Abstract
The interaction of two folding intermediate mimetics of the model protein substrate Fyn SH3 with the chaperonin GroEL, a supramolecular foldase/unfoldase machine, has been investigated by 15N relaxation-based nuclear magnetic resonance spectroscopy (lifetime line broadening, dark state exchange saturation transfer, and relaxation dispersion). The two mimetics comprise C-terminal truncations of wild-type and triple-mutant (A39V/N53P/V55L) Fyn SH3 in which the C-terminal strand of the SH3 domain is unfolded, while preserving the remaining domain structure. Quantitative analysis of the data reveals that a mobile state of the SH3 domain confined and tethered within the cavity of GroEL, possibly through interactions with the disordered, methionine-rich C-terminal tail(s), can be detected, and that the native state of the folding intermediate mimetics is stabilized by both confinement within and binding to apo GroEL. These data provide a basis for understanding the passive activity of GroEL as a foldase/unfoldase: the unfolded state, in the absence of hydrophobic GroEL-binding consensus sequences, is destabilized within the cavity because of its larger radius of gyration compared to that of the folding intermediate, while the folding intermediate is stabilized relative to the native state because of exposure of a hydrophobic patch that favors GroEL binding.
GroEL is a large (800 kDa) supramolecular machine that facilitates protein folding and protects against the damaging effects of misfolding and aggregation.1 GroEL comprises two heptameric rings, each of which encloses a large cavity accessible to protein substrates. The mechanism whereby GroEL exerts its effect encompasses a series of concerted allosteric transitions driven by the hydrolysis of ATP and the binding and dissociation of the co-chaperonin GroES that caps the open rings of GroEL. Apo GroEL, however, can also function as a passive anti-aggregation chamber as evidenced by both H/D and fluorescence-based folding experiments.2 Recently, we presented a quantitative study of the interaction of a model, metastable SH3 domain with apo GroEL3 by combined analysis of 15N Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion,4 dark state exchange saturation transfer (DEST),5 and lifetime line broadening (ΔR2)6 nuclear magnetic resonance (NMR) experiments.7 The model domain consisted of a triple (A39V/N53P/V55L) mutant of Fyn SH3 (SH3Mut) that, at a low temperature (10 °C), exists in equilibrium between the major native state (F) and a sparsely populated (~2%) folding intermediate (I) in which the C-terminal β-strand is disordered, exposing a hydrophobic patch.8 We showed that apo GroEL stabilizes the folding intermediate, accelerates the overall interconversion between the two states ~20-fold, and increases the rate constant for the F to I transition by ~500-fold.3 In this paper, we investigate two mimetics of the folding intermediate, one stable and the other metastable, and present NMR evidence of confinement and stabilization of the folding intermediate mimetics within the cavity of apo GroEL.
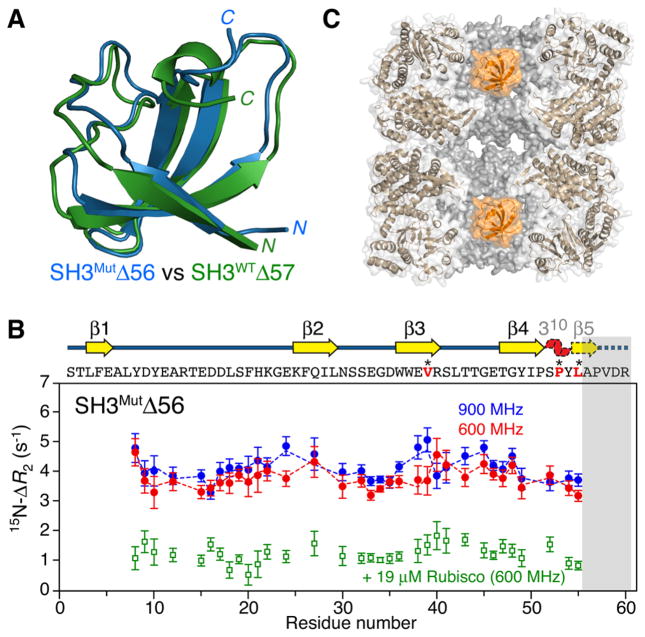
