Abstract
Background
Walking while performing another task (eg, talking) is challenging for many stroke survivors, yet its neural basis are not fully understood.
Objective
To investigate prefrontal cortex activation and its relationship to gait measures while walking under single-task (ST) and dual-task (DT) conditions (ie, walking while simultaneously performing a cognitive task) in stroke survivors.
Methods
We acquired near-infrared spectroscopy (NIRS) data from the prefrontal cortex during treadmill walking in ST and DT conditions in chronic stroke survivors and healthy controls. We also acquired functional magnetic resonance imaging (fMRI) and NIRS during simulated walking under these conditions.
Results
NIRS revealed increased oxygenated hemoglobin concentration in DT-walking compared with ST-walking for both groups. For simulated walking, NIRS showed a significant effect of group and group × task, being greater on both occasions, in stroke survivors. A greater increase in brain activation observed from ST to DT walking/ simulated walking was related to a greater change in motor performance in stroke survivors. fMRI revealed increased activity during DT relative to ST conditions in stroke patients in areas including the inferior temporal gyri, superior frontal gyri and cingulate gyri bilaterally, and the right precentral gyrus. The DT-related increase in fMRI activity correlated with DT-related change in behavior in stroke participants in the bilateral inferior temporal gyrus, left cingulate gyrus, and left frontal pole.
Conclusion
Our results provide novel evidence that enhanced brain activity changes relate to dual task motor decrements.
Keywords: prefrontal cortex, gait control, stroke, cognitive motor interference, dual task, near-infrared spectroscopy, fMRI, rehabilitation
Introduction
Most stroke survivors do not attain the locomotor capacity necessary for daily activities or community ambulation.1,2 Walking in the community is frequently experienced as much more difficult than walking at home, which contributes to restricted participation in meaningful activities and eventually reduced satisfaction with recovery.3 Some of the complex demands of community mobility can be studied in laboratory settings using dual-task (DT) paradigms, in which walking is performed with a concurrent cognitive task.4 Recent data from imaging studies show that walking itself places demand on brain resources that are used for cognitive functioning such as the prefrontal cortex (PFC).5–8 While the behavioral effects of DT walking are well described,4 the neural basis for cognitive-motor interference during DT walking after stroke is not. Evidence from brain lesions and imaging studies suggests that dual-tasking involves the PFC.9–12 Therefore, considering evidence from older adults,8 we hypothesized that PFC activation is associated with DT performance while walking after stroke. We chose to assess prefrontal activity using near-infrared spectroscopy (NIRS), a noninvasive optical imaging method that detects changes in blood oxygen levels through the skull. In the elderly, NIRS has shown the involvement of the PFC during walking and DT walking,8,13 and a relationship of age-related decline in gait capacity to gait speed control.5,14 However, while NIRS offers the advantage of being able to be acquired during real walking, it has modest spatial resolution and limited depth penetration. We therefore assessed DT-waking using NIRS during actual gait, and also using NIRS with simultaneous functional magnetic resonance imaging (fMRI) during simulated gait.15,16 Thus, the primary aim of the present study was to investigate PFC activation and relationships between PFC activation and gait measures while walking under single-task (ST) and DT conditions in stroke survivors and healthy controls.
Subjects and Methods
The present study consisted of 2 experiments. In experiment 1, we used NIRS to examine PFC activation of actual walking under ST and DT conditions. In experiment 2, we used NIRS and fMRI concurrently to explore brain activation patterns during simulated walking, under ST and DT conditions, in a subset of participants. Both experiments were approved by the local NHS Research Ethics Committee. All participants gave their informed consent to participate in the study according to the Declaration of Helsinki (2008).
Experiment 1
Participants
Nineteen individuals with chronic stroke (2 females and 17 males, mean age = 59.61 ± 15.03 years) and 20 healthy controls (8 females and 12 males, mean age = 54.35 ± 9.38 years) volunteered for participation. Table 1 summarizes the demographic details for all participants. Inclusion criteria for the stroke group were (1) more than 6 months after stroke (confirmed by CT or MRI brain scan) and medically stable; (2) able to walk for at least 5 minutes with or without mobility aids; (3) no cognitive, sensory, or psychological impairments precluding full engagement with experimental paradigm; and (4) 18 or more years old and able to give informed consent. Healthy participants did not report a history of neurological and/or psychiatric illness and were native English speakers.
Table 1.
