Abstract
Summary
Insulinomas are the most frequent cause of hyperinsulinaemic hypoglycaemia. Although surgical enucleation is the standard treatment, a few other options are available to high-risk patients who are elderly or present with co-morbidities. We present a case report of an 89-year-old female patient who was admitted to the emergency department due to recurrent hypoglycaemia, especially during fasting. Laboratory work-up raised the suspicion of hyperinsulinaemic hypoglycaemia, and abdominal CT scan revealed a 12 mm nodular hypervascular lesion of the pancreatic body suggestive of neuroendocrine tumour. The patient was not considered a suitable candidate for surgery, and medical therapy with diazoxide was poorly tolerated. Endoscopic ultrasound-guided ethanol ablation therapy was performed and a total of 0.6 mL of 95% ethanol was injected into the lesion by a transgastric approach; no complications were reported after the procedure. At 5 months of follow-up, no episodes of hypoglycaemia were reported, no diazoxide therapy was necessary, and revaluation abdominal CT scan revealed a pancreatic nodular lesion with a size involution of about half of its original volume. The patient is regularly followed-up at the endocrinology clinic and shows a significant improvement in her wellbeing and quality of life.
Learning points:
Insulinomas are the most frequent cause of hyperinsulinaemic hypoglycaemia.
Surgical enucleation is the standard treatment with a few other options available to high-risk patients.
Endoscopic ultrasound-guided ethanol ablation therapy is one feasible option in high-risk patients with satisfactory clinical outcomes, significant positive impact on quality of life and low complication rates related to the procedure.
Background
Insulinomas are the most frequent cause of hyperinsulinaemic hypoglycaemia. Surgical enucleation is the standard treatment, but medical therapy with diazoxide may be tried in patients who are not suitable candidates for surgery because of old age or co-morbidities. However there is a compelling need for further treatment options when pharmacological treatment is not tolerated or long-term management is seeked.
Endoscopic ultrasound (EUS)-guided ethanol ablation may be a safe and effective method for treating the patients with small lesions or who are poor surgical candidates.
Case presentation
A 89-year-old female patient with a history of acute myocardial infarction, stroke with motor sequelae, pacemaker carrier due to a second-degree atrioventricular block (Mobitz type II) and severe aortic valve disease was admitted to the 1st time to our emergency department (ED) due to acute prostration and confusion. At that time, a plasma glucose level of 50 mg/dL was detected with prompt relief of symptoms after the administration of endovenous glucose.
The patient was discharged with recommendation to monitor capillary blood glucose and restrict rapidly absorbing carbohydrates of her diet.
Ten-days later, she was re-admitted to our ED complaining of recurrent hypoglycaemia, especially during fasting, with the lowest capillary glucose level detected at home of 30 mg/dL. The patient reported no weight gain, no history of diabetes and no evidence of use or access to hypoglycaemic drugs at home.
Investigation
At the first episode, blood samples were collected to evaluate plasma levels of glucose, insulin and c-peptide: a low glucose value (45 mg/dL) with inappropriately elevated insulin (12 µIU/L) and c-peptide (1.6 µg/mL) raised the suspicion of hyperinsulinaemic hypoglycaemia, so the patient was admitted to the Endocrinology Department.
At the 1st day of hospitalization, the patient had another symptomatic hypoglycaemia at 14:00 h and new blood samples were collected confirming the previous results: plasma glucose 38 mg/dL, plasma insulin 10 µIU/L and c-peptide 1.9 µg/mL with negative anti-insulin antibodies.
During hospitalization, the patient had multiple daily episodes of hypoglycaemia mainly during fasting, before dinner and at early night (Fig. 1) requiring intravenous infusion of 5–10% dextrose during the night period and frequent oral feeding during daytime.
Figure 1.