The two mimetics of the Fyn SH3 folding intermediate comprise three-residue (SH3WTΔ57) and four-residue (SH3MutΔ56) C-terminal truncations of the full-length wild-type and triple-mutant domains, respectively (see the Supporting Information for full details of cloning, expression, and purification). The 1H–15N correlation spectra of SH3MutΔ56 and SH3WTΔ57 are well-resolved and characteristic of folded proteins (Figure S1). The global folds for both truncated constructs, determined using CS-Rosetta9 from backbone chemical shifts and residual dipolar couplings (Figure S2), are very similar to one another (Figure 1A) as well as to the native and folding intermediate states of full-length SH3Mut (Figure S3). The distinguishing characteristic of both truncation mutants and the folding intermediate8 of full-length SH3Mut is the unfolding of the C-terminal β-strand; the remaining domain structure, however, remains essentially unchanged relative to the native state. Analytical ultra-centrifugation (Figure S4) and 15N CPMG relaxation dispersion measurements (Figure S5) show that the folded (F) monomeric state of SH3MutΔ56 undergoes concentration-dependent exchange with a folded dimer (FD) and concentration-independent exchange with the fully unfolded (U) state, while SH3WTΔ57 is a stable folded monomer with no significant relaxation dispersion (Rex < 1 s−1). For SH3MutΔ56, for which large dispersions are observed, the calculated 15N chemical shifts for the unfolded state are fully consistent with those predicted for a random coil (Figure S5B); in addition, five residues have optimized values of >0.7 ppm for the 15N chemical shift differences between folded monomeric and dimeric states (Figure S5C). The latter, together with residues showing severely broadened 1H/15N correlations at a high concentration of SH3MutΔ56, form a single contiguous dimerization interface that largely coincides with the hydrophobic GroEL-binding surface3 (Figure S6).
Figure 1.
Interaction of SH3MutΔ56 with apo GroEL. (A) Ribbon diagrams showing a superposition of the structures of SH3MutΔ56 (blue) and SH3WTΔ57 (green), representing mimetics of a folding intermediate of the Fyn SH3 domain, determined from backbone chemical shifts and residual dipolar couplings using CS-Rosetta9 (see the Supporting Information and Figures S2 and S3). (B) 15N ΔR2 observed for 100 μM 15N-labeled SH3MutΔ56 in the presence of 120 μM (in subunits) apo GroEL at 900 MHz (blue circles) and 600 MHz (red circles) and 10 °C. The dashed lines represent the best fits obtained by simultaneously fitting all relaxation data (ΔR2, DEST, and relaxation dispersion) to the model shown in Figure 3. The ΔR2 effect is essentially abolished when the GroEL cavity is blocked by acid-denatured Rubisco (green circles). The gray bar indicates the residues deleted in SH3MutΔ56. The sequence (residues in red are sites of mutations) and secondary structure are shown above the panel; the 310 helix and β5 (dashed outline) are unfolded in SH3MutΔ56, but only β5 is unfolded in SH3WTΔ57 (see panel A). Error bars are one standard deviation. (C) Cross section through apo GroEL (Protein Data Bank entry 3E7610) illustrating the two cavities with a SH3MutΔ56 molecule (orange, space-filling model) confined in each cavity.
