Summary
In a randomised controlled trial of vitamin D during pregnancy, we demonstrated that women with lower self-efficacy were more likely to experience practical problems with taking the trial medication, and that this was associated with lower compliance and achieved 25(OH)-vitamin D concentrations.
Purpose
The relationship between self-efficacy (the belief that one can carry out a behaviour), compliance with study protocol and outcome was explored within a randomised, double-blind, placebo-controlled trial of vitamin D supplementation in pregnancy.
Methods
In the MAVIDOS trial, women with circulating plasma 25(OH)-vitamin D of 25-100nmol/l in early pregnancy were randomised to either 1000IU cholecalciferol/day or matched placebo from 14 weeks until delivery. Circulating 25(OH)-vitamin D concentrations were assessed at 14 and 34 weeks’ gestation. A sequential sub-sample completed Schwarzer’s General Self-Efficacy Scale at 14 and 34 weeks and the Problematic Experiences of Therapy Scale at 34 weeks. Women were interviewed about their experiences of the trial, and interview transcripts analysed thematically.
Results
In 203 women, those with higher self-efficacy were less likely to experience practical problems taking the study medication (OR 0.81(95%CI 0.69, 0.95), p = 0.01). Over half reported practical problems associated with poorer compliance with the protocol requiring women to take the medication daily. Compliance in women who experienced practical problems was 94% compared with 98% for those with no problems, (p < 0.001). Poorer compliance was also associated with lower concentrations of 25(OH)-D in late pregnancy in the treatment group (β=0.54nmol/l (95%CI 0.18,0.89), p =0.003). Thematic analysis suggested common difficulties were remembering to take the medication every day and swallowing the large capsules.
Conclusions
These findings suggest that differences in self-efficacy influence trial outcomes. Such information may help clinicians anticipate responses to routine vitamin D supplementation in pregnancy and identify those who may need more support to comply.
Trial registration
ISRCTN82927713, registered 11/04/2008
Keywords: self-efficacy, pregnancy, randomised controlled trial, vitamin D, compliance
Introduction
There is growing evidence that early growth and factors operating in utero or early infancy influence peak bone mass in adulthood, a known risk factor for osteoporosis.[1] A large, recent, population-based trial of vitamin D supplementation in pregnancy (MAVIDOS) has confirmed the efficacy of 1000IU/day cholecalciferol for maternal 25(OH)D repletion. Furthermore, although negative for the primary outcome of infant whole body bone mineral content by DXA, the trial demonstrated in a pre-specified secondary analysis, a greater than 0.5 SD increase in infant BMC in neonates born to supplemented compared to placebo mothers, and who delivered during the winter months.[2] The implication of these findings is that supplementing pregnant women with vitamin D may be an important population-wide public health intervention to help prevent osteoporosis. In order for vitamin D supplementation in pregnancy to have long-term impact on the prevalence of this disease, women have to be prepared to take supplements in sufficient quantity and with sufficient consistency that they achieve circulating 25(OH)D repletion throughout gestation. This paper describes an investigation of the individual factors that may present barriers to compliance with this requirement.
Individual psychological characteristics, such as a sense of self-efficacy (the belief that one is capable of carrying out a specific behaviour), are important determinants of behaviour and ultimately of health status.[3] Having a higher sense of self-efficacy and hence a greater sense of control over life has been shown repeatedly to predict better physical health and greater longevity.[4] Self-efficacy has also been shown to mediate the effect of dietary interventions on nutrient status and dietary quality.[5] Higher self-efficacy enabled participants to respond to a nutrition intervention by increasing their fruit and vegetable consumption, because they believed they were capable of doing so.[6] In the context of the vitamin D supplementation trial described above, a participant’s self-efficacy is likely therefore to describe her belief that she is able to take the action required by the study protocol, which specifies that she should take the study medication every day.[7]
The difficulties of achieving compliance with nutrient supplementation recommendations in pregnancy are well documented.[8,9] Factors known to affect compliance with such recommendations depend on the population under study, and include age, level of education and socio-economic status.[10–12] The most common reasons given for not taking supplements during pregnancy are simply forgetting to take them or being put off taking them by the side effects.[13,14] Compliance with supplementation regimes appears to be better in participants in clinical trials, though there may be features of the way clinical trials are run and how they are staffed that affect both compliance with the intervention protocol and trial outcomes.[15] Very little is understood, however, about the role played by trial participants’ psychological characteristics, and how they may affect compliance with trial protocols and hence change trial outcomes.