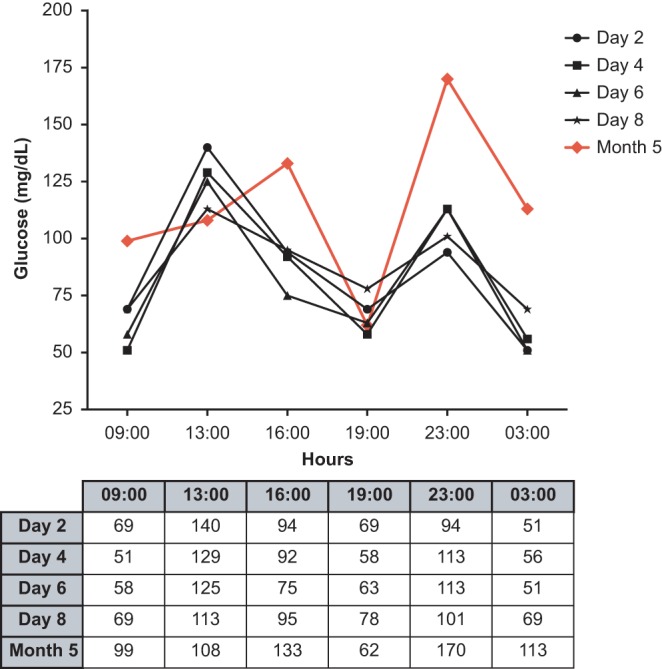
Capillary glucose (mg/dL) monitoring profile pre- and post-procedure: before therapy with diazoxide, with 5% dextrose continuous infusion at night and frequent oral feeding during daytime (D2); medical therapy with diazoxide associated with continuous nocturnal dextrose infusion and frequent oral feeding during daytime (D4–D8) and 5 months post EUS ethanol ablation therapy without any adjuvant medical therapy (M5).
The abdominal CT scan revealed a 12 mm nodular hypervascular lesion of the pancreatic body suggestive of pancreatic neuroendocrine tumour (Fig. 2A).
Figure 2.
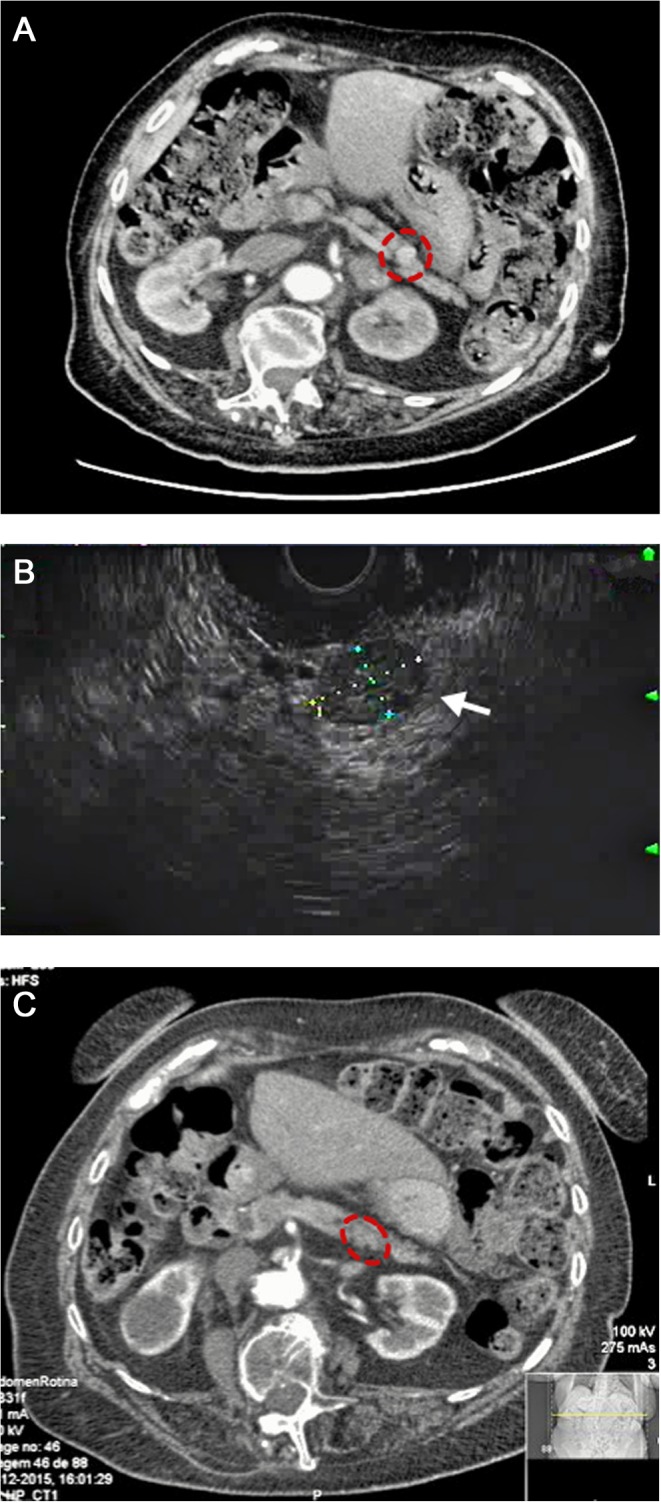
Imaging investigation. (A) Abdominal CT scan before EUS ethanol ablation therapy revealed a nodular hypervascular lesion with 12 mm size. (B) EUS-guided ethanol ablation therapy: a total of 0.6 mL of 95% ethanol was injected into the lesion by a transgastric approach; ¾ of the nodular lesion changed from hypoechoic to hyperechoic appearance after ethanol injection. (C) Post-procedure CT scan showing a residual pancreatic nodular lesion currently measuring 6 mm and less enhancement.