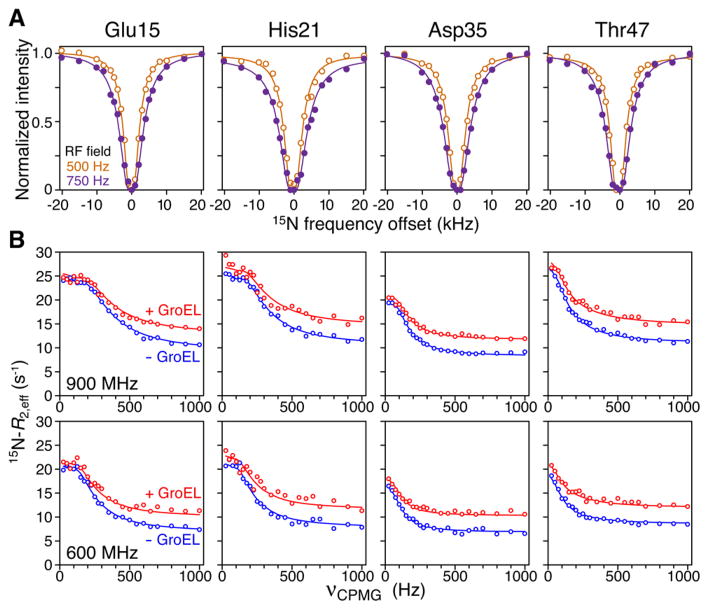
In the presence of apo GroEL, relatively uniform 15N lifetime line broadening is observed for both SH3MutΔ56 (Figure 1B) and SH3WTΔ57 (Figure S7A), which is largely abolished when the cavity of GroEL is blocked by acid-denatured Rubisco (Figure 1B), a protein substrate that binds with nanomolar affinity to GroEL.11 Significant broadening of 15N DEST profiles is also observed for both SH3MutΔ56 (Figure 2A) and SH3WTΔ57 (Figure S7B) in the presence of apo GroEL with widths at half-height of ~5 kHz at saturation field strengths of 500–750 Hz. No significant relaxation dispersions are observed for SH3WTΔ57 in the presence of apo GroEL, indicating that any unfolded GroEL-bound species remains below the level of detection. Thus, one can conclude that the ΔR2 and DEST effects arise from the binding of the folded truncated species to the high-molecular weight apo GroEL (presumably at the apical domain). The magnetic field dependence of ΔR2 is smaller than that expected for a relaxation mechanism based on the one-bond 1H–15N dipolar coupling interaction and a −170 ppm 15N chemical shift anisotropy: for SH3MutΔ56 (Figure 1B) and for SH3WTΔ57 (Figure S7A) compared to expected values of 1.3 and 1.2, respectively, indicating that exchange is in the slow-to-intermediate exchange regime on the relaxation time scale and the overall value of the pseudo-first-order rate constant for exchange between small (observable) and high-molecular weight “dark” species is close to the maximal value of ΔR2.5,6
Figure 2.
15N DEST and CPMG relaxation dispersion observed for 100 μM 15N-labeled SH3MutΔ56 in the presence of 120 μM (in subunits) apo GroEL at 10 °C. (A) Examples of 15N DEST profiles at RF field strengths of 500 MHz (orange circles) and 750 MHz (purple circles). (B) Examples of 15N CPMG relaxation dispersion curves in the presence (red circles) and absence (blue circles) of apo GroEL at 900 MHz (top) and 600 MHz (bottom). The solid lines represent best fits obtained by simultaneously fitting all relaxation data (ΔR2, DEST, and relaxation dispersion) to the model shown in Figure 3.
The 15N CPMG dispersion profiles for SH3MutΔ56 in the presence of apo GroEL are characterized by a decrease in Rex (i.e., the difference in R2,eff between the lowest and highest CPMG field strengths) relative to those for free SH3MutΔ56 (Figure 2B). This is manifested by only a small increase in R2,eff at the lowest CPMG field, while at high CPMG fields (1000 Hz), R2,eff is increased by ~ΔR2. The reduction in Rex is eliminated when GroEL is blocked by acid-denatured Rubisco (Figure S8). Direct binding of the folded state (F) to GroEL (i.e., exchange with a slowly tumbling species) cannot by itself lead to a decrease in Rex. To explain this observation, an additional observable state (FE) that does not exchange with unfolded state U in the presence of GroEL has to be invoked in which the folded conformation is tethered and stabilized upon confinement within the cavity of GroEL (Figure 3). In effect, the presence of state FE reduces the population of the species that can spontaneously unfold with a concomitant decrease in Rex. The confined state FE is considered to be an observable state with the same 15N chemical shifts and R2 values comparable to those of F.
Figure 3.
