Abstract
In obesogenic environments food-related external cues are thought to overwhelm internal cues that normally regulate energy intake. We investigated how this shift from external to internal stimulus control might occur. Experiment 1 showed that rats could use stimuli arising from 0 and 4h food deprivation to predict sucrose delivery. Experiment 2 then examined (a) the ability of these deprivation cues to compete with external cues and (b) how consuming a Western-style diet (WD) affects that competition. Rats were trained to use both their deprivation cues and external cues as compound discriminative stimuli. Half of the rats were then placed on WD while the others remained on chow, and external cues were removed to assess learning about deprivation state cues. When tested with external cues removed, chow-fed rats continued to discriminate using only deprivation cues, while WD-fed rats did not. The WD-fed group performed similarly to control groups trained with a noncontingent relationship between deprivation cues and sucrose reinforcement. Previous studies provided evidence that discrimination based on interoceptive deprivation cues depends on the hippocampus and that WD intake could interfere with hippocampal functioning. A third experiment assessed the effects of neurotoxic hippocampal lesions on weight gain and on sensitivity to the appetite-suppressing effects of the satiety hormone cholecystokinin (CCK). Relative to controls, hippocampal-lesioned rats gained more weight and showed reduced sensitivity to a 1.0 ug but not 2.0 or 4.0 ug CCK doses. These findings suggest that WD intake reduces utilization of interoceptive energy state signals to regulate appetitive behavior via a mechanism that involves the hippocampus.
1. Introduction
A longstanding idea is that the regulation of energy intake and body weight depends on the integrative control by external food-related cues in the environment and physiological signals arising from the internal milieu. In fact, according to several accounts, the ability of such environmental food cues to evoke appetitive and consummatory responding is held in check by interoceptive satiety signals that inhibit those behaviors (e.g., [1–3]). It is not surprising that, within this framework, excess intake and body weight gain have often been seen as a consequence of a reduction in the control of feeding by internal cues relative to that by external cues [4, 5].
A similar idea is expressed in Woods’s model of energy regulation [6]. This model proposes that in humans and other animals, meal initiation depends almost exclusively on the presence of environmental food cues, whereas meals are terminated, and presumably new intake is suppressed, by the emergence of interoceptive satiety cues. Thus, energy dysregulation leading to obesity can be seen as a failure of interoceptive satiety cues to adequately counter response evocation by food cues in the environment. That is, increased sensitivity to external food-related stimuli by obese compared to lean people may be based on insensitivity to internal signals that normally suppress the ability of external cues to evoke appetitive and consummatory responses.
Most of the world’s obese and overweight populations live in Western and Westernized societies in which environmental cues associated with highly-palatable, energy-dense foods and beverages are ubiquitous [7]. This combination of heightened sensitivity to food cues and the creation of an “obesogenic” environment where contact with these cues is almost unavoidable may have produced a calamitous “perfect storm” in the current obesity pandemic. The so-called “Western diet” is widely popular in these places and contains high levels of saturated fats and processed sugars [8, 9]. Intake of the Western diet is not only associated with excess energy intake and obesity, but also with increased incidence of brain pathology and cognitive dysfunction [10].
A variety of evidence shows that consuming a Western-style diet can impair the performance of rodents and humans on learning and memory problems that depend on the functional integrity of the hippocampus [11–15]. Other studies in our laboratory and elsewhere have demonstrated that the ability to use interoceptive food deprivation and hydrational stimuli to solve discrimination problems is also dependent on the hippocampus [16–19]. In contrast, there is little evidence that the hippocampus is required to learn about simple nonspatial discriminative stimuli [20, 21]. Considered together, this set of findings is consistent with the idea that a WD-induced interference with hippocampal function could diminish appetitive control by interoceptive relative to exteroceptive cues. In other words, such a loss of hippocampal function could promote overeating based on a weakened ability to use interoceptive satiety signals to counter response evocation by food and food-related external cues.
While external food cues in the obesogenic environment are often described as overwhelming the internal biological controls of intake, the mechanisms that underlie this phenomenon remain to be specified. The purpose of the present paper is to investigate the possibility that the weakening of internal relative to external stimulus control of appetitive behavior is a consequence of a Western diet-induced impairment in the ability to discriminate between interoceptive energy state signals.
Previous research in our laboratory has shown that rats can use the interoceptive stimulus consequences of different levels of food deprivation as discriminative cues for the delivery of either mild shock (e.g., [16, 22]) or sucrose pellets [23]. Evidence for this learning has been obtained after as few as three reinforced trials [17], and discriminative control generalizes from cues produced by food deprivation and satiation to hormonal manipulations such as exogenous administration of ghrelin [23], cholecystokinin-octapeptide (CCK-8) [24, 25], and leptin [25] that are known to promote or suppress feeding behavior. Experiment 1 expanded on these earlier studies by employing levels of food deprivation and satiation more comparable to what rats would experience as part of their normal meal patterning (i.e., 0 and 4h food deprivation) (e.g., [26]). Experiment 2 investigated (a) how cues arising from low levels of food deprivation control appetitive behavior in compound with discrete external cues, and (b) how consuming a Western diet affects discriminative control by food deprivation cues in the presence and absence of external stimuli. Experiment 3 compared rats with selective ibotenate lesions of the hippocampus and intact controls with respect to post-surgical body weight and sensitivity to the intake-suppressing effects CCK-8. CCK-8 has long been considered to be a hormonal satiety signal based on findings that the release of endogenous CCK from the intestine after eating is correlated with suppression of food intake, administration of exogenous CCK reduces food intake, and administration of CCK antagonists increases food intake (for review [27]). Further, studies of deprivation discrimination learning have shown that exogenous CCK produces interoceptive stimuli that generalize to cues arising from 0h food deprivation [24, 25].
2. Methods
2.1 Apparatus
All training and testing sessions were conducted in 8 identical conditioning chambers constructed of aluminum end walls and Plexiglas sidewalls, measuring 59.7 × 34.3 × 26.35 cm (Lafayette Instruments). The floors of the chamber consisted of stainless steel metal rods measuring .48 cm in diameter and 1.07 cm apart. The auditory stimulus was a tone (1500 Hz, 74–76 db, Sonalert, Lafayette Instruments). The light conditioned stimulus measured 2.4 cm in diameter and was located 5 cm to the left of and 6 cm above the recessed food magazine. A computer-operated infrared monitoring system located in the recessed food magazine was used to record food magazine entries. Reinforcers were 45 mg sucrose pellets (Research Diets, P.J. Noyes Company Inc., Lancaster, N.H.).
2.2. Experiment 1: Learning to use food deprivation states as discriminative cues for sucrose
2.2.1. Subjects
The subjects were 16 naïve male Sprague-Dawley rats (Harlan), weighing 250–300 g upon arrival. Rats were individually housed in exhaust ventilated plastic tub cages (Optirat). The colony room was maintained on a 10:14 hour light:dark cycle with lights on at 1330h. All rats were maintained on standard laboratory rodent chow (LabDiet, Formula 5001), which has a caloric density of 3.0 kcal/g (approximately 13% kcal from fat, 56% kcal from carbohydrates). All rats were maintained on ad libitum water throughout the experiment. All procedures for the care and treatment of the rats in this experiment were approved by the American University Institutional Animal Care and Use Committee.
