Abstract
Like elderly men, old male rhesus macaques show attenuated circulating levels of testosterone and dehydroepiandrosterone sulfate (DHEAS), and many of them also show reduced levels of daytime activity. It is unclear, however, if this age-associated behavioral change is causally related to the underlying decrease in circulating androgen levels. To test this possibility, old male rhesus macaques were given daily supplements of testosterone and DHEA for 6 months, designed to mimic the mean 24-hour circulating hormone patterns of young adults. Compared to the young adults, the old controls showed attenuated daytime activity levels. However, there was no difference between the androgen supplemented old animals and the aged-matched controls, even after 6 months of treatment. The data suggest that age-associated decreases in circulating androgen levels are unlikely to be a primary reason for altered activity-rest patterns in elderly men, and that androgen supplementation paradigms might not provide any obvious therapeutic benefit.
Keywords: Aging, Androgens, Dehydroepiandrosterone, Rhesus macaque, Testosterone
1. Introduction
The circulating concentrations of many steroid hormones decrease during aging, and may contribute to the etiology of age-related pathologies such a cognitive decline and immune senescence (Engleman et al., 2011; Kohama et al., 2016; Rapp et al, 2003a; 2003b). In women and female rhesus macaques the most dramatic hormonal changes occur after menopause, as a result of the precipitous decrease in synthesis and release of estradiol and progesterone from the ovaries, as well as a marked decrease in dehydroepiandrosterone sulfate (DHEAS) from the adrenal glands (Downs and Urbanski, 2006; Downs et al., 2008; Sorwell et al., 2012). Although men and male rhesus macaques show less dramatic age-related decreases in the circulating levels of DHEAS and testosterone (T), these androgens have well-defined circadian rhythms. Consequently, their decrease during aging contributes to attenuation of circadian signaling throughout the body, which in turn may play a key role in the etiology of perturbed activity-sleep patterns that are so prevalent in the elderly (Downs et al., 2007; 2008; Sitzmann et al., 2014; Sorwell and Urbanski, 2013; Urbanski and Sorwell, 2012; Urbanski et al., 2014).
The aim of the present study was to test whether providing old male rhesus macaques with daily supplements of testosterone and DHEA for 6 months would lead to improved sleep-wake patterns, defined as increased activity during the day and less disruption of rest during the night. The daily androgen supplementation paradigm that we employed was previously developed in the same old rhesus macaques, and was shown to recapitulate mean youthful circulating levels of DHEAS, estradiol, estrone, 5α-dihydrotestosterone and T, while preserving their characteristic circadian profiles but not the underlying pusatile patterns (Urbanski et al., 2014).
2. Materials and methods
2.1. Experimental animals
A total of six young adult (mean age at end of study = 12 years) and twelve old (mean age at end of study = 24 years) male rhesus macaques (Macaca mulatta) were used in this Institutional Animal Care and Use Committee approved study, and were cared for by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC) in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. They were housed indoors in a temperature controlled environment (24°C) under a 12L:12D photoperiod (lights on from 0700 h–1900 h). Daily meals at ∼0800 h and ∼1500 h (LabDiet High Protein Monkey Chow, St. Louis, MO, USA) were supplemented with fresh fruits or vegetables; fresh drinking water was available ad libitum.
