Abstract
Summary
A 65-year-old woman was admitted to the emergency unit with a 48 h history of generalised weakness and confusion. On examination, she had mild slurring of speech although there was no other focal neurological deficit. She had profound hyponatraemia (serum sodium level of 100 mmol/L) on admission with the rest of her metabolic parameters being within normal range. Subsequent investigations confirmed the diagnosis of small-cell lung cancer with paraneoplastic syndrome of inappropriate antidiuresis (SIAD). She was monitored closely in high-dependency unit with an attempt to cautiously correct her hyponatraemia to prevent sequelae associated with rapid correction. The patient developed prolonged psychosis (lasting over 2 weeks) and displayed delayed dyskinetic movements, even after a gradual increase in serum sodium levels close to 130 mmol/L. To our knowledge, delayed neurological recovery from profound hyponatraemia (without long-term neurological sequelae) has previously not been reported. This case should alert a clinician regarding the possibility of prolonged although reversible psychosis and dyskinetic movements in a patient presenting with profound symptomatic hyponatraemia.
Learning points:
Patients with profound hyponatraemia may develop altered sensorium, dyskinesia and psychotic behaviour.
Full recovery from psychotic symptoms and dyskinesia may be delayed despite cautious correction of serum sodium levels.
Careful and close monitoring of such patients can help avoid long-term neurological sequelae.
Background
Hyponatraemia is the most common electrolyte abnormality encountered in primary as well as secondary care clinical practice (1). It occurs as a result of dilution, or depletion, of the body’s sodium (Na+) stores. The most common cause of euvolaemic hyponatraemia is the syndrome of inappropriate antidiuresis (SIAD), characterised by the presence of hypotonic hyponatraemia along with inappropriately concentrated, high-sodium urine (2). Hyponatraemia is also a well-recognised adverse effect of several medications including commonly used anti-depressants and thiazide diuretics. Development of symptoms and signs secondary to low sodium typically correlates well with the severity and speed of the electrolyte drop, ranging from nausea and confusion (3) to seizures and coma in profound hyponatraemia (Na+ <115 mmol/L, normal range 135–145 mmol/L) (4).
Development of neurotic–psychotic symptoms secondary to an electrolyte imbalance is a well-established and extensively reported phenomenon. However, there is hardly any mention in available literature regarding these neuropsychiatric features persisting for a certain length of time despite normalisation of the initial metabolic trigger. Here, we report a case of delayed recovery from electrolyte-induced psychosis and dyskinesia resulting from profound hyponatraemia in a patient with newly diagnosed SIAD who was previously not known to have any mental health illness.
Case presentation
A 65-year-old woman was admitted to the emergency department with a 48 h history of generalised weakness and confusion. There was limited history available due to her altered mental state at admission, although she was reported to be previously well and living independently. There was no history of vomiting, diarrhoea, fever, cough, head injury or weight loss.
She had background history of type II diabetes mellitus, primary autoimmune hypothyroidism and hypertension. She was on atenolol, amlodipine, bendroflumethiazide, gliclazide, levothyroxine, metformin and ramipril therapy with no recent change in her medications.
On initial examination, she was apyrexial, normotensive and clinically euvolaemic. She was scoring 14 on Glasgow Coma Scale (due to mild confusion) with no focal neurological deficit. The rest of her systemic examination was unremarkable.
Investigations and course of hospital stay
Baseline investigations including full blood count, blood glucose, bone profile, inflammatory markers, renal and liver function test were within normal range. She had profound hyponatraemia (serum sodium 100 mmol/L) on admission. Her sodium levels were previously (checked about 6 months prior to admission) within normal range. Table 1 shows the results of her subsequent investigations.
Table 1.