Treatment
Due to the patient’s age and multiple co-morbidities, she was not considered a suitable candidate for surgical approach. Medical therapy with diazoxide was initiated, with 75 mg divided in three doses daily. The gradual titration of this drug was poorly tolerated due to the onset of signs and symptoms of congestive heart failure (oedema, hypotension and symptomatic bradycardia). No other medical therapy, with octreotide or calcium blocker channel, was tried because of the high risk of iatrogenic cardiac abnormalities also associated to these drugs.
EUS-guided ethanol ablation therapy was promptly considered as an option, and a total of 0.6 mL of 95% ethanol was injected into the body pancreatic lesion by transgastric approach. About three-quarters of the nodular lesion changed from hypoechoic to hyperechoic appearance after ethanol injection (Fig. 2B). No complication occurred after the ablation therapy including abdominal pain, fever or mild post-procedure pancreatitis (amylase: 31 U/L (Normal: 28-100) and lipase: 3 U/L (Normal: <67).
No hypoglycaemic episodes were identified after the treatment; therefore the patient was discharged with recommendation to comply with personalized dietary plan and to monitor capillary glucose.
Outcome and follow-up
One month after the procedure, no episodes of hypoglycaemia were reported and no diazoxide therapy was necessary. Laboratorial and radiological reassessments were scheduled at 5 months post-procedure.
She was re-admitted to the Endocrinology Department and a 24-h inward evaluation was done: no symptomatic episodes of hypoglycaemia were reported during this period; only a single asymptomatic capillary blood glucose episode of 62 mg/dL at 19:00 h was verified (Fig. 1).
A revaluation abdominal CT scan revealed a pancreatic nodular lesion currently measuring 6 mm and less enhancement compared with the first description reflecting a size involution of about half of its original volume (Fig. 2C).
The patient keeps regular follow-up at the endocrinology clinic with a significant improvement of her wellbeing and quality of life due to the disappearance of the hypoglycaemia-related symptoms. After 1 year of follow-up the patient is well and no hypoglycaemic events have been reported.
Discussion
Surgical enucleation is the treatment of choice for small and superficially located benign insulinomas. If the lesion is deeply seated or close to the main pancreatic duct, pancreatic resection is preferred. In pancreatic body and tail lesions, distal pancreatectomy is proposed and for lesions arising from pancreatic head through Whipple procedure (pancreaticoduodenectomy) is the chosen approach. Surgical approach is associated with considerable morbidity (abdominal abscess, wound infection, pancreatic duct injury and fistula, endocrine/exocrine insufficiency, perioperative haemorrhage) and mortality, which limits its indication in high-risk patients (1, 2, 3).
In addition, patients should be advised to shorten the number of hours between feedings and to follow a fractionated diet including complex carbohydrate with slow absorption to minimize hypoglycaemic episodes. During hypoglycaemic crisis, rapidly absorbable forms are indicated (3).
Medical pharmacological therapy focus mainly on the use of diazoxide, somatostatin analogues (as octreotide), calcium channel blockers and, in refractory hypoglycaemia, glucocorticoids, but with modest rates of success and important limitations related to iatrogenic side effects specially in elder patients as we report in our case (2, 3).
Ethanol ablation therapy is a minimally invasive alternative treatment with low complication rates despite scarce clinical experience (1, 4, 5, 6, 7, 8). The procedure is performed by using a linear echoendoscope and the volume of the 95% ethanol injection is calculated according to the size of the lesion. Usually, a transduodenal approach is adopted for pancreatic head lesions and a transgastric approach is used for pancreatic body or tail lesions (6).
EUS ablation therapy offers important advantages compared to percutaneous approach allowing real-time control of the procedure, full characterization of the lesion (size and surrounding structures), sample collection to cytology examination as well as delivering therapeutic agents to a target area (4, 5).