2.2 Androgen supplementation
Based on their age, and mean morning plasma T and DHEAS concentrations, the old animals were separated into two equal groups. Half of the old animals (n=6) received daily androgen supplementation, as previously described (Urbanski et al., 2014), while the other half (n=6) served as age-matched controls. The androgen supplementation comprised oral T administration (12 mg/kg body weight, at 1900 h) and two oral DHEA administrations (0.04 mg/kg body weight, at 0700 and 1000 h). Both T and DHEA were obtained from Sigma-Aldrich Corp (St. Louis, MO, USA). Previously, it had been shown that when T is administered orally in oil, significant quantities bypass the liver, presumably because of reduced passage into the hepatic portal system and increased uptake by the lymphatic system, and elevate circulating T concentrations (Amory and Bremner, 2005). Consequently, we suspended the T in sesame oil at a concentration of 120 mg/ml and then mixed it with ∼12 g of chocolate or placed it inside a 5-g cookie, based on the animal’s preference. Similarly, we suspended the DHEA in sesame oil (10 mg/ml) and mixed it with chocolate or placed it inside a cookie. The control animals did not receive control treats or vehicle but were otherwise exposed to the same diet and the same environmental conditions. At the end of the study, the mean (± SEM) body weights of the young (n=6), old controls (n=6), and old supplemented animals (n=6) were 9.5 ± 0.8 kg, 10.9 ± 0.6 kg and 12.1 ± 1.1 kg, respectively, and their mean testis weights were 26.1 ± 2.6, 28.4 ± 2.4 and 21.3 ± 2.5 g; there were no significant (p>0.05) between-group differences for either body weight or testis weight (Kuskal-Wallis 1-way ANOVA). As expected, mean (± SEM) plasma DHEAS concentrations were significantly (p<0.05) lower in the old controls (43.7 ± 12.7 ng/ml) than in the young animals (126.8 ± 18.1 ng/ml) (Urbanski et al., 2014), but significantly (p<0.01) elevated (359.2 ± 50.8 ng/ml) in the old animals ∼3 hours after DHEA supplementation (Mann-Whitney U-tests).
2.3. Monitoring of activity patterns
As previously described (Urbanski, 2011; Urbanski et al., 2012), each animal was fitted with an Actiwatch activity monitor (Philips-Respironics, Bend, OR, USA), worn inside a protective case that was attached to a lightweight loose-fitting aluminum collar (Primate Products, Inc., Immokalee, FL, USA). The animals were caged individually during the activity monitoring in a room that housed up to 16 animals. At the start of the study, continuous activity recordings were made for ~2 weeks and a characteristic baseline pattern was established for each animal. Approximately 6 months later, activity was again monitored for ~2 weeks. The actograms were subsequently analyzed using Actiware-Sleep (version 3.4) software (Cambridge Neurotechnology Ltd, Cambridge, UK). The mean total daily activity (defined as the average 24-hour activity) was calculated for each animal, as was the mean daytime activity (defined as activity during the period between 0700 h and 1900 h) and mean night-time activity (activity between 1900 h and 0700 h). Differences between treatment groups were compared using the Mann-Whitney U-test.
3. Results
Figure 1 shows representative mean 24-hour activity patterns from a young adult, an old control and an old androgen-supplemented animal after 6 months of study. All of the animals showed intense activity during the day and very little activity during the night (Table 1). The activity levels in the old controls and old androgen-supplemented animals did not change significantly between the start and the end of the study (Supplementary data Fig. S1). In both of these groups, however, the overall mean activity levels and mean daytime activity levels at the end of the study were significantly lower than in the young adults (p < 0.01). Importantly, there was no significant (p>0.05) difference in daytime or night-time activity levels between the old supplemented animals and the age-matched controls.
Fig. 1.
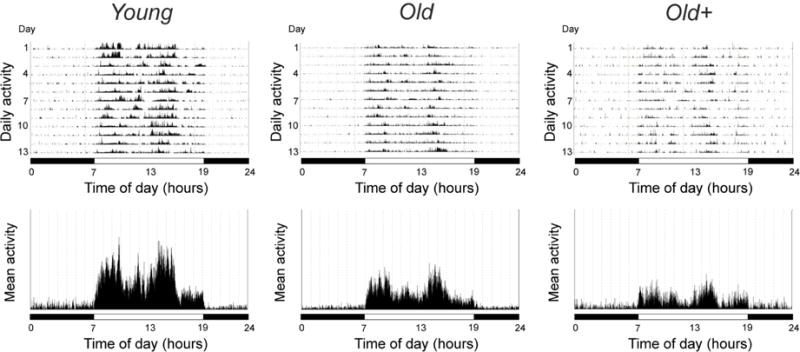
Representative actograms from a young adult and an old male rhesus macaque, as well as from an old male that received daily androgen supplementation (+) for 6 months. In the upper panels, the height of the vertical lines within the actograms is indicative of the intensity of physical activity at any particular time of day; the mean 24-hour activity profiles across the ~ 2 weeks are depicted in the lower panels. The horizontal black and white bars correspond to the times of night and day, respectively.