Demographic Details for All Participants.
| Stroke (N = 19) | Control (N = 20) | |
|---|---|---|
| Age, years, mean ± SD | 59.61 ± 15.03 | 54.35 ± 9.38 |
| Female, n (%) | 2 (10.5) | 8 (40) |
| Height, m, mean ± SD | 1.743 ± 0.052 | 1.735 ± 0.092 |
| Weight, kg, mean ± SD | 86.5 ± 17.16 | 80.2 ± 15.76 |
| Right-handed, n (%) | 18 (94.7) | 18 (90) |
| SSWS (m/s),* mean ± SD | 0.48 ± 0.338 | 1.01 ± 0.025 |
| Right-side hemiplegia, n (%) | 11 (57.9) | NA |
| Barthel Index, mean ± SD | 18.67 ± 1.85 | NA |
| Medications, mean ± SD | 4.83 ± 2.96 | NA |
| Time since onset, mo, mean ± SD | 26.5 ± 27.46 | NA |
| SOMCT, mean ± SD | 25.38 ± 3.24 | NA |
| BBS, mean ± SD | 50.81 ± 5.94 | NA |
| 10-m test, seconds, mean ± SD | 15.85 ± 10.61 | NA |
| PASE, mean ± SD | 78.1 ± 61.0 | NA |
Abbreviations: SSWS, self-selected walking speed; SOMCT, short orientation, memory, and concentration test; BBS, Berg Balance Scale; PASE, Physical Activity Scale of the Elderly; NA, not applicable.
P < .001 (2-sample t test).
Experimental Procedure
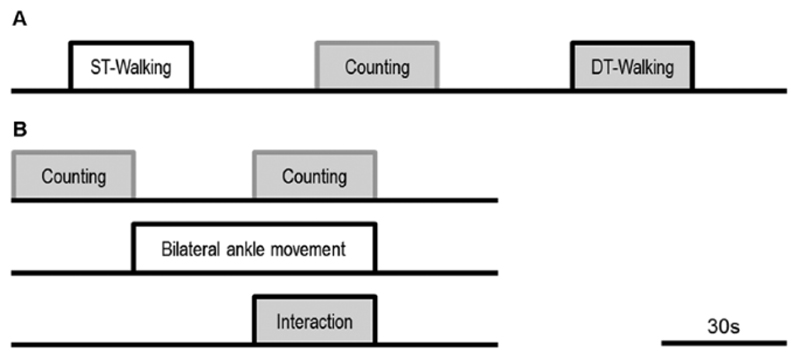
Initially, we familiarized participants with the treadmill walking task and introduced them to the cognitive task. Each participant then completed walking trials, under ST or DT conditions, on a treadmill (Woodway ELG 75, Weil am Rhein, Germany) at self-selected walking speed. Each walking task consisted of a 30-second task period repeated five times and alternated with rest periods or counting while standing in a pseudo-random order (Figure 1A). To avoid anticipation of the start of task periods, the duration of rest periods ranged from 25 to 45 seconds in a pseudo-random order. During the DT blocks, participants were asked to count backward in sevens from a random number between 291 and 299. The instructions before ST and DT blocks were standardized for all participants and they were not given advice as to which task to prioritize during DT-walking.
Figure 1.
Experimental procedure: (A) Experiment 1. Participants completed 5 trials of each of the following tasks; walking alone (ST-walking), counting while standing (Counting), and walking while counting (DT-walking). Each trial consisted of a 30-second task period and alternated with rest periods in a pseudo-random order. (B) Experiment 2; one ABCD cycle. This cycle was repeated 6 times giving a total paradigm length of 12 minutes. The top row indicates when participants engaged in the counting task, the second row indicates when participants were moving their feet and the third row indicates the interaction between the 2 (ie, blocks when the 2 tasks were performed simultaneously). ST, single task; DT, dual task.