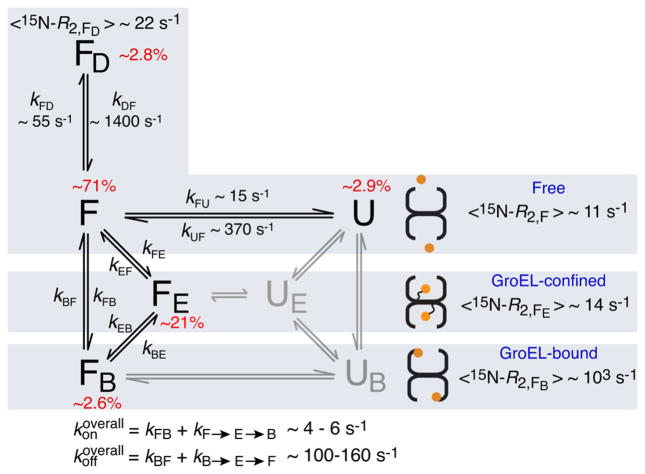
Kinetic scheme for the interaction of SH3MutΔ56 with apo GroEL. The populations of states UE and UB, colored gray, are below the limits of detection and were therefore not included in the fitting of the relaxation data (see the Supporting Information for theory and details of the fitting procedure). The rate constants and populations relate to experimental conditions of 100 μM SH3 domain and 120 μM (in subunits) GroEL at 10 °C. Binding of the dimer FD to GroEL is assumed to be undetectable because of both its size and the fact that dimerization occludes the GroEL-binding surface (Figure S6). For SH3WTΔ57, there is no evidence of the existence of an unfolded state as no relaxation dispersion is observed. The 15N ΔR2 and DEST data for SH3WTΔ57 (Figure S7) show binding of the folded state to GroEL and are explained by two-state exchange between states F and FB, with kFB and kBF values of ~7 and 500 s−1, respectively (corresponding to pB ~ 1.4%).
The full kinetic scheme describing all possible equilibria in the SH3MutΔ56/GroEL system is shown in Figure 3. The folded state F is in slow exchange with unfolded state U and the dimeric form of the protein (FD) in bulk solution. Observable folded (FE) and unfolded (UE) states confined within the GroEL cavity reorient rapidly; the corresponding “dark”, directly bound species, FB and UB, tumble with the same rotational correlation time as GroEL. In addition, both confined and directly bound species can potentially undergo inter-conversion between folded and unfolded states. The maximal possible fraction of UE, in the absence of stabilization of FE, is <1% as free unfolded species U is sparsely populated. If the FE ↔ UE interconversion is even slightly shifted toward FE, the fraction of UE becomes even smaller. Initial fitting of all the relaxation data (see the Supporting Information for full details of the fitting) to the full model shown in Figure 3 (including the parts colored light gray) indicated that the populations of UE and UB could not be defined and were driven to zero during minimization. We therefore conclude that states UE and UB are undetectable because of their very small populations (consistent with the absence of any sequential, hydrophobic GroEL-binding consensus sequences12), which, in effect, is equivalent to assuming that unfolding of SH3MutΔ56 does not occur within the GroEL cavity. Thus, the five-state model given by the states colored black in Figure 3 can fully account for the relaxation data for the SH3MutΔ56/GroEL system.
The complexity of the kinetic scheme in Figure 3 precludes the definition of all the rate constants. In addition, the population (pB) and average R2 value of FB are correlated in the slow-to-intermediate exchange regime so that pB can be defined only by assuming a value of 〈15N – R2,FB〉 consistent with the molecular weight (~800 kDa) of GroEL [~950 s−1 at 900 MHz and 10 °C (Supporting Information and Figure S9)]. This allows one to define the populations of the FE (~21%) and FB (~2.6%) states, as well as the overall rate constants for the interconversion of the F and FB states through direct (F ↔ FB) and indirect (F ↔ FE ↔ FB) pathways; the relative contributions of these two pathways, however, cannot be determined. The overall rate constants for the conversion of F to FB and the reverse FB to F process are given by13
and
with values in the range 4–6 and 100–160 s−1, respectively. (Note the small population of the “dark” FB state is fully consistent with the observation that no measurable reduction in cross-peak volumes can be detected in the 1H–15N correlation spectrum of SH3MutΔ56 in the presence of GroEL.)