2.2.2. Behavioral training
After acclimating to the colony room for approximately two weeks, the rats were assigned to one of two training groups (n = 8) matched on body weight. All rats were then placed on a daily alternating schedule of 0h and 4h food deprivation throughout the remainder of the study. Rats were given 24 hours access to ad lib chow on 0h days and deprived for 4 hours prior to training on 4h days. All rats were placed in the apparatus for 6 min training sessions. Rats in Group 0+ were reinforced under 0h deprivation (~24h ad libitum access to food), but not reinforced under 4h deprivation, while Group 4+ received the opposite contingency. When training sessions occurred under the rewarded deprivation, the feeder activated and dispensed five 45 mg sucrose pellets into the food magazine 4 min after the start of the session. When sessions occurred under the nonrewarded deprivation state, the pellet dispensers operated, but pellets were not dispensed into the food cup. Rats remained in the chambers for 2 additional minutes following pellet dispenser activation before being returned to their home cages. Throughout the experiment, the 4 min period that ended with feeder activation was further subdivided into twenty-four 10 s intervals. The percent of these intervals during which the infrared photobeam inside the food magazine was broken (i.e., percent beam breaks) was the index of appetitive behavior throughout the experiment. Training sessions were conducted at 1330h but were not held every day (5 training sessions per week) to prevent reinforcement delivery on a single alternating schedule. Rats were trained in squads of 8, with one animal assigned to each conditioning chamber. Training consisted of 72 sessions, 36 sessions under 0h food deprivation and 36 sessions under 4h food deprivation.
2.3. Experiment 2: Effects of Western diet on the relative salience of food deprivation and external discriminative stimuli
2.3.1. Subjects
The subjects were 32 naïve male Sprague-Dawley rats (Harlan) of the same description as those used for Experiment 1. The rats were also housed and treated in the same way as the rats described in Experiment 1 prior to the beginning of experimental manipulations.
2.3.2. Behavioral training
As in Experiment 1, following acclimation to the colony room for approximately two weeks, rats were assigned to one of two training groups (n = 16) matched on body weight and then placed on a daily alternating schedule of 0h and 4h food deprivation. Group Deprivation+ (Dep+) was trained with deprivation states and external light and tone cues as compound discriminative stimuli. These rats were reinforced during training sessions when they were under 4h food deprivation in the presence of one external cue and were not reinforced when they were under 0h food deprivation in the presence of the other external cue. Rats in Group Deprivation Noncontingent (DepN) were trained on the same deprivation schedule as Group Dep+. However, for Group DepN only the external cues and not their deprivation cues were trained as relevant discriminative stimuli. That is, one external cue signaled reinforcement and the other signaled nonreinforcement, whereas the probability of reinforcement was the same under each deprivation level. The identities of the reinforced and nonreinforced external cues were counterbalanced for both groups. The presentation of stimuli, training schedule, and index of appetitive behavior were the same as those used in Experiment 1. Training sessions were conducted at 1330h. Training consisted of 56 sessions, 28 sessions under 0h food deprivation and 28 sessions under 4h food deprivation.
The day after asymptotic discrimination performance was achieved, a deprivation cue test was conducted to assess learning about interoceptive deprivation cues during training. For four test sessions, two under each deprivation level, discriminative control of appetitive responding by deprivation cues was assessed in the absence of external light and tone cues. Beginning the day following the conclusion of the deprivation cue test, the rats were tested for discriminative control by their external cues when their deprivation state was held constant at 0h for four test sessions. While deprivation state cannot be removed (i.e., a rat will always be under some deprivation state), deprivation state was held constant to assess learning about external cues during training.
To re-establish baseline discrimination prior to dietary manipulations, discriminative performance using deprivation and external cues as compound discriminative stimuli was reassessed (i.e, retraining). After four off-days following the external cue test, rats were retrained for 16 sessions, 8 under each deprivation state.
2.3.3. Dietary treatment
The day after discrimination performance with the original compound discriminative stimuli was re-established in retraining, half of the rats were placed on WD. For diet assignment, each contingency group was further subdivided into two groups matched on terminal retraining discrimination performance and body weight. Two of these groups (Dep+ Chow and DepN Chow) remained on their standard low fat chow diet (same as that described for Experiment 1). The remaining two groups (Dep+ WD and DepN WD) were placed on a western-style diet (WD) with a caloric density of approximately 4.4 kcal/g and a macronutrient composition of ~ 42% kcal from fat, 37% kcal from carbohydrates, and 19 % kcals from protein. The WD contained the following (g/kg): 270g casein, 220.5g dextrose, 120g maltodextrin, 170g lard, 15g safflower oil, 15g soybean oil, 80g corn starch, and 50g cellulose.
2.3.4. Post-diet testing
Following the shift of half of the rats in each contingency group to WD, external cues were removed from the discrimination to assess learning about interoceptive cues. Specifically, the day after the last sessions of retraining with the compound discriminative stimuli, no training occurred and rats were given WD. The next day, rats were trained without external cues, while the interocpetive cue schedule remained the same (i.e., Dep+ and DepN). While Group Dep+ would only be able to use the deprivation cue contingency, Group DepN did not receive a predictive contingency during this phase. Deprivation cues only were present during behavioral testing for 16 sessions, 8 under each deprivation state (duration of 21 days). All rats consumed all of the dispensed sucrose pellets regardless of diets.
After one off-day, external cues were re-introduced into the discrimination for four sessions, two under each deprivation state. When both deprivation and external cues were again present in compound, sucrose delivery was contingent on both deprivation and external cues for Groups Dep+ WD and Dep+ Chow; while Groups DepN WD and DepN Chow underwent the same deprivation regimen, sucrose delivery was only contingent on the external cues.
2.4. Experiment 3: Hippocampal lesions attenuate appetite-suppressing effects of CCK
2.4.1. Subjects
The subjects were 24 male Sprague-Dawley rats, weighing 325–350 g at the outset of the experiment. The rats were maintained under a 12:12 light:dark cycle with the light phase beginning at 0700h. Prior to the beginning of the current study, all rats participated in a simple Pavlovian conditioning experiment in which a brief auditory stimulus signaled the presentation of sucrose pellets. In preparation for that study, food rationing was used to reduce all rats to 85% of their free-feeding weights. The rats were maintained at that weight for approximately 14 days before being returned to ad libitium feeding (Lab Diets 5001) for 6 days, during which time all rats met or exceeded their original free-feeding weights. The rats were then assigned to surgical treatment conditions which were matched on body weight.
Rats were housed in stainless steel hanging wire cages with a metal food hopper and a glass water bottle fastened to the front of each cage. In addition, 50 ml hard plastic centrifuge tubes were fastened to the cage front in place of the food hopper on test days when intake of the liquid dietary supplement Chocolate Ensure Plus ® (referred to hereafter as Ensure) was recorded. This study was conducted at Purdue University and the care and use of the rats was reviewed and approved by the Purdue University Animal Care and Use Committee.
2.4.2. Surgery
The rats were assigned to three groups matched on mean body weight calculated based on the two days immediately prior to the beginning of surgery. One group of rats (n = 8) received lesions of the total hippocampus using multiple focal injections of small amounts of the selective neurotoxin ibotenic acid (IBO: Biosearch Technologies). Operated control rats (n = 8) underwent the same surgical procedures for rats with lesions except that no IBO was injected. The final group of rats consisted of 8 unoperated control animals.