Data Acquisition
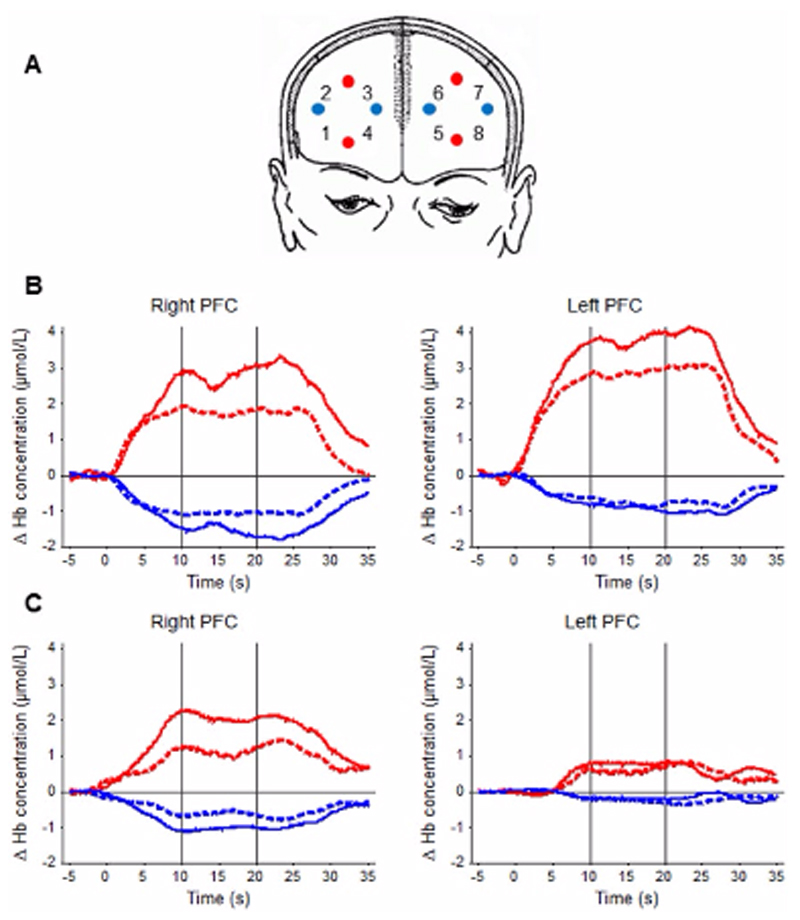
Prefrontal cortex activation was measured with the Oxymon Mk III system (Artinis Medical Systems, PW Elst, the Netherlands). The continuous-wave multi-channel system uses 2 wavelengths at 782 and 859 nm to estimate relative changes in the concentration of oxygenated (oxy-Hb) and deoxygenated (deoxy-Hb) hemoglobin, respectively. Two identical plastic holders consisting of 4 optodes each (2 sources, 2 detectors) in a 4-channel arrangement with an interoptode separation of 30 mm were placed on each participant’s forehead (Figure 2), covering the area linking Fp1, F3, and F7 and the area linking Fp2, F4, and F8 according to the 10-20 electroencephalography electrode system, corresponding to the left and the right PFC, respectively.17 Optical signals were continuously sampled at 10 Hz, and stored according to their wavelength and location, resulting in values for changes in the concentration of oxy-Hb and deoxy-Hb from each of the 8 channels. Optical data were used to quantify task-related changes of oxy-Hb and deoxy-Hb based on the modified Beer-Lambert law.18 Spatiotemporal gait parameters were assessed using an inertial measurement unit comprising a triaxial accelerometer, gyroscope, and magnetometer (MTx, Xsens, AN Enschede, the Netherlands) that was attached over the skin of the fourth lumbar vertebra,19 corresponding to the participant’s projected center of mass (CoM) during walking.20 Counting backward performance was assessed by rate (enu-merated numbers per 30 seconds) and accuracy (percentage of correct answers). Finally, systematic blood pressure was measured (Dinamap, GE Healthcare, Waukesha, WI, USA) at the beginning and after the end of each trial.
Figure 2.
Near-infrared spectroscopy (NIRS) measurement: (A) Schematic representation of NIRS probe location. The red (transmitters) and blue (detectors) circles indicate positions of optodes. Numbers indicate the positions of channels defined as a midway between neighboring transmitter-detector pairs. The probe holder covered the area linking Fp1, F3, and F7 and the area linking Fp2, F4, and F8 according to the international 10-20 electroencephalography (EEG) system, which is correspondent to the left and right prefrontal cortex (PFC), respectively. (B and C) Representative examples of PFC activation in a stroke patient (B) and a healthy control (C) under single task (ST; dashed lines) and dual task (DT; solid lines) walking. Time course of the grand average across all blocks averaged over all measured probe positions (left and right PFC). The y-axis displays relative concentration changes of hemoglobin in millimoles per liter. The increase in [oxy-Hb] is indicated by the red line, the decrease in [deoxy-Hb] by the blue line. Vertical lines across the graphs indicate the 10-second time window used for further statistical analysis.