This paper examines the relationship between women’s self-efficacy and their circulating vitamin D concentration in late pregnancy in the context of a supplementation trial. It explores factors that may affect compliance with the study protocol and how this might affect the trial outcome. Analysis reported in this paper tests the hypothesis that having higher self-efficacy is associated with better compliance and with factors affecting compliance, which in turn increase women’s vitamin D status at the end of the trial.
Methods
A detailed description of The Maternal Vitamin D Osteoporosis Study (MAVIDOS) has been previously published[16]. Briefly, MAVIDOS is a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial of vitamin D supplementation in pregnancy in the United Kingdom. The study was conducted in accordance with guidelines laid down in the Declaration of Helsinki and was approved by the Southampton and South West Hampshire Research Ethics Committee. MAVIDOS was registered prospectively (ISRCTN:82927713; EUDRACT:2007-001716-23); full approval from UK MHRA was granted, and written, informed consent obtained from all participants.
Participants
The MAVIDOS trial was run at three study sites in the UK. Pregnant women were recruited when in attendance for early pregnancy ultrasound screening. The participants in the sub-study reported in this paper were from the sample of 1207 recruited at one of these sites [University Hospital Southampton NHS Foundation Trust, Southampton, UK]. Women were recruited if they were aged over 18 years, were having a singleton pregnancy, were of a gestation less than 17 weeks based on last menstrual period and had 25(OH)D between 25 and 100nmol/l and serum calcium<2.75mmol/l. Women with known metabolic bone disease, renal stones, hyperparathyroidism or hypercalciuria, those taking medication known to interfere with fetal growth, with fetal anomalies on ultrasonography and those taking more than 400 IU/day vitamin D supplementation were excluded. Eligible women with were recruited and randomised at 14 weeks’ gestation (or as soon as possible before 17 weeks’ gestation if recruited later) to either cholecalciferol 1000IU/day or matched placebo taken until delivery of the baby. Recruitment to this sub-study began part-way through the trial. A further sub-sample of women who had taken part in the trial were written to asking if they were prepared to be interviewed about their experience of taking part in the trial . The number of interviews was determined by the point at which saturation of topics was reached.
Measures
Biochemical
Reference blood samples were collected from the mother at 14 and 34 weeks’ gestation, and stored at -80°C after processing. Measurement of plasma 25(OH)-D (Liaison RIA automated platform, Diasorin, Minnesota, USA) was undertaken centrally (MRC Human Nutrition Research, Cambridge, UK) in a single batch at the end of the study.
Questionnaires
Women self-completed Schwarzer’s General Self-Efficacy Scale at 14 and 34 weeks of pregnancy. The General Self-efficacy Scale used was a shortened, 5-item scale derived from the original 10-item scale, and measured the general sense that one can perform novel or difficult tasks, or cope with adversity in various domains of human functioning. [17] This shortened version has been used in previous studies of women of child-bearing age in the UK, has internal consistency (Cronbach’s alpha statistic = 0.85), and has been shown to predict quality of diet.[18] Example items are ‘I can always manage to solve difficult problems if I try hard enough’ and ‘I am calm when things are difficult because I know I can cope’. Women were asked to indicate on a 4-point scale how much they agreed with each item. Responses were coded from 1 (strongly disagree) to 4 (strongly agree) and summed to give an overall score. A higher score indicated higher levels of self-efficacy, with a range of possible scores from 5 to 20. At 34 weeks of pregnancy, women also completed a modified version of the Problematic Experiences of Therapy Scale (PETS).[19] This scale is a brief self-report measure that assesses the extent to which respondents perceive that they have been prevented from carrying out an intervention for common and plausible reasons. Three of the four sub-scales of this assessment were used and adapted to address issues women may have had taking the trial medication. Example scale items used in this study are ‘I could not take my capsules because I was unsure how to do it properly’ (uncertainty about medication sub-scale), ‘I skipped taking my capsules because I was not sure if it was helping’ (doubts about medication sub-scale), and ‘lack of time prevented me from taking my capsules’ (practical problems sub-scale). At the end of this questionnaire, women were then asked in an additional question how they had found taking the study medication and given space to write about their experiences.