For lesioned rats, the IBO was dissolved in phosphate buffered saline (pH 7.4) at a concentration of 10 mg/ml. The rats were anesthetized with intraperitoneal injections of equithesin (a combination of pentobarbital and chloral hydrate) and placed in a Kopf stereotaxic apparatus. Following the procedure described in detail previously [28, 29], an incision was made in the scalp, and the bone overlying the area to be lesioned was removed. Injections of IBO were made with a 5-μl Hamilton syringe mounted on the stereotaxic frame and held in a Kopf microinjector unit (Model 5000). A small diameter glass micropipette was glued onto the end of the needle of the syringe in order to minimize damage to the cortex overlying the area to be lesioned. Injections were made over approximately 1 min at each site, and the pipette was left in place for approximately 1 min to prevent spread of the neurotoxin up the tract. Stereotaxic coordinates for the lesions and the amounts injected at each site are shown in Table 2. Rats in the lesioned group (n = 8) received focal injections at 38 different sites for a total of 2.3 ul of IBO. All surgeries were conducted over a three-day period. The number of rats that received surgery on each day was equated for each surgical group.
Table 2.
Coordinates for Complete Hippocampal Lesions
| AP | ML +/− | DV | Amount (μl) |
|---|---|---|---|
| −2.4 | 1.0 | −3.4 | 0.07 |
| −3.1 | 1.4 | −3.6 | 0.07 |
| −2.8 | 0.06 | ||
| 3.0 | −3.5 | 0.10 | |
| −4.0 | 2.5 | −3.6 | 0.07 |
| −2.8 | 0.06 | ||
| 3.6 | −3.8 | 0.05 | |
| −3.1 | 0.05 | ||
| −4.9 | 4.9 | −7.5 | 0.12 |
| −6.0 | 0.05 | ||
| −4.4 | 0.05 | ||
| 2.6 | −3.6 | 0.05 | |
| −2.7 | 0.05 | ||
| −5.7 | −5.0 | −7.5 | 0.05 |
| −6.5 | 0.05 | ||
| −5.5 | 0.05 | ||
| −4.5 | 0.05 | ||
| −3.6 | −7.5 | 0.05 | |
| −4.0 | 0.05 |
2.4.3. Body weight
After the lesioned rats achieved a post-surgery body weight that equaled or surpassed that of ad lib-fed controls, recovery from surgery was deemed complete (see [30]). Body weight gain was calculated daily beginning when this recovery criterion was achieved. The rats were maintained on ad libitum feeding throughout the study except for six periods that took place between post-recovery Days 40 and 60 in which the rats were fasted overnight prior to collection of tail blood for glucose testing. Body weights did not differ significantly for hippocampal lesioned compared to control groups during these overnight deprivation periods or during ad lib feeding when body weights recovered following overnight deprivation.
2.4.4. CCK
At the conclusion of the study, the rats’ intake after receiving i.p. injections of 4, 2, and 1 μg/kg CCK-8 (Peptides International, Louisville, KY) in descending order was compared to intake after isotonic saline injections. The rats were food deprived for 24h prior to each test. Each test comprised of two sessions in which half of the rats in each group received CCK before saline, and half received the reverse order of treatment. Each rat was injected i.p. with CCK-8 or saline, and approximately 15 min later, tubes containing 30 ml of Ensure were attached to the front of each cage. Amount consumed was measured for each rat 30 and 60 min later. The rats were given free access to food and water beginning immediately after the conclusion of this 60 min period of intake measurement. The next session did not begin until the rats had returned to their pre-deprivation level of body weight. For all rats, the first (4 ug/kg dose), second (2 ug/kg dose) and final (1 ug/kg dose) tests with CCK-8 began 75, 83, and 91 days post-surgery, respectively.
2.4.5. Histology
At the end of the experiment, all rats were administered an overdose of anesthetic and perfused transcardially with a mixture of buffered physiological saline followed by 10% formaldehyde solution. The brains were removed, embedded in egg yolk, cryoprotected in a 30% solution of sucrose-formalin, and subsequently cut on a cryostat into 40-μm sections. Every fifth section was saved for histology. A cresyl violet cell body stain was used to determine cell loss and gliosis resulting from the lesions.
3. Results
3.1 Experiment 1: Learning to use food deprivation states as discriminative cues for sucrose
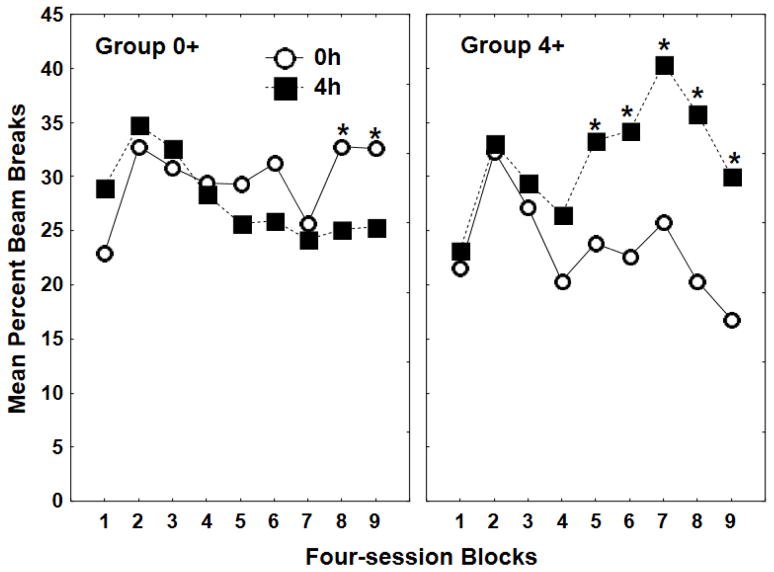
Figure 1 shows that by the end of nine four-session blocks of training, rats in both Groups 0+ and 4+ responded significantly more during sessions under their rewarded compared to their nonrewarded level of food deprivation. Analysis of variance (ANOVA) obtained a significant Group × Deprivation level × Block interaction (F(8, 104) = 4.13, p < .01). Post hoc tests (Fisher’s Least Significant Difference) revealed that Group 0+ responded significantly more on 0h compared to 4h sessions during the last two blocks of training, whereas Group 4+ responded significantly more under 4h compared to 0h sessions during each of the last five blocks of training (all ps < .05).
Figure 1.
Mean ± S.E.M. percent magazine entries during the 4-min period preceding sucrose delivery of reinforced (+) and nonreinforced (−) four-session blocks for Group 0+ (left panel) and Group 4+ (right panel) during Experiment 1 acquisition.
3.2. Experiment 2: Effects of Western diet on the relative salience of food deprivation and external discriminative stimuli
3.2.1. Training prior to diet manipulations
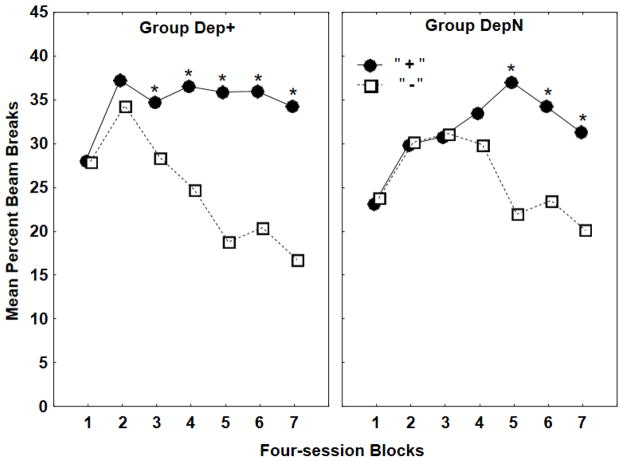
Both groups learned their discrimination problems with significant discriminative responding emerging somewhat earlier for Group Dep+ than for Group DepN (see Figure 2). ANOVA over all of training revealed that responding on rewarded trials was significantly greater than on nonrewarded trials for both groups (F(1, 30) = 74.68, p < .01). Post hoc Newman-Keuls tests found that this difference was significant on Blocks 3–7 for Group Dep+ and on Blocks 5–7 for Group DepN (all ps < .05).