Data Processing
Near-infrared spectroscopy data processing was performed using the OxySoft software (version 2.1.6). Standard preprocessing and individual-level statistical analysis was applied. To remove high-frequency noise such as cardiac pulsation, NIRS signals were then low-pass filtered at a 0.67-Hz cutoff frequency using the Matlab software. For the analysis of brain activation, the middle 10 seconds of task block was selected (the time between 6 and 16 seconds after stimulus onset) and the average concentration changes within this time window was used for further analysis. To identify channels exhibiting task-related acti-vation, average concentration changes were compared with the average baseline-corrected activation by means of t tests for all tasks, and active channels were defined as statistically different relative to baseline. Data from active channels were used for subsequent analyses.Postprocessing and analysis of the inertial measurement unit (IMU) was performed using a customized program written in LabVIEW 8.5 (National Instruments, Austin, TX, USA).19 Spatial gait parameters were then estimated according to the inverted pendulum gait model.21,22
Experiment 2
Participants
Nine individuals with chronic stroke (all males, mean age = 66.2 ± 8.3 years; mean time since onset = 26.4 ± 16.6 months) and 10 healthy controls (4 females and 6 males, mean age = 56.2 ± 9.5 years), volunteered for participation. In addition to the previously mentioned inclusion criteria, participants had to be able to perform a bilateral reciprocal feet tapping task and to pass MR-safety screen-ing assessments.
Experimental Procedure
Participants were given full verbal instructions and trained on the tasks before entering the scanning room. The 2 tasks were (1) counting backward covertly in threes from a given number and (2) reciprocal feet movement. Movements and counting were both cued at the movement rate established during familiarization, where participants performed reciprocal feet movement at their comfortable pace for a minute. Participants performed movement at 80% of this rate inside the scanner. If participants could not match the movement rate while counting backward by threes, different numbers were used so that they were able to perform the counting task (ie, counting back by twos or ones). In the scanner, participants performed 4 different conditions: (A) single task, counting only; (B) single task, feet movement only; (C) dual task, counting and feet movement concurrently; and (D) rest (Figure 1B). The paradigm consisted of 30-second blocks of the tasks in ABDC cycle repeated 6 times, giving a total paradigm length of 12 minutes. Task performance was paced by a flashing symbol projected onto a screen at the foot of the scanner tube. Participants were instructed to count back one digit (condition A), move feet once (condition B), or both (condition C), each time a symbol flashed on the screen. Each 30-second block was preceded by an instruction screen. For each counting block a different number was given to participants at the starting point. Reciprocal bilateral feet movements were restricted to one degree of freedom (extension-flexion at the ankle joint), using a custom-built plastic apparatus.
Cognitive task performance was recorded during simulated walking. Participant’s compliance with the movement task was confirmed by a potentiometer fitted to the feet apparatus. The signals from the potentiometer as well as a record of stimulus presentation and button press were collected and analyzed off-line.
Data Acquisition and Processing
Near-infrared spectroscopy data were acquired and proceed as described in experiment 1. MRI data were acquired using a 3.0-T TrioTim Syngo (Siemens, Erlangen, Germany) MRI system. A total of 256 T2*-weighted blood oxygen level–dependent (BOLD) sensitive echo planar imaging (EPI) images were acquired during task performance (45 axial slices per volume, TR = 3000 ms, TE = 30 ms, matrix = 64 × 64, in-plane resolution = 3 x 3 mm2, flip angle = 87°). A T1-weighted anatomical image was also acquired (TR = 3000 ms, TE = 4.71 ms, TI [inversion time] = 1100 ms, flip angle 8°, field of view = 256 × 256, matrix = 256 × 256).Image analysis was carried out using tools from the FMRIB Software Library (Analysis group, FMRIB, Oxford, UK, www.fmrib.ox.ac.uk/fsl). Standard preprocessing and individual-level statistical analysis was applied.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 (IBM SPSS, Inc, Armonk, NY, USA). Behavioral data were analyzed by conducting a mixed-design analysis of variance (ANOVA) with task (2 levels; ST and DT) as the independent variable for each measure separately, and participants’ group (stroke vs control) as the between-subject factor. For the NIRS data statistical analysis, in both experiments, the average of combined activated channels was calculated for each task and side (left and right PFC). Data from all participants were analyzed by conducting a mixed-design ANOVA with task (3 levels; counting, ST-walking (or simulated walking), and DT-walking (or simulated) and hemisphere (2 levels; left and right) as the independent within-subjects variables for oxy-Hb and deoxy-Hb separately, while participant group (stroke vs control) was the between-subjects factor. For all statistical tests, alpha level was set at .05 a priori, and SPSS-generated Bonferroni adjusted P values are quoted. To account for baseline differences in ST performance, DT-related changes in both PFC activation and spatiotemporal gait measured was calculated as relative changes by the following formula23:
In experiment 2, statistical analysis of the images was performed in 2 stages. NIRS data acquisition was synchronized with the fMRI measurements using a trigger from the MRI scanner and signals were preprocessed and analyzed as described before.