The population of the confined state FE corresponds to an occupancy of approximately one molecule of SH3MutΔ56 per cavity. Given a cavity volume of ~85000 Å,3,14 the concentration of FE within the cavity is ~20 mM, corresponding to a 200-fold enrichment relative to the concentration in the bulk solution (100 μM). The simplest explanation for this phenomenon is that the FE state of SH3MutΔ56 is tethered to the end of one or more of the 23-residue, methionine-rich C-terminal tails (each containing five methionines in the last 11 residues of the sequence). Because the tails are intrinsically disordered (invisible in the apo GroEL electron density map)10,14 and highly mobile [as evidenced by a set of very sharp, superimposed, methionine methyl cross-peaks at the random coil position in a 1H–13C TROSY correlation spectrum of a [13Cmethyl]methionine-labeled sample (unpublished data)], the effective rotational correlation time and hence R2 values of FE would be very similar to those of the F state in bulk solution.
Why is the folded state of SH3MutΔ56 stabilized within the GroEL cavity? The radius of gyration (Rgyr) of fully unfolded SH3MutΔ56 is estimated to be 20–24 Å.15 The radius of the cylindrical cavity of GroEL is ~30 Å.10,14 Thus, one would predict, from purely entropic considerations, that the folded state of SH3MutΔ56 (Rgyr ~ 12–14 Å) would be preferred over the fully unfolded state upon confinement in the GroEL cavity in the absence of any compensatory interactions of the unfolded state with the cavity walls.16 Using the random-flight Gaussian chain model developed by Zhou and Dill17 to describe the thermodynamics of stabilization of protein folded states upon confinement within a cavity, the energy of stabilization (ΔΔG) for folded SH3MutΔ56 is calculated to be −2.2 kcal mol−1, corresponding to a population of ~0.017% for the confined unfolded state UE (Figure 3A) (see the Supporting Information). Such small fractions cannot be detected by relaxation-based NMR techniques and can be safely neglected in analysis. In addition, numerical simulations of relaxation dispersion profiles for the full system in Figure 3 (encompassing a total of seven exchanging states) show that the lower bound for the fraction of UE detectable under the experimental conditions used in this work is approximately 0.2–0.3%.
In conclusion, through the combined analysis of 15N lifetime line broadening, DEST, and CPMG relaxation dispersion, we have shown that protein substrates can be confined and tethered within the cavity of GroEL (probably through interaction with the disordered C-terminal tails) and that the folding intermediate of the Fyn SH3 domain, represented by the SH3MutΔ56 truncation mutant, is stabilized upon confinement within the GroEL cavity relative to the unfolded state. In conjunction with our previous work3 on the interaction of SH3Mut with GroEL, we have now obtained a more complete picture of the passive effect that GroEL exerts on these particular protein substrates. Specifically, GroEL stabilizes folding intermediates relative to both folded (in the case of SH3Mut) and unfolded (in the case of SH3MutΔ56) states and, in addition, increases the rate of interconversion between fully folded and folding intermediate states. The unfolded state of the SH3 domain, which does not contain any hydrophobic GroEL consensus binding sequences,12 is disfavored within the GroEL cavity because of its larger radius of gyration, while the folding intermediate is favored over the native state by exposure of a GroEL binding-competent hydrophobic patch upon unfolding of the C-terminal β5 strand of the SH3 domain.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Intramural Program of NIDDK, NIH, and by the AIDS Targeted Antiviral program of the Office of the Director of the NIH (to G.M.C.).
We thank Drs. Baber, Garrett, and Ying for technical support.
Footnotes
ORCID
G. Marius Clore: 0000-0003-3809-1027
Author Contributions
D.S.L. and V.T. contributed equally to this work.
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem. 6b01237.