Figure 2.
This graph depicts the results of discrimination training for Experiment 2, showing mean ± S.E.M. percent magazine entries during the 4-min period preceding sucrose delivery for four-session blocks for Groups Dep+ (left panel) and DepN (right panel).
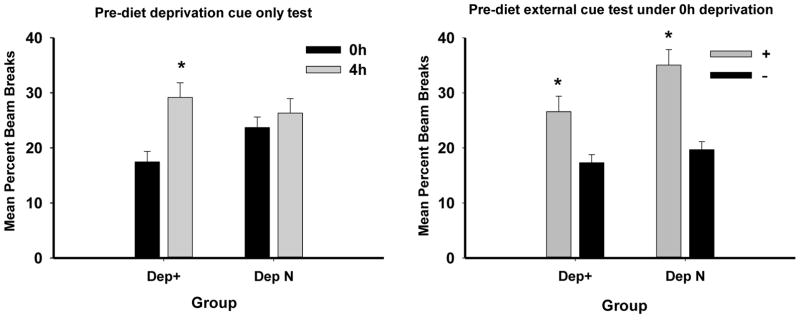
Each contingency group was then given two test sessions with their 0 and 4h deprivation cues alone (external cues removed). Group Dep+ maintained significant discriminative responding in the absence of external cues, whereas Group DepN did not (see left panel of Figure 3). ANOVA obtained a significant main effect of Deprivation level (0 vs. 4h) (F(1, 30) = 11.16, p < .01), as well as a significant Deprivation level × Contingency (Dep+ vs. DepN) interaction (F(1, 30) = 4.52, p < .05). Post hoc Bonferroni tests confirmed that Group Dep+ responded more when tested under 4h compared to 0h food deprivation (p < .01), whereas Group DepN did not. Because both groups were tested under the same 0 and 4h food deprivation conditions, this pattern of results indicates that (a) performance during testing with deprivation cues alone was based on the discriminative contingencies between deprivation cues and sucrose pellets established during training and that (b) the rats in Group Dep+ learned to use their deprivation state cues as discriminative stimuli even when exteroceptive stimuli were also relevant signals for reward.
Figure 3.
The left panel shows mean ± S.E.M. percent magazine entries during 4-min period preceding sucrose delivery during testing when external cues were removed for two sessions following compound cue training. The right panel shows mean ± S.E.M. percent magazine entries when all rats were tested under 0h deprivation for two test sessions with their rewarded and two sessions with their nonrewarded external cues.
Immediately following this test, discriminative control of the previously trained external stimuli was assessed for both groups when food deprivation was held constant at 0h (two sessions each under the rewarded and nonrewarded external cues). Both groups responded more to their rewarded compared to their non-rewarded external cue, although this difference was marginally larger for Group DepN (see right panel of Figure 3). ANOVA obtained a significant main effect of rewarded compared to nonrewarded cue (F(1, 30) = 48.84, p < .01). However, the interaction of this factor did not vary significantly as a function of Contingency (F(1, 30) = 3.02, p = .09). Combined with the results of the prior test with deprivation cues alone, the results of the test with external cues indicate that for Group Dep+, both food deprivation and external cues acquired discriminative control over appetitive responding. In contrast, for Group DepN, external cues, but not food deprivation cues, were sufficient to maintain significant appetitive discriminative responding.
3.2.2. Body weight
The rats maintained on WD gained substantially more weight compared to the chow-fed controls from the time the WD was introduced until the end of the study (see Table 1). Immediately prior to the introduction of WD, body weights for rats that would receive WD did not differ from rats that would remain on chow. However, after 28 days on WD, WD-fed rats weighed more than chow-fed rats, yielding a significant Diet × Period (Pre- vs. Post-WD) interaction (F(1, 28) = 200.52, p < .01). Newman-Keuls tests revealed that differences between the diet conditions were significant only during the post-WD period (p < .05). The variable of Contingency (Dep+ vs. DepN) did not yield a significant main effect or significant interaction with Diet or Period (largest F(1, 28) = 1.98, p > 0.17 for the Contingency × Period interaction).
Table 1.
Body Weight for Rats Exposed to WD and Chow Diets
| Group | Last Day Pre WD (± SEM) | Last Day Post WD (± SEM) |
|---|---|---|
| Dep+Ext+ Chow | 495.25 (12.93) | 512.50 (14.53) |
| Dep+Ext+WD | 489.38 (10.67) | 554.88 (12.11) |
| DepNExt+ Chow | 490.63 (6.93) | 505.63 (6.52) |
| DepNExt+WD | 491.25 (12.71) | 549.88 (14.09) |
3.2.3. Post-diet testing
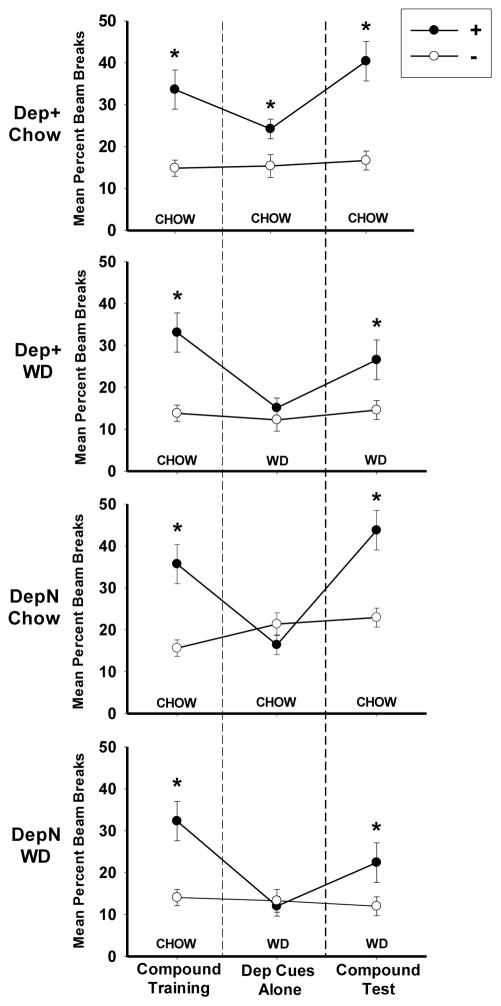
Figure 4 shows discrimination performance for all groups during (a) the last two-session block of retraining with both deprivation and external cues (to the left of the dashed line); (b) the first block of two 0-hr and two 4-hr sessions when all groups were tested with their deprivation cues alone on the day following the introduction of WD for the Dep+ WD and DepN WD groups; and (c) when the original training contingencies with the deprivation/external cue compound were reinstated. Discrimination performance for all groups was comparable at the end of the retraining phase. However, when the rats were tested with external cues removed, Group Dep+ Chow maintained discriminative responding based on their deprivation cues, but Group Dep+ WD did not. In fact, Group Dep+ WD’s discrimination performance based on their food deprivation cues did not differ significantly from that of Groups DepN Chow and DepN WD for which deprivation cues were not trained as valid discriminative stimuli. When the rats were tested again with their original food deprivation and external cue compounds, discrimination was maintained for Group Dep+ Chow and returned to significance for each of the remaining three groups.
Figure 4.
This graph shows the mean ± S.E.M. percent magazine entries during the 4-min period preceding sucrose delivery on (a) the last two-session block of both deprivation and external cues present (i.e., “Compound Retraining” leftmost), (b) the first two-session block of deprivation cues alone (i.e., “Dep Cues Alone”, center), and (c) the two-session block with the original deprivation/external cue compound (i.e., “Compound Test”, rightmost) for Groups Dep+ Chow, Dep+ WD, DepN Chow, and DepN WD during Experiment 2.