The main procedure-associated complications reported are: mild pancreatitis (1, 4, 7), pancreatic haematoma (1, 7), pancreatic necrotic lesion (8) and abdominal pain (4). In case of the remaining symptomatic patients, EUS ablation therapy can be repeated as a strategy to minimize the symptoms with a few contraindications and complications (5, 9, 10).
Of all the reported cases in the literature, our patient is the eldest and none of these complications were detected after the procedure.
Although there is a low rate of complications in most referred patients, it is important to emphasize that few patients underwent this procedure in this specific context and this option must be done in experienced centres with strict regulations regarding the notification of complications.
Nevertheless, some issues related to the technique require further investigation, such as the standardization of target area to ethanol injection and adequate ethanol dose to achieve successful ablation without causing severe complications (5, 10). Furthermore, EUS sensitivity is dependent on tumour location and size, which is a limitation especially on tumours from the pancreatic tail or extra-pancreatic tumours. Also, the EUS as an ethanol ablation technique, is highly dependent on the operator’s experience, which may cause both false-positive and false-negative results (2).
This local-regional ablative therapy seems to have great potential in patients not considered suitable candidates for surgery (because of old age, co-morbidities or high-anaesthetic risk), patients who did not tolerate pharmacological treatment, as well as those who did not desire or refused to undergo surgical treatment.
In high-risk patients, this may be one of the only feasible options with satisfactory clinical outcomes, significant positive impact on quality of life and low level of complications.
The use of this technique in a larger number of patients with longer follow-up will allow the evaluation of its indications and complications before its widespread use in clinical practice.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
The patient signed a written informed consent for publication of the submitted article and accompanying images.
Author contribution statement
A S L followed the patient while she was electively admitted for investigation at the Endocrinology Department, doing so under the tutelage of P O; and was also responsible for conception, interpretation of data and drafting the article, which was reviewed by I P and P O and subsequently by F C. I P followed the patient in endocrinology consultation and added substantial contribution to revising the manuscript critically for important intellectual content. F C reviewed, guided and approved all the processes of investigation and all the medical decisions applied and gave final approval of the version to be published. F P performed the elective therapeutic procedure through ethanol ablation therapy. All the authors read and approved the final manuscript.
Reference
- 1.Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. 2012. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointestinal Endoscopy 75 200–206. ( 10.1016/j.gie.2011.09.019) [DOI] [PubMed] [Google Scholar]
- 2.Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. 2013. Diagnosis and management of insulinoma. World Journal of Gastroenterology 19 829–837. ( 10.3748/wjg.v19.i6.829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davi MV, Pia A, Guarnotta V, Pizza G, Colao A, Faggiano A, NIKE Group 2017. The treatment of hyperinsulinemic hypoglycaemia in adults: an update. Journal of Endocrinological Investigation 40 9–20. ( 10.1007/s40618-016-0536-3) [DOI] [PubMed] [Google Scholar]
- 4.Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. 2006. EUS-guided alcohol ablation of an insulinoma. Gastrointestinal Endoscopy 63 1059–1062. ( 10.1016/j.gie.2005.10.034) [DOI] [PubMed] [Google Scholar]
- 5.Zhang WY, Li ZS, Jin ZD. 2013. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World Journal of Gastroenterology 19 3397–3403. ( 10.1016/j.gie.2005.10.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin SY, Lu XP, Jiang HX. 2014. EUS-guided ethanol ablation of insulinomas: case series and literature review. Medicine 93 e85 ( 10.1097/MD.0000000000000085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deprez PH, Claessens A, Borbath I, Gigot JF, Maiter D. 2008. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastro-Enterologica Belgica 71 333–337. [PubMed] [Google Scholar]
- 8.Muscatiello N, Nacchiero M, Della Valle N, Di Terlizzi F, Verderosa G, Salcuni A, Macarini L, Cignarelli M, Castriota M, D’Agnessa V, et al. 2008. Treatment of a pancreatic endocrine tumor by ethanol injection (PEI) guided by endoscopic ultrasound. Endoscopy 40 (Supplement 2) E83 ( 10.1055/s-2007-995540) [DOI] [PubMed] [Google Scholar]
- 9.DiMaio CJ, DeWitt JM, Brugge WR. 2011. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas 40 664–668. ( 10.1097/MPA.0b013e3182128d06) [DOI] [PubMed] [Google Scholar]
- 10.Siperstein AE, Berber E. 2001. Cryoablation, percutaneous alcohol injection, and radiofrequency ablation for treatment of neuroendocrine liver metastases. World Journal of Surgery 25 693–696. ( 10.1007/s00268-001-0015-6) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a