Results of investigations to ascertain the cause of hyponatraemia.
| Investigation | Results | Reference range |
|---|---|---|
| Serum osmolality | 219 mmol/kg | 275–295 |
| Urinary osmolality | 452 mmol/kg | – |
| Urinary sodium | 53 mmol/L | – |
| Random cortisol | 1389 nmol/L | >400 nmol/L |
The random cortisol level was high as it was checked on admission while she was in a state of extreme physiological stress. The high cortisol level practically excluded adrenal/pituitary insufficiency as an underlying aetiology for her hyponatraemia. Her thyroid function test was within normal range. Her low serum osmolality in the presence of high urinary osmolality and sodium levels was consistent with a biochemical diagnosis of SIAD.
The patient was transferred to high dependency unit for the first 72 h of admission. She was given saline infusion with close monitoring of hemodynamic and metabolic parameters with the aim for a controlled increase in serum sodium concentration by 8–10 mmol/L/24 h. Bendroflumethiazide tablets were stopped on admission.
Computed tomography (CT) of head did not identify any obvious cause for her ongoing confusion. The chest X-ray showed a bulky hilum and a presumptive diagnosis of bronchogenic carcinoma leading to paraneoplastic SIAD was made. A subsequent computed tomography (CT) of chest identified a 4 cm left hilar mass invading the mediastinum with level 4 lymph node enlargement. The morphological and immunophenotypic appearance of the ultrasound-guided lymph node biopsy confirmed the suspected diagnosis of small-cell bronchogenic carcinoma.
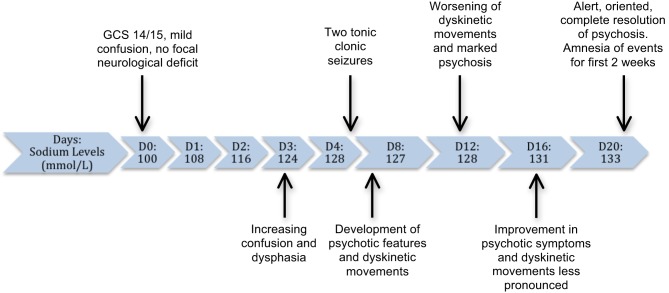
The patient’s sodium levels showed gradual improvement, although she developed worsening of her symptoms, as shown in Fig. 1.
Figure 1.
Timeline of sodium levels correlated with symptom progression during hospital admission.
Her confusion persisted despite relative normalisation of her sodium levels and the absence of any other metabolic, infective or inflammatory pathology to explain her ongoing symptoms. She subsequently developed dysphasia, was unable to recognise her own name and gave inappropriate answers to simple questions. She had good eye contact during this phase and was not distressed, although her ability to recall recent events was severely impaired with a lack of insight.
On day 5 of admission, she had two generalised tonic–clonic seizures. She developed dyskinetic movements such as repetitive facial tics and grimacing in addition to showing further behavioural changes such as generalised apathy. These involuntary, repetitive movements affecting her face and limbs were most pronounced during the 2nd week of her admission. At the peak phase of her psychosis, she required help with all self-care activities and needed prompting with even basic functions such as eating. She had no clinical features suggestive of central pontine myelinolysis including absence of gaze palsy, spastic paraparesis and dysphagia. She had a formal neurologist review during this phase of her illness, with the recommendation of magnetic resonance imaging (MRI) brain, which showed no metastasis, demyelination or meningeal enhancement. Her cerebrospinal fluid (CSF) examination was also normal.
The cognitive function as well as dyskinetic movements showed gradual improvement from week 3 onwards although she had persistent retrograde amnesia for the events in the first 2 weeks of admission. In view of normal biochemical and radiological investigation, her prolonged psychosis and dyskinetic symptoms were attributed to a delayed recovery from profound hyponatraemia.
Outcome and follow-up
The patient was discharged home on day 32 of admission with normal cognition and no residual neurological sequelae. Currently, she is undergoing chemotherapy under oncology follow-up.