ANOVAs comparing performance on rewarded (+) versus nonrewarded (−) sessions among the groups for each of the three phases confirmed this pattern of results. For the last two sessions of the retraining phase, ANOVA obtained a significant main effect of + vs. − (F(1, 28) = 69.50, p < .01), which did not vary significantly by Contingency (F(1, 28) < 1 for the Group × + vs. − interaction), thereby confirming that all groups showed comparable significant discrimination performance at the end of retraining. However, during the first two 0-hr and 4 –hr sessions when external cues were removed to test discriminative control by deprivation state cues alone, the + vs. − × Diet × Contingency interaction was significant (F(1,28) = 4.29, p < .05). Post hoc Bonferroni tests showed that Group Dep+ Chow (p < .05) maintained significant discriminative responding when only deprivation cues were available, while Group Dep+ WD and the deprivation noncontingent groups (Groups DepN Chow and DepN WD) did not. A session-by-session analysis of the data from all eight test sessions with deprivations cues alone showed a similar pattern of results (data not shown). During these sessions with deprivation cues alone, ANOVA obtained a Diet × + vs. − × Contingency interaction for all sessions (F(1, 28) = 8.39, p < .01). Post hoc Bonferroni tests revealed that Group Dep+ Chow discriminated significantly (p < .01) based on energy state cues during this phase, while Groups Dep+ WD and deprivation noncontingent groups did not. Neither Contingency nor Diet interacted significantly with Sessions during testing with deprivation cues alone. During the final test phase when food deprivation and external cues were reinstated for two sessions as compound discriminative stimuli, ANOVA yielded a + vs. − × Diet interaction, (F(1, 28) = 5.43, p < .05), which indicated that the magnitude of discrimination was larger for chow-fed than WD-fed rats. However, in the absence of any interaction with or main effect of Contingency (F(1, 28) < 1 for the Diet × + vs. − × Contingency interaction), post hoc Bonferroni tests confirmed that both diet groups showed significant discrimination (ps < .05).
3.3 Experiment 3: Hippocampal lesions attenuate appetite-suppressing effects of CCK
3.3.1. Body weight
Mean body weights for unoperated and operated controls differed little on pre-surgery Day 1 (unoperated = 355.1 g; operated = 351.9 g, SEM ± 3.12) and on post-surgery Day 34 (unoperated = 422.3 g; operated = 424.3 g, SEM ± 7.99). These differences failed to yield a significant main effect of Group or Group × Day interaction (largest F(1,14) < 1). Furthermore, cumulative mean body weight gain did not differ significantly (F(1,14) = 1.86, p > .19) between these groups at the conclusion (Day 140) of the study (unoperated = 61.0 g; operated = 71.7 g, SEM ± 5.63). Therefore, the data from the unoperated and operated controls were combined for the remaining analyses.
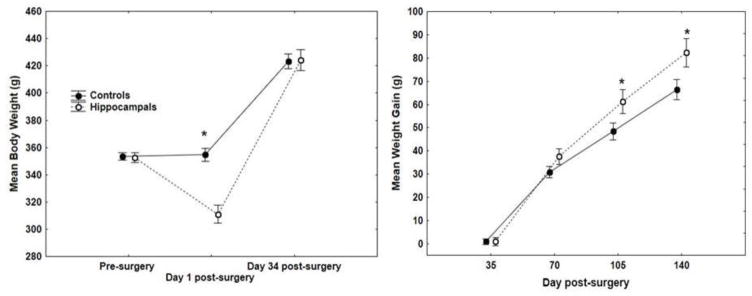
The left panel of Figure 5 shows that while rats assigned to hippocampal lesioned and control groups were matched on body weight immediately prior to surgery, the hippocampal lesioned rats showed a marked weight loss on the day after surgery. Body weight for the lesioned rats did not recover to control levels until post-surgery Day 34. These differences yielded a significant Group × Days interaction (F(2, 44) = 16.01, p < .01), and post hoc Newman-Keuls tests showed that body weight was significantly lower for hippocampal lesioned rats compared to controls only on post-surgical Day 1. However, subsequent weight gain was greater for hippocampal rats compared to controls (right panel of Figure 5). ANOVA on body weight gain obtained a significant Group × Days interaction (F(3, 66) = 3.28, p < .05), and post hoc Newman-Keuls tests showed that hippocampal lesioned rats’ weight gain was significantly greater than controls on post-surgical Days 105 and 140 (ps < .05). As we have reported previously [31], hippocampal lesioned rats exhibit a pattern of initial rapid weight loss followed by steady weight gain that ultimately exceeds that of controls.
Figure 5.
The left panel shows mean body weight ± S.E.M. of hippocampal lesioned and control rats prior to surgery, the day after surgery, and 34 days after surgery, when lesioned rats’ body weight was recovered. The right panel shows mean body weight gain ± S.E.M. of hippocampal lesioned and control rats following surgery.
3.3.2. CCK
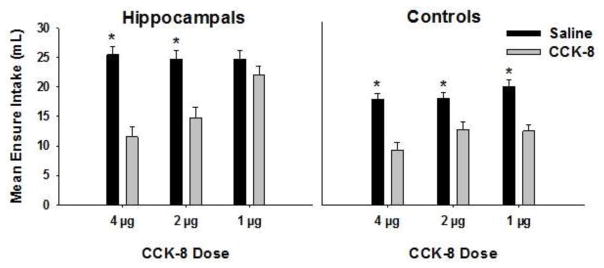
Figure 6 shows that, compared to saline, CCK-8 injections suppressed Ensure intake over the 60 min test period for both lesioned and control groups at 4 and 2 μg/kg doses. However, at the 1 μg/kg dose, CCK-8 suppressed intake for controls but not for the rats with hippocampal lesions. ANOVA on Ensure intake 60 minutes following injections yielded a Group × Drug × Dose interaction, (F(2, 40) = 7.67, p < .01). Post hoc Newman-Keuls tests confirmed that hippocampal lesioned rats’ Ensure intake following 1 μg/kg CCK did not differ significantly from intake following saline, while lesioned rats at the two higher doses and intact controls at all doses consumed significantly less Ensure following CCK-8 injection than following saline (ps < .01). Collapsed across Groups, Ensure intake following CCK-8 injections was significantly less than intake following saline, yielding a main effect of Drug, (F(1, 20) = 82.33, p < .01). In addition, hippocampal lesioned rats consumed more Ensure overall compared to controls, as indicated by a significant main effect of Group, (F(1, 20) = 25.67, p < .01).
Figure 6.
This graph shows mean Ensure intake of hippocampal-lesioned (left panel) and intact control (right panel) rats following injections of CCK-8 (grey bars) relative to saline (black bars) at 4, 2, and 1 ug/kg doses.
3.3.3. Histology
The extent of excitotoxic lesions of the complete hippocampus is shown in Figure 7 with schematic reconstructions of coronal sections of a representative rat. Included are sections showing the minimal (black) and maximal (grey) areas of damage. Approximate stereotaxic coordinates of the coronal sections are with reference to bregma using the atlas of Paxinos and Watson (1997). The brains from rats in the hippocampal lesioned group were characterized by almost complete removal of all CA1 – CA3 pyramidal cells, hilar cells, and the granule cells in dentate gyrus. Several animals in this group had small islands of normal appearing cells, but these were few in number, and the spared areas were generally isolated in terms of inputs and/or outputs. Almost all hippocampal lesioned rats had considerable enlargement of the ventricles, and several animals showed obvious thinning of the cortex overlying hippocampus. Further, there was damage to subiculum in most animals, though this damage was limited primarily to one hemisphere. The cell loss extended into entorhinal cortex for several rats, but this was unilateral in extent. One animal had unilateral damage that included amygdala, but the behavioral measures for this rat were similar to others in the lesioned group. This extrahippocampal damage was greater in extent than that found in our previous papers [31, 32], in which IBO totaling 2.08 ul was injected at 30 sites, while in the current study 2.30 ul of IBO was injected at 38 sites.