Details of sample preparation, NMR experiments, and fitting of the experimental data (Figures S1–S9) (PDF)
References
- 1.(a) Thirumalai D, Lorimer GH. Annu Rev Biophys Biomol Struct. 2001;30:245–269. doi: 10.1146/annurev.biophys.30.1.245. [DOI] [PubMed] [Google Scholar]; (b) Horwich AL, Fenton WA. Q Rev Biophys. 2009;42:83–116. doi: 10.1017/S0033583509004764. [DOI] [PubMed] [Google Scholar]; (c) Hartl FU, Bracher A, Hayer-Hartl M. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 2.(a) Robinson CV, Gross M, Eyles SJ, Ewbank JJ, Mayhew M, Hartl FU, Dobson CM, Radford SE. Nature. 1994;372:646–651. doi: 10.1038/372646a0. [DOI] [PubMed] [Google Scholar]; (b) Zahn R, Spitzfaden C, Ottiger M, Wuthrich K, Pluckthun A. Nature. 1994;368:261–265. doi: 10.1038/368261a0. [DOI] [PubMed] [Google Scholar]; (c) Zahn R, Perrett S, Stenberg G, Fersht AR. Science. 1996;271:642–645. doi: 10.1126/science.271.5249.642. [DOI] [PubMed] [Google Scholar]; (d) Zahn R, Perrett S, Fersht AR. J Mol Biol. 1996;261:43–61. doi: 10.1006/jmbi.1996.0440. [DOI] [PubMed] [Google Scholar]; (e) Priya S, Sharma SK, Sood V, Mattoo RU, Finka A, Azem A, De Los Rios P, Goloubinoff P. Proc Natl Acad Sci U S A. 2013;110:7199–7204. doi: 10.1073/pnas.1219867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libich DS, Tugarinov V, Clore GM. Proc Natl Acad Sci U S A. 2015;112:8817–8823. doi: 10.1073/pnas.1510083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer AG., 3rd J Magn Reson. 2014;241:3–17. doi: 10.1016/j.jmr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawzi NL, Ying J, Ghirlando R, Torchia DA, Clore GM. Nature. 2011;480:268–272. doi: 10.1038/nature10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fawzi NL, Ying J, Torchia DA, Clore GM. J Am Chem Soc. 2010;132:9948–9951. doi: 10.1021/ja1048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthis NJ, Clore GM. Q Rev Biophys. 2015;48:35–116. doi: 10.1017/S0033583514000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neudecker P, Robustelli P, Cavalli A, Walsh P, Lundstrom P, Zarrine-Afsar A, Sharpe S, Vendruscolo M, Kay LE. Science. 2012;336:362–366. doi: 10.1126/science.1214203. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Lange O, Delaglio F, Rossi P, Aramini JM, Liu G, Eletsky A, Wu Y, Singarapu KK, Lemak A, Ignatchenko A, Arrowsmith CH, Szyperski T, Montelione GT, Baker D, Bax A. Proc Natl Acad Sci U S A. 2008;105:4685–4690. doi: 10.1073/pnas.0800256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiser PD, Lorimer GH, Palczewski K. Acta Crystallogr, Sect F: Struct Biol Cryst Commun. 2009;65:967–971. doi: 10.1107/S1744309109032928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Vies SM, Viitanen PV, Gatenby AA, Lorimer GH, Jaenicke R. Biochemistry. 1992;31:3635–3644. doi: 10.1021/bi00129a012. [DOI] [PubMed] [Google Scholar]
- 12.Stan G, Brooks BR, Lorimer GH, Thirumalai D. Proc Natl Acad Sci U S A. 2006;103:4433–4438. doi: 10.1073/pnas.0600433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein AV. J Phys Chem B. 2015;119:158–163. doi: 10.1021/jp5109703. [DOI] [PubMed] [Google Scholar]
- 14.Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 15.Fitzkee NC, Rose GD. Proc Natl Acad Sci U S A. 2004;101:12497–12502. doi: 10.1073/pnas.0404236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal J, Best RB. Proc Natl Acad Sci U S A. 2008;105:20233–20238. doi: 10.1073/pnas.0807742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou HX, Dill KA. Biochemistry. 2001;40:11289–11293. doi: 10.1021/bi0155504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.