At 14 weeks of pregnancy, women were asked about their age, their level of education and amount of time spent outdoors, and their weights and heights were measured.[16] Compliance with the medication was assessed by pill count at the end of the trial and was defined as the percentage of pills taken of the number of pills each woman was expected to take. If this information was not available, proportions of pills taken at 19 or 34 weeks was used, whichever was the latest count. At the end of the trial, women who consented were interviewed at home by a member of the research team about their experiences of taking part in the trial and the interview transcripts subjected to a thematic analysis.
Statistical analysis
This sub-study was carried out in all women who provided data on self-efficacy at 14 weeks and had vitamin D data at 34 weeks. Distribution of circulating 25(OH)D at 34 weeks was checked to assess normality. Self-efficacy score and compliance were analysed as continuous variables. The three scores derived from the PETS, defined as "practical problems", "uncertainty about medication" and "doubts about medication" were dichotomised into having no problems or having at least one problem. The free text responses to the question about how they found taking the study medication were coded into one of six types of response: "all fine", "ok, built into daily routine", "occasionally forgot tablets", "issue with capsule size", "issue with illness", "forgot for short periods because of change in routine".
Analyses were performed separately for intervention and placebo groups, and then for the two groups combined. The associations between predictors and 25(OH)D at 34 weeks were explored with linear regression. The results are displayed as β-coefficients with 95% confidence intervals (95% CI). Logistic regression was used to explore the association between self-efficacy and PETS variables describing problems with taking the medication, with estimates displayed as ORs (95% CIs). Mediation analyses were conducted to test the mediation effect of experiences of taking the study medication on the relationship between self-efficacy and 25(OH)D at 34 weeks. The mediation test was conducted according to the method proposed by Baron and Kenny.[20] Three regression models were fitted: first, the effect of self-efficacy on 25(OH)D at 34 weeks was tested; second, experiences of taking study medication (the mediators) were regressed on self-efficacy; and third, 25(OH)D at 34 weeks was regressed on both the mediators and self-efficacy. The mediation effect test is for an attenuation of the association between self-efficacy and 25(OH)D at 34 weeks after adjusting for experiences of taking the study medication. Analyses were carried out using Stata v 14.0 (Statacorp, College Station, Texas, USA).
Results
A total of 344 women completed the self-efficacy questionnaire at 14 weeks of pregnancy. Of those women, 328 provided 25(OH)-vitamin D measurements at 14 weeks and 218 at 34 weeks. Table 1 provides data on the age of this group of women, and their level of education. Mean self-efficacy at 14 weeks, and 25(OH)-vitamin D at 14 and 34 weeks of pregnancy are also shown, as are scores on the PETS scale and their percent compliance. The data are presented for control and treatment groups separately and for the whole group. The table shows that, in this sub-sample there were no differences between groups in these variables except in 25(OH)D at 34 weeks. Higher 25(OH)-vitamin D levels in the treatment group demonstrate the effectiveness of the intervention and suggest that this group of women was compliant with the trial protocol. Compliance with the medication regime was generally high, averaging 96% of pills taken during pregnancy. The table also shows, however, that of the 203 women who completed the PETS at 34 weeks, over half reported practical problems with taking the trial medication whereas fewer reported doubts or uncertainties about the medication.