Results
Experiment 1: NIRS During Single- and Dual-Task Treadmill Walking
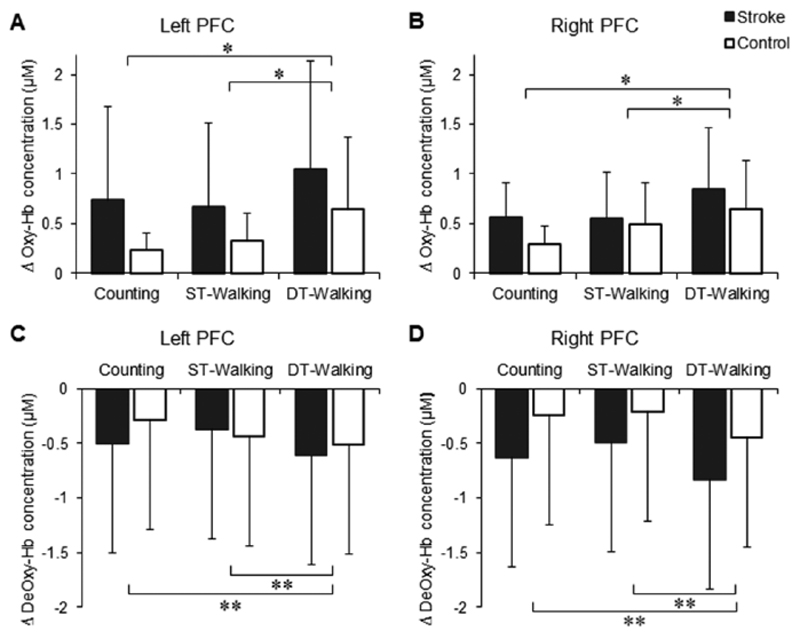
We first examined whether treadmill walking would interfere with counting performance. ANOVA revealed significant main effects of task and group on counting rate while only a significant group effect on counting accuracy (Table 2). Then we examined the effects of cognitive demand on walking. ANOVA revealed significant main effects of task and group on stride length and cadence (Table 2).Near-infrared spectroscopy data showed a significant main effect of task on oxy-Hb concentration changes, F(1, 37) = 7.483, P = .01, which increased (reflecting increased PFC activation) during DT-walking compared with ST-walking for both groups and hemispheres (Figure 3). Similarly, there was a significant main effect of task on deoxy-Hb concentration changes, F(1, 37) = 11.346, P = .002, which decreased (also reflecting increased PFC activation) during DT-walking compared with ST-walking for both groups and hemispheres (Figure 3).For both oxy-Hb and deoxy-Hb changes there were no significant main effects of either group or hemisphere and no significant interactions between task, group, and hemisphere. Finally, there were no significant changes in systematic blood pressure or heart rate due to the walking.
Table 2.
Dual-Task–Related Changes in Counting Performance and Gait Spatiotemporal Measures.a
| Stroke (N = 19) | Control (N = 20) | ||||||
|---|---|---|---|---|---|---|---|
| ST | DT | ST | DT | Task Sig. | Group Sig. | Interaction Sig. | |
| Counting rate | 17.6 ± 8.7 | 16.1 ± 8.2 | 24.5 ± 8.8 | 24.4 ± 8.7 | .046 | .009 | .109 |
| Counting accuracy | 84.3 ± 13.8 | 81.4 ± 20.1 | 97.2 ± 2.4 | 94.8 ± 5.6 | .158 | .001 | .894 |
| Stride length (m) | 0.71 ± 0.26 | 0.76 ± 0.25 | 1.08 ± 0.33 | 1.09 ± 0.30 | .015 | .001 | .061 |
| Cadence (steps/min) | 67.4 ± 21.1 | 63.0 ± 23.3 | 100.7 ± 23.3 | 97.2 ± 25.3 | .001 | .000 | .615 |
Abbreviations: ST, single task; DT, dual task; Sig., significance.
Values in boldface indicate statistical significance.
Figure 3.
Group data (mean ± SD) of task-related changes in oxy-Hb (A and B) and deoxy-Hb (C and D) concentrations in the prefrontal cortex (PFC) during single-task (ST) and dual-task (DT) walking.