Discussion
The points of interest in our patient include the severity of hyponatraemia, development of prolonged psychosis and dyskinetic symptoms with delayed although complete recovery from these over a period of 4 weeks. Our patient had severe and profound hyponatraemia on admission as per the symptom-based and biochemical classification (Tables 2 and 3) proposed by the European Hyponatraemia Guideline Development Group in 2015 (5). The hyponatraemia resulted from SIAD probably secondary to ectopic antidiuretic hormone (ADH) secretion from small-cell lung cancer. An inappropriately excess ADH secretion from tumour cells leads to unregulated increase in water reabsorption at the renal tubules with this being a well-established paraneoplastic syndrome. She was on bendroflumethiazide, a thiazide diuretic, prior to admission, which potentially could have contributed to hyponatraemia although previous sodium levels as checked around 8 months prior to admission were within normal range (while she was on long-term bendroflumethiazide tablets).
Table 2.
Classification of hyponatraemia based on biochemical severity (according to guidance from European Hyponatraemia Guideline Development Group) (5).
| Classification | Sodium levels (mmol/L) |
|---|---|
| Mild | 130–135 |
| Moderate | 125–129 |
| Profound | <125 |
Table 3.
Classification of hyponatraemia based on symptoms (according to guidance from European Hyponatraemia Guideline Development Group) (5).
| Classification | Symptoms |
|---|---|
| Moderately severe symptoms | Nausea |
| Confusion | |
| Headache | |
| Severe symptoms | Vomiting |
| Abnormal somnolence | |
| Seizures | |
| Coma |
The challenging aspect of managing severe symptomatic hyponatraemia remains in balancing the risk of cerebral oedema due to uncorrected hyponatraemia vs the development of osmotic demyelination if sodium levels are corrected too rapidly (6, 7). The European Hyponatraemia Guideline Development Group have written comprehensive clinical practice guidelines (5), which recommend use of hypertonic saline in patients with moderately severe or severe symptoms regardless of whether the imbalance is acute or chronic, as were recorded in our patient. The target rate of increase in sodium concentration should be limited to a total of 10 mmol/L during the first 24 h and an additional 8 mmol/L during every 24 h thereafter (5).
The diagnosis of hyponatraemia-induced psychosis and dyskinetic movements in this case was diagnosis of exclusion as the rest of her biochemical tests, CSF examination and radiological investigations were normal. Although a link between low sodium levels and altered mental state due to cerebral neuronal oedema is well known, there is limited literature on hyponatraemia-induced psychosis. In 2014, Novac and coworkers suggested only 6 previously reported similar cases. They reported a case of hyponatraemia-induced catatonia, which was treated with clonazepam and risperidone with subsequent resolution of altered neurological state within 48–72 h (8). Kreuger and coworkers report a similar case of catatonia resulting from low sodium, which only corrected after administration of lorazepam (9). The neurological abnormalities exhibited in our patient closely resemble dyskinesia, which was observed by Nagaratnam and coworkers in 1997. These movements were hypothesised to be secondary to basal ganglia hypoxia as a result of hyponatraemia with the patient showing recovery from these signs within 72 h after sodium increase (10).
The possibility of central pontine myelinolysis (CPM) was considered although the sodium correction in our patient was gradual. CPM is believed to be more common in patients with chronic hyponatraemia with excess alcohol intake, poor nutrition and liver disease being the factors predisposing to it (11). Our patient had none of these risk factors, and she did not display the classical signs and symptoms such as spastic paraparesis, dysphagia and gaze palsies, which have been described in patients with CPM. Her magnetic resonance imaging scan also did not reveal any demyelination changes.
The factor making our case particularly unusual is the prolonged psychosis and delayed onset of dyskinesia, even after sodium levels increased to 124 mmol/L. A 1-year retrospective review of hyponatraemic patients by Ellis and coworkers in 1995, reported 76% of such patients experiencing clouding of consciousness and 0.5% developing acute psychosis. Neurological sequelae were also noticed after sodium correction, including 1% of patients developing post-correction seizures, typically associated with relatively faster rates of sodium correction (12). Our patient presented with a significantly low sodium level (100 mmol/L). She developed 2 tonic–clonic generalised seizures despite gradual sodium correction. Cerebral oedema was considered as a possible aetiology for these seizures although the initial CT scan did not show radiological features consistent with it. Moreover, she subsequently went on to develop dyskinetic movements and psychosis. These are generally not considered as characteristic features associated with cerebral oedema. We did consider the possibility of rapid alteration in her electrolyte homeostasis and intra-neuronal oedema as potential mechanisms for her tonic–clonic seizures. Curiously, the severity of her psychosis and dyskinetic symptoms was most profound during the 2nd week of admission. The complete resolution of the signs and symptoms within 4 weeks of hospital admission was consistent with a reversible metabolic derangement rather than a paraneoplastic syndrome such as Lambert–Eaton syndrome, which in most cases is irreversible and/or progressive.