Table 1. Characteristics of study participants, mean self-efficacy, vitamin D at 14 and 34 weeks, percent compliance and numbers(%) of women experiencing problems with taking the study medication by treatment group.
| Variables | N | Placebo | Cholecalciferol 1000 IU/d | Whole group |
|---|---|---|---|---|
| Age (years), mean (SD) | 328 | 31.1 (5.3) | 30.8 (19.3) | 31.0 (5.1) |
| Educational attainment ≥ A level, N (%) | 326 | 122 (75.3) | 130 (79.3) | 252 (77.3) |
| 14 week Self-efficacy, mean (SD) | 328 | 15.3 (1.9) | 15.1 (1.8) | 15.2 (1.9) |
| 14 week 25(OH)D (nmol/l), mean (SD) | 328 | 46.5 (16.5) | 47.5 (18.2) | 47.0 (17.3) |
| 34 week 25(OH)D (nmol/l), mean (SD)* | 218 | 43.4 (19.4) | 67.4 (19.7) | 55.5 (22.9) |
| Compliance (%), median (IQR) | 216 | 95.2 (89.6-98.8) | 96.5 (90.1-99.2) | 96.0 (89.8-98.9) |
| At least a practical problem, N (%) | 202 | 56 (56.6) | 51 (49.5) | 107 (53.0) |
| At least an uncertainty about medication, N (%) | 203 | 15 (15.2) | 17 (16.4) | 32 (15.8) |
| At least a doubt about medication, N (%) | 203 | 14 (14.1) | 16 (15.4) | 30 (14.8) |
p<0.001 for difference between placebo and active group. All other p-values for differences between placebo and active group >0.05
Table 2 shows that women entering the trial with higher self-efficacy were less likely (OR<1) to experience practical problems with taking the study medication, though they did not seem to be more likely to have doubts or uncertainties about whether it was right for them to be taking it. Neither was their lower self-efficacy associated with poorer compliance with the trial protocol.
Table 2. Associations between self-efficacy scores at 14 weeks, PETS and compliance.
| Placebo | Cholecalciferol 1000 IU/d | Whole group | ||||
|---|---|---|---|---|---|---|
| Outcomes | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Practical problems (Yes vs. No) | 0.81 (0.64,1.02) | 0.07 | 0.80 (0.64,1.01) | 0.06 | 0.81 (0.69,0.95) | 0.01 |
| Uncertainty (Yes vs. No) | 0.90 (0.67,1.22) | 0.51 | 1.00 (0.75,1.33) | 0.99 | 0.95 (0.77,1.17) | 0.64 |
| Doubts (Yes vs. No) | 0.91 (0.66,1.24) | 0.54 | 0.87 (0.65,1.18) | 0.38 | 0.89 (0.71,1.10) | 0.28 |
| r | r | r | ||||
| Compliance (SD) | 0.04 | 0.66 | 0.07 | 0.49 | 0.05 | 0.43 |
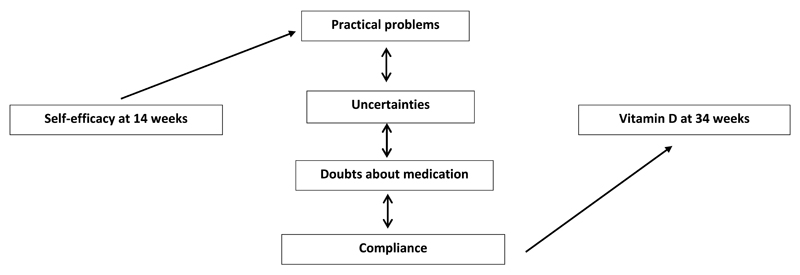
Experiencing practical problems, having doubts and uncertainties about the medication and rate of compliance were all strongly associated with one another (p < 0.05 for all). Women who experienced no doubts or uncertainties about taking the medication tended also to have no practical problems, and those who experienced no doubts, uncertainties or practical problems had higher rates of compliance (Table 3). For women in the treatment group, Table 4 shows that the more compliant they were, the higher their 25(OH)-vitamin D levels at 34 weeks. Figure 1 summarises the relationships between self-efficacy, experiences taking the study medication and 25(OH)-vitamin D status at 34 weeks. There were no mediation effects of experiences of taking study medication on the relationship between self-efficacy at 14 weeks of pregnancy and vitamin D levels at 34 weeks.