Experiment 2: fMRI and NIRS During Simulated Walking
Functional Magnetic Resonance Imaging Results
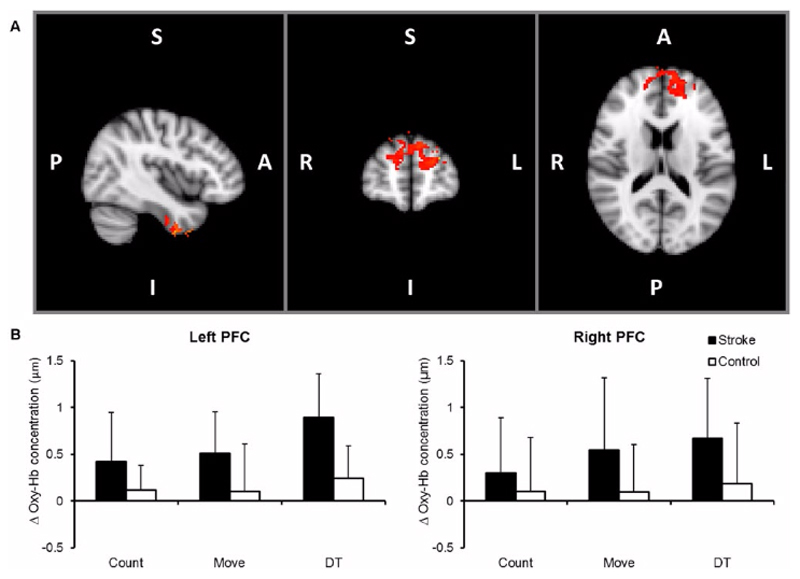
For both stroke patients and healthy controls, reciprocal bilateral ankle movement produced expected patterns of bilateral activity, including sensorimotor areas. Here, we focus on results of the positive interaction between the counting backward and ankle movement tasks. Positive interaction refers to increased activity during the DT performance over and above the linear sum of activations in the 2 ST conditions.In healthy controls, positive interaction was seen only in the lingual gyri bilaterally. Areas showing increased positive interaction in healthy controls compared to stroke patients include the lingual gyri bilaterally, cerebellum, right angular gyrus, right precuneus cortex, left postcentral gyrus, and posterior division of the right inferior temporal gyrus. In stroke participants, areas showing a significant positive interaction included bilateral superior frontal gyrus, bilateral inferior temporal gyrus and left caudate nucleus. Areas showing increased positive interaction in stroke patients compared with healthy controls include the inferior temporal gyri bilaterally, the superior frontal gyri bilaterally, the cingulate gyri bilaterally, and the right precentral gyrus.Correlations between DT-related increase in fMRI signal change and behavior were found only in stroke participants in the bilateral inferior temporal gyrus, left cingulate gyrus and left frontal pole; with increased DT-related behavioural changes associated with increased activation in the stroke group (Figure 4).
Figure 4.
(A) Dual-task (DT)–related activation in stroke; thresholded images (Z > 2.3) of group for the positive interaction. Significant clusters defined according to extent (at corrected P < .01). (B) Group data (mean ± SD) of task-related changes in oxy-Hb concentrations in prefrontal cortex (PFC) during counting, ankle movement and DT as measured by near-infrared spectroscopy (NIRS) simultaneously with functional magnetic resonance imaging (fMRI).
Near-Infrared Spectroscopy Results
Oxy-Hb concentration varied significantly with task, F(2, 32) = 4.884, P = .014, and group, F(1, 16) = 7.669, P = .014, such that the higher concentration was found during DT compared with ST and in stroke participants compared with controls. In addition, the task × group interaction was significant, F(2, 32) = 3.542, P = .041. The pairwise comparisons of the DT performance with counting and moving conditions were statistically significant (P = .001 and P = .048, respectively), while the comparison of the counting with the moving condition was nonsignificant (Figure 4; middle).To further explore the task × group interaction, separate ANOVAs were conducted for each group. A significant main effect of task was found in stroke patients, F(2, 14) = 9.321, P = .003, but not in healthy controls, F(2, 18) = 1.121, P = .348, demonstrating that the interaction reflected greater effects of DT in patients than controls.Similarly, post hoc analysis revealed significant differ-ence between the 2 groups during DT performance task in the right, F(1, 16) = 4.682, P = .047, and the left, F(1, 16) = 11.545, P = .004, PFC. In other words, bilateral PFC was activated more in stroke patients compared to controls under DT conditions. For deoxy-Hb changes there were no significant main effects of task, group, hemisphere or interactions between task, group, and hemisphere.
