris N, Lea T. Should i stay or should i go? Coming off methadone and buprenorphine treatment. Int J Drug Policy. 2011;22:77–81. doi: 10.1016/j.drugpo.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Bentzley BS, Barth KS, Back SE, Aronson G, Book SW. Patient perspectives associated with intended duration of buprenorphine maintenance therapy. J Subst Abuse Treat. 2015;56:48–53. doi: 10.1016/j.jsat.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, Jiang H, Zhang Q, Du J, Wang Y, Zhao M. Brain-derived neurotrophic factor serum levels in heroin-dependent patients after 26 weeks of withdrawal. Compr Psychiatry. 2016;65:150–155. doi: 10.1016/j.comppsych.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 34•.Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 2016;5 doi: 10.12688/f1000research.8369.1. This review presents the hypothesis that opioid addiction is perpetuated by a learned association of relief of aversive states following relapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill CM, Cook C, Pickens S. Migraine and reward system-or is it aversive? Curr Pain Headache Rep. 2014;18:410. doi: 10.1007/s11916-014-0410-y. [DOI] [PubMed] [Google Scholar]
- 36.Cahill CM, Taylor AM, Cook C, Ong E, Moron JA, Evans CJ. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madhavan A, Bonci A, Whistler JL. Opioid-induced gaba potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci. 2010;30:14029–14035. doi: 10.1523/JNEUROSCI.3366-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor AM, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–1198. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nature neuroscience. 2016;19(2):220–222. doi: 10.1038/nn.4199. This paper provides evidence that chronic pain influences striatal output by selectively increasing the excitability of medium spiny neurons in the indirect pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagheddu C, Aroni S, De Felice M, Lecca S, Luchicchi A, Melis M, Muntoni AL, Romano R, Palazzo E, Guida F, et al. Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology. 2015;97:383–393. doi: 10.1016/j.neuropharm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct bidirectional mu-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34:14707–14716. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, et al. Essential role of bdnf in the mesolimbic dopamine pathway in social defeat stress. Science (New York, NY) 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 48.Walsh JJ, Han MH. The heterogeneity of ventral tegmental area neurons: projection functions in a mood-related context. Neuroscience. 2014;282:101–108. doi: 10.1016/j.neuroscience.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanti A, Belzung C. Open questions in current models of antidepressant action. Br J Pharmacol. 2010;159:1187–1200. doi: 10.1111/j.1476-5381.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb C, Gunn JM, Potiriadis M, Everall IP, Bousman CA. The brain-derived neurotrophic factor val66met polymorphism moderates the effects of childhood abuse on severity of depressive symptoms in a time-dependent manner. Front Psychiatry. 2016;7:151. doi: 10.3389/fpsyt.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M, Chang H, Xiao X. Bdnf val66met polymorphism and bipolar disorder in european populations: a risk association in case-control, family-based and gwas studies. Neurosci Biobehav Rev. 2016;68:218–233. doi: 10.1016/j.neubiorev.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 52.A converge consortium: sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–591. doi: 10.1038/nature14659. http://dx.doi.org/10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, et al. Bdnf is a negative modulator of morphine action. Science (New York, NY) 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, Mehrabani S, Wu J, Levitt P, Olmstead MC, De Koninck Y, et al. Neuroimmune regulation of gabaergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology. 2016;41:949–959. doi: 10.1038/npp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bus BA, Tendolkar I, Franke B, de Graaf J, den Heijer M, Buitelaar JK, Oude Voshaar RC. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer B, Jones W, Rehm J. High correlations between levels of consumption and mortality related to strong prescription opioid analgesics in British Columbia and Ontario, 2005–2009. Pharmacoepidemiol Drug Saf. 2013;22:438–442. doi: 10.1002/pds.3404. [DOI] [PubMed] [Google Scholar]
- 57.Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron glia biology. 2011;7:99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal cl(–) homeostasis. Nat Neurosci. 2013;16:183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. First report that different immune cells contribute to chronic pain progression in males and females. Microglia contribute to pain hypersensitivity only in males. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor AM, Mehrabani S, Liu S, Taylor AJ, Cahill CM. Topography of microglial activation in sensory- and affect-related brain regions in chronic pain. J Neurosci Res. 2016 doi: 10.1002/jnr.23883. http://dx.doi.org/10.1002/jnr.23883Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 61.Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain. 2014;155:1762–1772. doi: 10.1016/j.pain.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 62.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. Bdnf from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 64.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, Schmidt C, Larson EB, Thomas MJ. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci U S A. 2016;113:757–762. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Husain MI, Chaudhry IB, Rahman RR, Hamirani MM, Qurashi I, Khoso AB, Deakin JF, Husain N, Young AH. Minocycline as an adjunct for treatment-resistant depressive symptoms: study protocol for a pilot randomised controlled trial. Trials. 2015;16:410. doi: 10.1186/s13063-015-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Sumitani M, Ueda H, Hozumi J, Inoue R, Kogure T, Yamada Y, Kogure T. Minocycline does not decrease intensity of neuropathic pain intensity, but does improve its affective dimension. J Pain Palliat Care Pharmacother. 2016;30:31–35. doi: 10.3109/15360288.2014.1003674. Clinical trial to indicate blocking microglial activation in chronic pain patients improves affective, not sensory, elements of pain. [DOI] [PubMed] [Google Scholar]