Table 3. Median (IQR) compliance rate by PETS.
| Compliance (%) | p-value * | |
|---|---|---|
| Median (IQR) | ||
| No practical problems | 98.0 (96.4-99.5) | <0.001 |
| Practical problems | 92.4 (83.7-97.4) | |
| No uncertainties | 96.8 (92.1-99.3) | 0.02 |
| Uncertainties | 90.4 (77.2-98.8) | |
| No doubts | 96.8 (91.8-99.3) | 0.03 |
| Doubts | 91.4 (77.2-98.8) |
Wilcoxon rank-sum test
Table 4. Association between 25(OH)D (nmol/l) at 34 weeks, self-efficacy at 14 weeks, problems taking the study medication and compliance.
| Placebo | Cholecalciferol 1000 IU/d | Overall | ||||
|---|---|---|---|---|---|---|
| Predictors | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p |
| Self-efficacy at 14 weeks (continuous score) | -0.3 (-2.2,1.7) | 0.80 | 1.1 (-1.0,3.2) | 0.32 | -0.3 (-2.0,1.3) | 0.68 |
| Practical problems (Yes vs. No) | 0.2 (-8.8,9.2) | 0.96 | -6.2 (-14.7,2.3) | 0.15 | -5.9 (-13.1,1.3) | 0.11 |
| Uncertainty (Yes vs. No) | 8.2 (-3.3,19.8) | 0.16 | -7.2 (-18.5,4.0) | 0.21 | 0.4 (-9.2,10.0) | 0.93 |
| Doubts (Yes vs. No) | 8.4 (-3.5,20.3) | 0.16 | -8.3 (-20.2,3.6) | 0.17 | -0.3 (-10.4,9.7) | 0.95 |
| Compliance (%) | 0.01 (-0.25,0.28) | 0.93 | 0.54 (0.18,0.89) | 0.003 | 0.29 (0.05,0.54) | 0.02 |
Figure 1. Conceptual model summarising the relationships between self-efficacy, vitamin D at 34 weeks, compliance with trial protocol, practical problems taking the study medication, uncertainty and doubts about taking the medication.
In the combined group of women in both treatment and placebo groups, those who had higher self-efficacy on entering the study tended to have higher 25(OH)D status at 14 weeks of pregnancy (β = 1.04, 95%CI 0.05,2.03, p=0.04). As Table 4 shows, this was not the case by late pregnancy (34 weeks) in either the treatment or placebo groups. Circulating 25(OH)D levels increased less between 14 and 34 weeks in women with higher self-efficacy irrespective of their allocation, possibly because women with higher self-efficacy entered pregnancy with higher vitamin D levels (β = -1.73 nmol/l, 95%CI -3.40, -0.07, p = 0.04).
In response to the additional question about how they had found taking the study medication, 78% of the women reported no issues or said that they had developed a daily routine that helped them to remember to take the medication. A small percentage (11%) said they occasionally forgot to take a tablet, and the remainder said that the size of the tablet was a problem, or that illness or a change in routine, such as a holiday, had prevented them from taking the tablets for a period.