Discussion
The 2 experiments reported here sought to examine the role of the PFC in gait control after stroke. We hypothesized that difficulties that stroke survivors experience with walking under DT conditions may reflect increased cognitive demand posed by walking after stroke. In support of this, we observed that stroke survivors exhibited increased PFC activation compared with healthy controls under both ST and DT walking conditions and that DT walking was associated with increased PFC activation both in stroke patients and in healthy controls. We also found that counting performance during walking not only affected gait measures in both groups, but the converse was also true; even among healthy controls, walking altered counting rate. Suggesting that the counting task was cognitively demanding even for the healthy controls, however, the effect of such a cognitive ask on gait as revealed by increased PFC activation was prominent more among stroke participants.
Taken together, these findings suggest that there is increased requirement for PFC activity during walking after stroke, and that this demand increases further in the presence of a concurrent cognitive task. These findings, for the first time, provide evidence of the neural correlates of real-life walking in DT conditions and demonstrate how increased cognitive demands, as indexed by PFC activity, may limit walking in real life conditions after stroke.
For both groups, PFC activation increased (ie, increased oxy-Hb and decreased deoxy-Hb) during DT-walking compared with ST-walking in both hemispheres. In addition, experiment 2 found that stroke patients showed significantly increased PFC activity compared with controls, in both ST and DT walking. The increased PFC activation in the stroke group might be related to its role in attention to action24 or in planning movement after stroke.25 In the elderly, greater PFC activation has been associated with age-related functional declines in cognition26 and in gait capacity5 and an effort to obtain better performance with aging.27 In young adults, DT walking has been associated with no change13 or increased PFC activity alongside maintained gait and lower-limb reflex control,28 suggesting an effective adaptive mechanism. However, in the healthy elderly, PFC activity has been shown to increase during talking but reduce when performing the challenge of a visually demanding DT activity; possibly reflecting a shift in processing resources from the prefrontal cortex to other brain regions.8,13 In the elderly, DT activities may also lead to decrements on tasks.5 These studies considered alongside our findings suggest adaptive brain control mechanisms are affected by the individual’s capability and condition, the demand placed on the individual, and the nature of the competing tasks.
We also found that stroke patients, but not controls, showed significant correlations between the change in PFC activity and change in behavior in DT conditions. We believe that reduced cognitive and/or motor control of locomotion in patients with the greatest behavioral “cost” of DT walking led to compensatory PFC recruitment that involves top-down control to direct responses according to task goals and environmental constraints.29
In the fMRI study, we observed increased PFC activation, along with increased activation in the inferior temporal cortex and basal ganglia, under DT condition for the stroke group only. It is likely that the control group might have found the combined DT was not cognitively demanding enough to induce further activation other than in the visual cortex. While the study used ankle movement, actual and purposeful human locomotion depends more on complicated control mechanisms compared with isolated ankle dorsiflexion.
The observation of a greater dependence on top-down control for walking after stroke has implications for rehabilitation. Traditional approaches to gait rehabilitation after stroke emphasize the explicit avoidance of dual-tasking while walking (ie, less automatic patterns) and explicitly focus attention on moving safely. Results from this study question these approaches and suggest it may be more beneficial and probably safer for stroke patients to learn walking under DT conditions (ie, more automatic and less conscious gait pattern).
This study has methodological limitations that need to be acknowledged. For example, we have used a continuous-wave type of NIRS, which cannot fully determine the opti-cal properties of tissue and hence measures only relative changes in blood oxygenation, rather than absolute concentrations.30 However, continuous-wave NIRS remains a practical and affordable choice—providing a safe means of examining brain activation in a range of individuals and tasks—because the signal type and acquisition timing requirements are less demanding compared with frequency- and time-resolved types.30
Near-infrared spectroscopy signals might also be affected by skin blood flow and other physiological noise from the superficial tissues.31 While the removal of these artifacts from cerebral signals could have been possible by employing several different experimental considerations and data processing,32 we have used a customized filter to get rid of as much physiological noise as we could.
Finally, interpretation of our results might also be limited by the small sample size and heterogeneity of subject characteristics. While rigorous participants’ selection has the advantage of minimizing variance and reducing com-mon confounds,33 it also restricts the generalization of interpretation to other patients.