Figure 7.
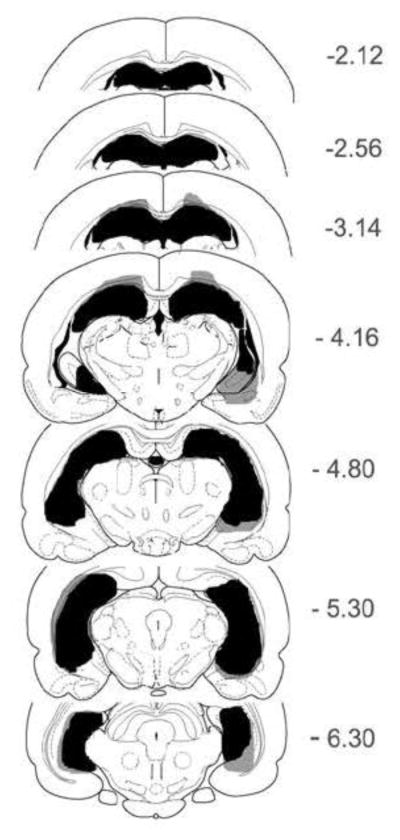
Schematic reconstructions of coronal sections depict the extent of excitotoxic lesions of the complete hippocampus of a representative rat. Minimal areas of damage are shown in black, while maximal areas of damage are shown in grey.
4. Discussion
Extending earlier findings with higher levels of food deprivation (e.g., 24h), Experiment 1 showed that rats can use cues arising from 0 and 4h food deprivation, approximately those encountered during free-feeding conditions, as discriminative stimuli. Experiment 2 showed that these deprivation state cues also exhibit strong discriminative control over appetitive responding when they are trained in compound with external stimuli that are equally, if not more, valid discriminative signals. Specifically, when external cues were removed from the compound, the rats continued to exhibit significant discrimination based on deprivation cues alone. A control group for which external auditory cues, but not the level of food deprivation, signalled sucrose delivery provided evidence that stimulus control by deprivation cues was based on the learned contingency between deprivation cues and reinforcement. Furthermore, the results showed that the rats also learned about the external cues during compound training. When tested with their deprivation state held constant, rats maintained significant discriminative control by external cues. Thus, both food deprivation cues and more punctate external stimuli contributed to the control of discriminative responding.
Experiment 2 then assessed the effects of shifting to a Western-style diet (WD) on the ability of rats to discriminate based on their deprivation cues alone. Half of the rats were trained with both deprivation cues and external cues as valid discriminative stimuli, and half were trained with only external cues as valid discriminative signals. Half of the rats in each of these contingency groups were shifted to WD, while the remaining rats continued to receive chow. Control rats maintained on chow and trained with deprivation cues as relevant discriminative stimuli (Group Dep+ Chow) retained significant discriminative responding when they were tested with the external cues removed, whereas the chow-fed controls trained with food deprivation cues in a noncontingent relationship with reinforcement (Group DepN Chow) failed to maintain discriminative responding in the absence of the external cues. Because both of these groups were tested under the same deprivation conditions, this outcome confirms that responding during the deprivation cue alone test was based on the contingency between deprivation cues and reinforcement used in training rather than any motivational or other nonspecific effects of differences in the 0 and 4h deprivation conditions.
In contrast, for rats maintained on WD, discrimination performance was abolished during testing with deprivation cues alone for both the group trained with deprivation cues as valid discriminative cues (Group Dep+ WD) and the group trained with their deprivation cues in a noncontingent relationship with reinforcement (Group DepN WD). Furthermore, discrimination performance for both of these WD-fed groups was significantly worse compared to chow-fed rats (Group Dep+ Chow) for which deprivation cues had been trained as valid discriminative signals. Discrimination performance for all rats was then retested when the originally trained external cues were reintroduced in compound with the deprivation stimuli. During this test, WD-fed rats regained significant discriminative responding, but at a somewhat lower level than the rats maintained on chow. Our lab previously reported a similar pattern of results in which a shift to WD impaired discrimination of 0 and 24h food deprivation. In this experiment, WD-fed rats were able to discriminate when external cues were subsequently trained as additional predictors of sucrose, but discrimination was again disrupted when external cues were removed [33].
Experiment 3 investigated a potential role of the hippocampus as a substrate for processing deprivation state information. Rats with hippocampal lesions gradually exhibited excess weight gain relative to rats with an intact hippocampus, confirming previous findings [30]. In addition, hippocampal lesioned rats showed attenuated intake suppression at the lowest dose of CCK-8, suggesting that increased weight gain may result from reduced sensitivity to satiety signaling by CCK. The reduced sensitivity of hippocampal lesioned rats compared to controls at only the lowest CCK-8 dose suggests a higher threshold level for intake suppression, in which higher levels of endogenous CCK may be required to suppress intake under normal feeding conditions. Consistent with this possibility, hippocampal lesioned rats also exhibited higher intake of Ensure than non-lesioned controls following saline injections.
The finding in Experiment 2 that the ability to use food deprivation cues to control appetitive conditioned responding was abolished for rats shifted to WD, whereas discriminative control by external conditioned stimuli was not, merits further consideration. At least two related hypotheses could account for this outcome. First, the shift to WD prior to testing with deprivation cues alone may have changed the sensory properties of the cues produced by 0 and 4h food deprivation, thereby making them less discriminable. This explanation would be consistent with our finding that discriminative performance based on deprivation cues alone was disrupted after only 1–4 days of exposure to WD. Given that responding under 0h food deprivation did not increase after the shift to WD, either in absolute terms or relative to responding by the chow-fed rats, it seems unlikely that the sensory properties of the cues produced by 0h food deprivation became more like those arising from 4h without food. On the other hand, WD-fed rats may have been more satiated under 4h food deprivation compared to chow-fed rats, potentially reducing the ability of WD-fed rats to differentiate between the cues produced by 0 and 4h food deprivation periods. While this possibility cannot be ruled out based on the present data, there are considerations that bring this interpretation into question. For example, evidence from rodent [34, 35] and human [36] studies indicates that diets higher in fat are less rather than more satiating than diets containing lower levels of fat. If this is the case, one might expect that compared to 4h deprivation from low-fat chow, 4h deprivation of WD might produce cues that are less similar to 0h food deprivation cues. Consistent with this account, many rats overeat and gain more weight when consuming a high-fat Western diet compared to standard chow. These findings would need to be reconciled with the notion that WD is more satiating compared to chow prior to interpreting the present results solely in terms of changes in the internal cue properties of 0 or 4h food deprivation.