Qualitative interviews with 14 women who had taken part in the trial confirmed that there were no major difficulties in complying with the requirement to take the study medication every day. If the women did mention problems, they tended to be practical problems associated with taking the medication, primarily the large size of the capsules and remembering to take them every day, rather than experiencing doubts or uncertainties about the study or the medication. In response to a question about issues she might have had taking part in the study, one woman said:
“The massive pills maybe. They were pretty hefty to try and get down.” (ID 3744, lines 244-245)
Another said that she had no difficulty swallowing the tablets, but sometimes forgot them:
“The tablets were easy to take. It was actually … trying to remember that I had to take them.”(ID 4081, lines 135-136)
Both these issues may have affected their compliance with the study protocol. Some of the women who described experiencing these practical problems with the study medication found that developing a daily routine helped:
“I was already taking Pregnacare, so I just took everything together.” (ID 3718, line 71)
Discussion
This study was carried out in a sub-group of 203 women taking part in a large, multi-centre randomised, placebo-controlled trial of the effects of vitamin D supplementation in pregnancy on neonatal bone mineral content. Women who entered the study with a lower sense of self-efficacy tended to have lower levels of circulating 25(OH)D in early pregnancy, though not in late pregnancy in either treatment or control groups. The vitamin D levels of these women were more likely to increase over the course of pregnancy than those of women with higher self-efficacy, however, presumably because they had lower levels at baseline. Responses to the adapted PETS indicated that more than half the women experienced problems taking the study medication, but not because they doubted its usefulness or were uncertain how to take it. Problems taking the study medication tended to be about the size of the capsules or difficulty remembering to take them, but qualitative data suggested that women usually found ways of overcoming the practical problems they experienced. The data partially support the study hypothesis that having higher self-efficacy is associated with better compliance and with factors affecting compliance, which in turn improves vitamin D status. Though there was no direct effect of women’s self-efficacy on their compliance, women with lower self-efficacy were more likely to experience practical problems taking the study medication. Experiencing practical problems was associated with poorer compliance with the study protocol which required women to take the medication every day; and poorer compliance was associated with lower levels of vitamin D in late pregnancy in the treatment group. Qualitative data indicated that the major factors affecting compliance were difficulties in remembering to take the study medication every day and in swallowing the tablets which came in large capsules.
Strengths and limitations
The women who took part in this study were a highly educated group as were the original MAVIDOS study participants with approximately three quarters in both the sub study and original study having A level qualifications or above.[2] As a highly educated group, participants might be expected to have both higher self-efficacy and 25(OH)D status than the general population and therefore not represent the general population of women of child-bearing age in the UK.[21] Women in this sub-study had, in fact, a comparable mean self-efficacy score to women in previous studies who had a wider range of educational attainment.[22] The high levels of compliance with the study protocol, however, suggest that women in this study were highly motivated, and that their responses to taking the supplement may not represent those of the general population to routine maternal vitamin D supplementation in pregnancy.
Implications of the study
Women largely complied with the protocol of the trial described in this paper. Those with lower self-efficacy experienced more problems, and those who experienced more problems took less of the study medication. This finding suggests that improving the self-efficacy of participants in clinical trials may indirectly improve compliance. Ensuring compliance is a major issue in the success of clinical trials; substantial resources are invested in monitoring how well participants adhere to study protocols, because compliance has been shown to have significant effects on trial outcomes.[23] It is possible to improve self-efficacy through intervention.[24,25] Self-efficacy, or the belief that one is capable of carrying out a specific behaviour, is acquired through a combination of watching others or learning about other’s experience of achieving these behavioural goals, being told that these goals are possible and achievable, past experience of being able to behave in this way, and current physiological or mood state.[3] Increasing self-efficacy in order to improve compliance with trial protocols could therefore be achieved through an exploratory conversation between participants and members of staff recruiting them. Applying these findings to routine vitamin D supplementation in pregnancy, conversations to increase self-efficacy for taking pregnancy supplements would need to explore the practical problems that the participant might have in taking and remembering to take the medication, and to support them to identify ways of overcoming them. Some practical problems might relate to the physiological and mood changes associated with being pregnant, and examples of ways in which others have overcome these difficulties might increase participants’ self-efficacy beliefs about their ability to adhere to the protocol.