Table 1.
Characterization of 24-hour activity patterns in male rhesus macaques
| YOUNG | OLD | OLD+ | |
|---|---|---|---|
| Average 24-hour activity | 120.0 ± 8.2 | 83.0 ± 12.9** | 88.6 ± 29.7** |
| Average daytime activity | 215.3 ± 15.3 | 147.6 ± 24.2** | 159.7 ± 57.9** |
| Average nighttime activity | 24.6 ± 1.9 | 18.3 ± 3.0 | 17.5 ± 3.0 |
Values represent means ± SEM (n=6 animals per group).
OLD+ = 6 months of daily androgen supplementation.
P<0.01, versus young animals (Mann-Whitney U-test).
4. Discussion
As expected, circulating androgen levels and daytime activity were significantly lower in the old controls than in the young animals. Despite 6 months of daily androgen supplementation, however, the daytime activity levels of the old treated animals were significantly lower and no different than the activity levels observed in the old controls. These data demonstrate that androgen supplementation in the elderly has no obvious beneficial effect on 24-hour activity levels, even when administered in a relatively physiological circadian manner and for an extended period. They are therefore consistent with a recent report that 1 year of testosterone supplementation in older men offered no obvious benefit with respect to vitality or walking distance (Snyder et al., 2016).
Supplementary Material
Fig. S1 Correletions between baseline activity levels and activity levels observed after 6 months of study. Data from young adult, old controls, and old androgen-supplemented male rhesus macaques are represented by yellow, green and orange symbols, respectively.
Acknowledgments
This work was supported by NIH grants: AG-036670, OD-010426 and OD-011092. Daily care of the animals was performed by Jamie Garten, Vasilios Garyfallou, Katie Moore, Kevin Mueller, Krystina Sorwell, Alison Weiss and Brandee Winstead, with additional help from the ONPRC Division of Comparative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The author declares no conflicts of interest.
References
- Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride in men: A pharmacokinetic study. J Clin Endocrinol Metab. 2005;90:2610–2617. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman F, Barron A, Urbanski HF, Neuringer M, Kohama SG, Messaoudi I. Accelerated immune senescence and reduced response to vaccination in ovariectomized female rhesus macaques. Age (Dordr) 2011;33:275–289. doi: 10.1007/s11357-010-9178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Renner L, Landauer N, Weiss AR, Urbanski HF, Park B, Voytko ML, Neuringer M. Effect of ovarian hormone therapy on cognition in the aged female rhesus macaque. J Neurosci. 2016;36:10416–10424. doi: 10.1523/JNEUROSCI.0909-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;2:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Sitzmann BD, Brown DI, Garyfallou VT, Mattison JA, Ingram DK, Roth GS, Ottinger MA, Urbanski HF. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta) Age (Dordr) 2014;36:183–197. doi: 10.1007/s11357-013-9563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487.e1–e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Urbanski HF. Causes and consequences of age-related steroid hormone changes: insights gained from nonhuman primates. J Neuroendocrinol. 2013;25:1062–1069. doi: 10.1111/jne.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinol. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dordr) 2012;34:1111–1121. doi: 10.1007/s11357-011-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG, Garyfallou VT, Garten J, Weiss A, Renner L, Neuringer M, Kohama SG. Androgen supplementation during aging: development of a physiologically appropriate protocol. Rejuv Res. 2014;17:150–153. doi: 10.1089/rej.2013.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Correletions between baseline activity levels and activity levels observed after 6 months of study. Data from young adult, old controls, and old androgen-supplemented male rhesus macaques are represented by yellow, green and orange symbols, respectively.