The second hypothesis is that consuming WD alters not the sensory properties of food deprivation cues, but rather the ability to process or utilize the information those cues provide. As noted previously, hippocampal damage impairs the ability of rats to use cues arising from their deprivation state as discriminative stimuli, and Experiment 3 confirmed previous findings that interference with hippocampal functioning is associated with reduced control of energy intake and body weight regulation. In addition, new findings have identified an excitatory neural pathway from the ventral hippocampus to the lateral septum that suppresses food intake [37]. Furthermore, Kanoski and his colleagues demonstrated ventral hippocampal regulation of feeding through activations of anorectic (leptin [38]; GLP-1 [39]) and orexigenic (ghrelin [40]) signaling pathways. Additional recent work shows that ventral hippocampus is an important component of a neural circuit involved in the inhibition of approach responses under conditions in which conflicting approach and avoidance tendencies are experienced [41]. We have previously proposed that the decision to eat or refrain from eating involves these types of conflicts, and that the hippocampus is a key substrate for resolving them [42, 43]. Importantly, impairments in hippocampal function and the emergence of hippocampal pathophysiology have also been linked to consumption of WD. Rats that become obese when maintained on WD exhibit impaired performance on hippocampal-dependent learning and memory tasks, hippocampal inflammation, reduced brain-derived neurotrophic factor, and compromised hippocampal blood-brain barrier integrity (see [44, 45]). Furthermore, several reports indicate that maintaining rats on this type of diet attenuates satiety signaling by CCK-8 (e.g., [46, 47]). These findings, added to those of our present paper, support the hypothesis that hippocampal dysfunction contributes to the adverse effects of WD on both weight control and on the ability to use satiety signals to inhibit appetitive conditioned responding. When considered together, these findings enhance the plausibility of the idea that WD consumption interferes with the hippocampal-dependent utilization of deprivation cue information.
Our finding that discrimination based on food deprivation cues was impaired a few days after the shift to WD might be viewed as problematic for this interpretation. However, Kanoski and Davidson [48] reported that rats’ performance on a hippocampal-dependent spatial memory problem in a radial maze was impaired within 3 days of a shift to WD. This shift did not produce impairment on a nonspatial cued version of the task that does not depend on an intact hippocampus. More recently, Jais and his colleagues [49] found brain glucose uptake is suppressed in mice following 3 days of exposure to a high-fat diet. This altered brain energy metabolism corresponded with the reduced expression of the brain glucose transporter GLUT-1. Given that our rats had 4 days of exposure to WD (2 trials under 0h and 2 under 4h deprivation) by the completion of the first test block without external cues, the duration of diet exposure used by Kanoski and Davidson [48] and Jais et al. [49] overlaps with the length of WD exposure used in our current study. Furthermore, another recent study from our research group [13] showed that expression of hippocampal GLUT-1 was reduced in rats following 10 days of WD (shortest period assessed), and this effect was accompanied by impaired performance on a hippocampal-dependent spatial alternation task. Overall, this set of results indicates that impaired hippocampal-dependent learning and memory can result from acute WD exposure, potentially through alterations in brain glucose metabolism.
Viewed within our conceptual framework, it will be important to study not only how WD intake interferes with hippocampal functioning, but also which types of hippocampal-dependent processes are involved with the utilization of information provided by energy state signals. For example, WD may have its most detrimental effects on the processing of contextual information. Many studies provide evidence that the ability of contextual stimuli to signal the reinforcement of other events is dependent on the hippocampus [50–52]. We have also proposed that the interoceptive state of satiety serves as a context that signals when consummatory behavior evoked by environmental food-related cues will not be followed by rewarding postingestive consequences [47, 48]. This contextual signaling of nonreward inhibits appetitive behavior by opposing the capacity of external cues to excite the memory of the rewarding postingestive consequences of eating. Alternatively, in the absence of satiety cues, internal or external cues may provide a context that supports the behavioral expression of the excitatory association between environmental food cues and reinforcing postingestive outcomes. Thus, it is possible that the WD-induced disruption of discriminative control by 0 and 4h food deprivation stimuli is attributable, at least in part, to interference with the processing of contextual stimuli associated with those deprivation levels.
This analysis may also speak to the finding that discriminative responding when external cues were reintroduced on the final compound cue test was weaker (although still significant) for WD-fed rats (Groups Dep+ WD and DepN WD) compared to rats maintained on chow (Groups Dep+ Chow and DepN Chow). This pattern could be based on a direct weakening effect of WD on the ability of rats to use external cues as simple discriminative stimuli. However, this conclusion may be questioned if one accepts that deprivation states can serve as contextual stimuli that aid in the retrieval of information about the association of other (e.g., external) stimuli with reinforcement. In the final compound retraining phase of Experiment 2, testing of external stimuli for all groups occurred against a contextual background provided by interoceptive food deprivation cues and other types of stimuli. If WD intake impaired the retrieval of the context in which the external cues were trained, this could have interfered with discriminative responding based on external cues for both the Dep+ WD and DepN WD groups. In other words, external discriminative cues may have exhibited weakened control over appetitive responding as a consequence of the effects of WD on the ability to use contextual stimuli. Therefore, the effects of WD on discriminative responding in the final phase of Experiment 2 may represent another manifestation of impairment in the utilization of contextual cues, independent of any direct effects on control by external food-related stimuli.
5. Conclusions
The results of our experiments have implications for several theoretical perspectives. Schachter’s 1968 [53] externality hypothesis proposed that obesity was associated with a hypersensitivity to external cues and a hyposensitivity to internal state signals. While Schacter’s proposal has been challenged on many grounds (e.g., [54]), findings of enhanced behavioral and neural food cue reactivity in individuals with excess weight have renewed interest in the externality idea (e.g., [55, 56]; [57–59]). Recent evidence of differential conditioning to food cues in obese and lean individuals also suggests alterations in associative learning processes [60, 61] (see [62] for review). Considering the integration of internal and external controls of behavior, several studies with human (e.g., [63–66]) and nonhuman animals [67] indicate that overeating in the face of powerful external food cues may be a consequence of reduced responsivity to satiety signals. For example, maintenance on a high-fat diet reduced the potency of meal cessation signals to suppress intake compared to chow-fed controls [67]. Our finding that WD intake reduces stimulus control by satiety signals offers a mechanism for how impaired interoception may contribute to overweight and obesity.
Environments are thought to become obesogenic when the power of foods and food-related events to evoke appetitive and eating behaviors becomes stronger than the power of internal satiety signals to inhibit those responses. The results of the present experiments suggest a mechanism for how this shift in the balance of internal to external control may occur. We demonstrated that rats can learn to use cues arising from food satiety and relatively low levels of hunger as discriminative stimuli to control appetitive conditioned responses. Control by interoceptive cues is not overshadowed or abolished in the face of competition with external stimuli that are equally valid, if not better, predictors of food reward. However, our findings also show that this balance may be altered in rats maintained on a Western-style diet. That is, consuming a diet high in saturated fat and sugar disrupted discriminative control by internal food deprivation cues alone, while the return of external discriminative stimuli led to the recovery of some of that appetitive control. Further, we showed that selective hippocampal lesions reduce the ability to use satiety signals to suppress intake.
Based on the idea that the initiation of eating is primarily under exteroceptive rather than interoceptive (i.e., hunger) stimulus control, whereas the reverse is true for termination of eating, the environment would become obesogenic if the ability to discriminate between interoceptive hunger and satiety cues is impaired and/or the ability of the hippocampus to use satiety signals as contextual cues is diminished. As a result of either or both of these deficits, the ability of satiety cues to counter the evocation of eating and appetitive behavior by food-related environmental cues would be weakened. Increased intake and ultimately weight gain is the expected outcome of this relative shift from internal to external stimulus control. Further research will be needed to separate the effects of WD intake on the sensory properties of hunger and satiety cues from the effects on the learning and memory mechanisms that may underlie the ability of those cues to control eating and appetitive behavior.
Acknowledgments
This research was supported by Grant R01HD028792 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. We thank Ms. Jennie Mak for her assistance with data collection.