The reasons women gave for not taking the medication in this study are similar to those given by women to explain why they do not take folic acid or iron supplements during pregnancy. Women say they forget or that the side effects put them off.[13,14] Though a proportion of the women in this study did say they forgot to take the tablets, few were worried about medication side-effects or had doubts about the value of the research in which they were taking part. This is important to know and encouraging, in the context of making recommendations for routine maternal vitamin D supplementation in pregnancy as a public health intervention for the purpose of improving maternal and infant health outcomes.
Conclusions
Women’s level of self-efficacy is indirectly associated with their compliance with a trial protocol requiring them to take daily vitamin D supplements. Women who had higher self-efficacy had higher 25(OH)D levels when they came into the trial and experienced fewer problems taking the study medication. The more compliant the women in the treatment group were with the study protocol, the higher their vitamin D levels in late pregnancy. These findings have implications for understanding differences between participants, particularly in their level of self-efficacy, and the effect of these differences on trial outcomes, and for anticipating responses to recommendations for routine maternal vitamin D supplementation in pregnancy.
Acknowledgements
We thank all our funders for supporting this work (Arthritis Research UK, Medical Research Council, Bupa Foundation, National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford) and the women who so kindly gave us their time and took part in this study.
Funding: This work was supported by grants from the Arthritis Research UK (17702), Medical Research Council (4050502589), Bupa Foundation, National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. The work leading to these results was supported by the European Union's Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreements numbers 289346 and 613977. We are extremely grateful to Merck GmbH for the kind provision of the Vigantoletten supplement. Merck GmbH had no role in the trial execution, data collection, analysis or manuscript preparation. The authors had full access to all study data.
Footnotes
Conflicts of interest: CC reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfi zer, Roche, Servier, and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, AMGen, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare, and Internis Pharma, outside the submitted work. HMI reports grants from the Medical Research Council (MRC), Arthritis Research UK, and European Union’s Seventh Framework Programme, during the conduct of the study; and while not directly receiving funding from other bodies, members of her team have received funding from the following companies for other work: Danone, Nestec, and Abbott Nutrition. MB, JB and WL report funding from Danone Nutricia Early Life Nutrition for other work than that reported here. All other authors declare no competing interests.
References
- 1.Harvey N, Dennison E, Cooper C. Osteoporosis: a lifecourse approach. J Bone Miner Res. 2014;29(9):1917–1925. doi: 10.1002/jbmr.2286. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D'Angelo S, Crozier SR, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. The Lancet Diabetes & Endocrinology. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandura A. Self-efficacy: the exercise of control. W.H.Freeman and Company; New York: 1997. [Google Scholar]
- 4.Ross CE, Wu C. The links between education and health. Am Soc Rev. 1995;60:719–745. [Google Scholar]
- 5.Luszczynska A, Haynes C. Changing nutrition, physical activity and body weight among student nurses and midwives: effects of a planning intervention and self-efficacy beliefs. Journal of health psychology. 2009;14(8):1075–1084. doi: 10.1177/1359105309342290. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe A, Perkins-Porras L, Rink E, Hilton S, Cappucio FP. Psychological and social predictors of changes in fruit and vegetable consumption over 12 months following behavioural and nutrition education counseling. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2004;23(6):574–581. doi: 10.1037/0278-6133.23.6.574. [DOI] [PubMed] [Google Scholar]