Conclusion
Findings from our experiments offer a theoretical basis for better understanding of behavioral deficits and developing strategies that optimize safe mobility after stroke. Our observation of top-down control of walking after stroke has important implications for rehabilitation that may challenge current approaches to walking rehabilitation and raises relevant questions. Finally, the results suggest that NIRS may offer an optimum tool to measure PFC activation as a therapeutic target in stroke.
Supplementary Material
Acknowledgments
The authors thank members of the Movement Science Group of Oxford Brookes University for their support.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Stroke Association UK (H.D., H.J.-B., E.A.); The University of Jordan, Amman (E.A.); Wellcome Trust (H.J-B.); Elizabeth Casson Trust (H.D.); and National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals Trust and Oxford University (H.D., H.J.-B.).
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Pound P, Gompertz P, Ebrahim S. A patient-centred study of the consequences of stroke. Clin Rehabil. 1998;12:338–347. doi: 10.1191/026921598677661555. [DOI] [PubMed] [Google Scholar]
- 2.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 3.Robinson CA, Shumway-Cook A, Ciol MA, Kartin D. Participation in community walking following stroke: subjective versus objective measures and the impact of personal factors. Phys Ther. 2011;91:1865–1876. doi: 10.2522/ptj.20100216. [DOI] [PubMed] [Google Scholar]
- 4.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35:715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193:445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- 6.Koenraadt KL, Roelofsen EG, Duysens J, Keijsers NL. Cortical control of normal gait and precision stepping: an fNIRS study. Neuroimage. 2014;85(pt 1):415–422. doi: 10.1016/j.neuroimage.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 7.Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi T, Makizako H, Shimada H, et al. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res. 2013;25:539–544. doi: 10.1007/s40520-013-0119-5. [DOI] [PubMed] [Google Scholar]
- 9.D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 10.Collette F, Olivier L, Van der Linden M, et al. Involvement of both prefrontal and inferior parietal cortex in dual-task performance. Cogn Brain Res. 2005;24:237–251. doi: 10.1016/j.cogbrainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- 12.Dreher J-C, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3(9):e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. 2014;92:122–128. doi: 10.1016/j.ijpsycho.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66A:879–887. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21:568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Leff DR, Elwell CE, Orihuela-Espina F, et al. Changes in prefrontal cortical behaviour depend upon familiarity on a bimanual co-ordination task: an fNIRS study. Neuroimage. 2008;39:805–813. doi: 10.1016/j.neuroimage.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Delpy D, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33:1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- 19.Esser P, Dawes H, Collett J, Howells K. IMU: inertial sensing of vertical CoM movement. J Biomech. 2009;42:1578–1581. doi: 10.1016/j.jbiomech.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Kerrigan DC, Viramontes BE, Corcoran PJ, LaRaia PJ. Measured versus predicted vertical displacement of the sacrum during gait as a tool to measure biomechanical gait performance. Am J Phys Med Rehabil. 1995;74:3–8. doi: 10.1097/00002060-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Zijlstra W. Assessment of spatio-temporal parameters during unconstrained walking. Eur J Appl Physiol. 2004;92:39–44. doi: 10.1007/s00421-004-1041-5. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez RC, Alvarez D, Lopez AM, Alvarez JC. Modified pendulum model for mean step length estimation. Eng Med Biol Soc 29th Annu Int Conf IEEE. 2007:1371–1374. doi: 10.1109/IEMBS.2007.4352553. [DOI] [PubMed] [Google Scholar]
- 23.Abernethy B. Dual-task methodology and motor skills research: some applications and methodological constraints. J Hum Mov Study. 1988;14:101–132. [Google Scholar]
- 24.Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease. Brain. 2002;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- 25.Sharma N, Baron J-C, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009;66:604–616. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 27.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 28.Meester D, Al-Yahya E, Dawes H, Martin-Fagg P, Pinon C. Associations between prefrontal cortex activation and H-reflex modulation during dual task gait. Front Hum Neurosci. 2014;8:78. [Google Scholar]
- 29.Turner GR, McIntosh AR, Levine B. Prefrontal compensatory engagement in TBI is due to altered functional engagement of existing networks and not functional reorganization. Front Syst Neurosci. 2011;5:9. doi: 10.3389/fnsys.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholkmann F, Kleiser S, Metz AJ, et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85(pt 1):6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Takikawa Y, Kawagoe R, Shibuya S, Iwano T, Kitazawa S. Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage. 2011;57:991–1002. doi: 10.1016/j.neuroimage.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Naseer N, Hong K-S. fNIRS-based brain-computer interfaces: a review. Front Hum Neurosci. 2015;9:3. doi: 10.3389/fnhum.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.