In summary, this case adds to the limited available literature regarding delayed and prolonged development of psychosis and dyskinetic symptoms in a patient with profound hyponatraemia. It also supports close monitoring of such patients during the phase of metabolic recovery with suggestion of possible spontaneous recovery.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient for publication of this case report, with signature of the consent form.
Author contribution statement
Victoria John is a foundation year doctor who had a key role in preparing the initial draft of the case report, as well as collecting patient related information. Atul Kalhan contributed to the writing of the report and produced the final edit. Philip Evans was also involved in the editing process, and both consultants had a major role in patient management during admission.
References
- 1.Sherlock M, Thompson C. 2010. The syndrome of inappropriate antidiuretic hormone: current and future management options. European Journal of Endocrinology 162 13–18. ( 10.1530/EJE-09-1057) [DOI] [PubMed] [Google Scholar]
- 2.Verbalis J, Goldsmith S, Greenberg A, Korzelius C, Schrier R, Sterns R, Thompson C. 2013. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. American Journal of Medicine 126 S1–S42. ( 10.1016/j.amjmed.2012.11.010) [DOI] [PubMed] [Google Scholar]
- 3.Weiner M, Epstein F. 1970. Signs and symptoms of electrolyte disorders. Yale Journal of Biology and Medicine 43 76–109. [PMC free article] [PubMed] [Google Scholar]
- 4.Arieff A, Guisado R. 1976. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney International 10 104–116. ( 10.1038/ki.1976.82) [DOI] [PubMed] [Google Scholar]
- 5.Spasovski G, Vanholder R, Alollio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, et al. 2014. Clinical practice guideline on diagnosis and treatment of hyponatraemia. European Journal of Endocrinology 170 G1–G47. ( 10.1530/EJE-13-1020) [DOI] [PubMed] [Google Scholar]
- 6.Grant P, Avuk J, Boulox P, Cohen M, Cranston I, Murray RD, Rees A, Thatcher N, Grossman A. 2015. The diagnosis and management of inpatient hyponatraemia and SIADH. European Journal of Clinical Investigation 45 888–894. ( 10.1111/eci.12465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell P. 2015. Diagnosis and management of hyponatraemia: AGREEing the guidelines. BMC Medicine Online 13 31 ( 10.1186/s12916-015-0277-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novac AA, Bota D, Witkowski J, Lipiz J, Bota RG. 2014. Special medical conditions associated with catatonia in the internal medicine setting: hyponatremia-inducing psychosis and subsequent catatonia. Permanente Journal 18 78–81. ( 10.7812/TPP/13-143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krueger A, Shebak S, Kavuru B. 2015. Catatonia in the setting of hyponatremia. Primary Care Companion for CNS Disorders 17 10 ( 10.4088/PCC.15l01808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaratnam N, Icao E, Peric H. 1997. Abnormal movements associated with severe hyponatraemia. Postgraduate Medicine Journal 73 503–504. ( 10.1136/pgmj.73.862.503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh TD, Fugate JE, Rabinstein AA. 2014. Central pontine and extrapontine myelinolysis: a systematic review. European Journal of Neurology 21 1443–1450. ( 10.1111/ene.12571) [DOI] [PubMed] [Google Scholar]
- 12.Ellis SJ. 1995. Severe hyponatraemia: complications and treatment (Abstract). QJM Journal of Medicine 88 905–909. ( 10.1093/oxfordjournals.qjmed.a069024) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a