- 7.Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, Schoenmakers I, Prentice A, Cooper C. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. The MAVIDOS Study Group. Trials. 2012;13:13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143(4):486–492. doi: 10.3945/jn.112.169987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultink W, van der Ree M, Matulessi P, Gross R. Low compliance with an iron-supplementation program: a study among pregnant women in Jakarta, Indonesia. Am J Clin Nutr. 1993;57(2):135–139. doi: 10.1093/ajcn/57.2.135. [DOI] [PubMed] [Google Scholar]
- 10.Khanal V, Adhikari M, Karkee R. Low compliance with iron-folate supplementation among postpartum mothers of Nepal: an analysis of Nepal Demographic and Health Survey 2011. Journal of community health. 2014;39(3):606–613. doi: 10.1007/s10900-013-9806-6. [DOI] [PubMed] [Google Scholar]
- 11.McGuire M, Cleary B, Sahm L, Murphy DJ. Prevalence and predictors of periconceptional folic acid uptake--prospective cohort study in an Irish urban obstetric population. Human reproduction (Oxford, England) 2010;25(2):535–543. doi: 10.1093/humrep/dep398. [DOI] [PubMed] [Google Scholar]
- 12.Mithra P, Unnikrishnan B, Rekha T, Nithin K, Mohan K, Kulkarni V, Kulkarni V, Agarwal D. Compliance with iron-folic acid (IFA) therapy among pregnant women in an urban area of south India. African health sciences. 2013;13(4):880–885. doi: 10.4314/ahs.v13i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebremedhin S, Samuel A, Mamo G, Moges T, Assefa T. Coverage, compliance and factors associated with utilization of iron supplementation during pregnancy in eight rural districts of Ethiopia: a cross-sectional study. BMC Public Health. 2014;14:607. doi: 10.1186/1471-2458-14-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbour RS, Macleod M, Mires G, Anderson AS. Uptake of folic acid supplements before and during pregnancy: focus group analysis of women's views and experiences. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2012;25(2):140–147. doi: 10.1111/j.1365-277X.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 15.Shankar AV, Asrilla Z, Kadha JK, Sebayang S, Apriatni M, Sulastri A, Sunarsih E, Shankar AH. Programmatic effects of a large-scale multiple-micronutrient supplementation trial in Indonesia: using community facilitators as intermediaries for behavior change. Food and nutrition bulletin. 2009;30(2 Suppl):S207–214. doi: 10.1177/15648265090302S204. [DOI] [PubMed] [Google Scholar]
- 16.Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, Schoenmakers I, Prentice A, Cooper C. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. Trials. 2012;13(1):13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: A user's portfolio. Causal and control beliefs. NFER-Nelson; Windsor, UK: 1995. pp. 35–37. [Google Scholar]
- 18.Lawrence W, Schlotz W, Crozier S, Skinner TC, Haslam C, Robinson S, Inskip H, Cooper C, Barker M. Specific psychological variables predict quality of diet in women of lower, but not higher, educational attainment. Appetite. 2011;56:46–52. doi: 10.1016/j.appet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby S, Donovan-Hall M, Yardley L. Measuring barriers to adherence: validation of the Problematic Experiences of Therapy Scale. Disability and rehabilitation. 2014;36(22):1924–1929. doi: 10.3109/09638288.2013.876106. [DOI] [PubMed] [Google Scholar]
- 20.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 21.Leganger A, Kraft P. Control constructs: do they mediate the relation between educational attainment and health behaviour? Journal of health psychology. 2003;8(3):361–372. doi: 10.1177/13591053030083006. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence W, Schlotz W, Crozier S, Skinner TC, Haslam C, Robinson S, Inskip H, Cooper C, Barker M. Specific psychological variables predict quality of diet in women of lower, but not higher, educational attainment. Appetite. 2011;56(1):46–52. doi: 10.1016/j.appet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czobor P, Skolnick P. The Secrets of a Successful Clinical Trial: Compliance, Compliance, and Compliance. Molecular Interventions. 2011;11(2):107–110. doi: 10.1124/mi.11.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in Healthy Eating and Physical Activity Interventions: a meta-regression. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28(6):690–701. doi: 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- 25.Prestwich A, Kellar I, Parker R, MacRae S, Learmonth M, Sykes B, Taylor N, Castle H. How can self-efficacy be increased? Meta-analysis of dietary interventions. Health Psychol Rev. 2014;8(3):270–285. doi: 10.1080/17437199.2013.813729. [DOI] [PubMed] [Google Scholar]