References
- 1.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proceedings of the Nutrition Society. 2012;71:478–87. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–20. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 4.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. American Journal of Clinical Nutrition. 2008;88:22–9. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. International Journal of Obesity. 2009;33:S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286:G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- 7.Popkin BM. The world is fat. Scientific American. 2007;297:88–95. doi: 10.1038/scientificamerican0907-88. [DOI] [PubMed] [Google Scholar]
- 8.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 9.Paradis AM, Godin G, Perusse L, Vohl MC. Associations between dietary patterns and obesity phenotypes. International Journal of Obesity. 2009;33:1419–26. doi: 10.1038/ijo.2009.179. [DOI] [PubMed] [Google Scholar]
- 10.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Frontiers in Aging Neuroscience. 2014:6. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, et al. Interrelationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–22. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–28. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Hargrave SL, Davidson TL, Lee TJ, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015 doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 15.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behavioral & Neural Biology. 1993;59:167–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 17.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh R, Leber B, Gillman K. Fornix fibers and motivational states as controllers of behavior: a study stimulated by the contextual retrieval theory. Behav Biol. 1978;22:463–78. doi: 10.1016/s0091-6773(78)92583-x. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience. 2004;24:6979–85. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichenbaum H. The hippocampal system and declarative memory in animals. J Cogn Neurosci. 1992;4:217–31. doi: 10.1162/jocn.1992.4.3.217. [DOI] [PubMed] [Google Scholar]
- 21.Gilboa A, Sekeres M, Moscovitch M, Winocur G. Higher-order conditioning is impaired by hippocampal lesions. Curr Biol. 2014;24:2202–7. doi: 10.1016/j.cub.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101:198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- 23.Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–10. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Davidson TL, Carretta JC. Cholecystokinin, but not Bombesin has Interoceptive Sensory Consequences like 1-h Food deprivation. Physiology & Behavior. 1993;53:737–45. doi: 10.1016/0031-9384(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 25.Kanoski SE, Walls EK, Davidson TL, Kanoski SE, Walls EK. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28:988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treesukosol Y, Moran TH. Analyses of meal patterns across dietary shifts. Appetite. 2014;75:21–9. doi: 10.1016/j.appet.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayegh AI. The role of cholecystokinin receptors in the short-term control of food intake. Prog Mol Biol Transl Sci. 2013;114:277–316. doi: 10.1016/B978-0-12-386933-3.00008-X. [DOI] [PubMed] [Google Scholar]
- 28.Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–9. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 29.Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12:405–14. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- 30.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–52. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarrard LE, Davidson TL, Bowring B. Functional differentiation within the medial temporal lobe in the rat. Hippocampus. 2004;14:434–49. doi: 10.1002/hipo.10194. [DOI] [PubMed] [Google Scholar]
- 32.Jarrard LE, Meldrum BS. Selective excitotoxic pathology in the rat hippocampus. Neuropathol Appl Neurobiol. 1993;19:381–9. doi: 10.1111/j.1365-2990.1993.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 33.Sample CH, Martin AA, Jones S, Hargrave SL, Davidson TL. Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite. 2015;93:13–23. doi: 10.1016/j.appet.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FL, AS Differential reinforcing and satiating effects of intragastric fat and carbohydrate infusions in rats. Phsyiology & Behavior. 1999;66:381–8. doi: 10.1016/s0031-9384(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 35.ZSW, CMM, KJB, SJS Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. American Journal of Phsyiology - Regulatory, Integrative, and Comparative Phsyiology. 2000;278:R196–R200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins M, Gibbons C, Caudwell P, Blundell JE, Finlayson G. Differing effects of high-fat or high-carbohydrate meals on food hedonics in overweight and obese individuals. Br J Nutr. 2016;115:1875–84. doi: 10.1017/S0007114516000775. [DOI] [PubMed] [Google Scholar]
- 37.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 2015;6:10188. doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–70. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40:327–37. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73:915–23. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumacher A, Vlassov E, Ito R. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 2016;26:530–42. doi: 10.1002/hipo.22542. [DOI] [PubMed] [Google Scholar]
- 42.Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a ‘Gray’ area? Neurosci Biobehav Rev. 2004;28:261–71. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiology of Learning and Memory. 2014;108:172–84. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hargrave SL, Jones S, Davidson TL. The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci. 2016;9:40–6. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duca FA, Zhong L, Covasa M. Reduced CCK signaling in obese-prone rats fed a high fat diet. Hormones and Behavior. 2013;64:812–7. doi: 10.1016/j.yhbeh.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2009;296:R1681–R6. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- 48.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process. 2010;36:313–9. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 49.Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, et al. Myeloid-Cell-Derived VEGF Maintains Brain Glucose Uptake and Limits Cognitive Impairment in Obesity. Cell. 2016;165:882–95. doi: 10.1016/j.cell.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 50.Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Opitz B. Memory function and the hippocampus. Front Neurol Neurosci. 2014;34:51–9. doi: 10.1159/000356422. [DOI] [PubMed] [Google Scholar]
- 52.Smith DM, Bulkin DA. The form and function of hippocampal context representations. Neurosci Biobehav Rev. 2014;40:52–61. doi: 10.1016/j.neubiorev.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161:751–6. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- 54.Herman CP, Polivy J. External cues in the control of food intake in humans: the sensory-normative distinction. Physiol Behav. 2008;94:722–8. doi: 10.1016/j.physbeh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes (Lond) 2011;35:142–9. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- 56.Scharmuller W, Ubel S, Ebner F, Schienle A. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett. 2012;518:106–10. doi: 10.1016/j.neulet.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 57.Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr. 2014;1:7. doi: 10.3389/fnut.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79:388–95. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35:7964–76. doi: 10.1523/JNEUROSCI.3884-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer MD, Risbrough VB, Liang J, Boutelle KN. Pavlovian conditioning to hedonic food cues in overweight and lean individuals. Appetite. 2015;87:56–61. doi: 10.1016/j.appet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z, Manson KF, Schiller D, Levy I. Impaired associative learning with food rewards in obese women. Curr Biol. 2014;24:1731–6. doi: 10.1016/j.cub.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 62.Boutelle KN, Bouton ME. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015;93:62–74. doi: 10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carnell S, Wardle J. Appetitive traits in children. New evidence for associations with weight and a common, obesity-associated genetic variant. Appetite. 2009;53:260–3. doi: 10.1016/j.appet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Mata F, Verdejo-Roman J, Soriano-Mas C, Verdejo-Garcia A. Insula tuning towards external eating versus interoceptive input in adolescents with overweight and obesity. Appetite. 2015;93:24–30. doi: 10.1016/j.appet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 65.Mooreville M, Davey A, Orloski A, Hannah EL, Mathias KC, Birch LL, et al. Individual differences in susceptibility to large portion sizes among obese and normal-weight children. Obesity (Silver Spring) 2015;23:808–14. doi: 10.1002/oby.21014. [DOI] [PubMed] [Google Scholar]
- 66.Wansink B, Payne CR, Chandon P. Internal and external cues of meal cessation: the French paradox redux? Obesity (Silver Spring) 2007;15:2920–4. doi: 10.1038/oby.2007.348. [DOI] [PubMed] [Google Scholar]
- 67.Cavanaugh AR, Schwartz GJ, Blouet C. High-fat feeding impairs nutrient sensing and gut brain integration in the caudomedial nucleus of the solitary tract in mice. PLoS One. 2015;10:e0118888. doi: 10.1371/journal.pone.0118888. [DOI] [PMC free article] [PubMed] [Google Scholar]