Abstract
Background
Long-term immunosuppression in transplant patients has significant morbidity, poorer quality of life, and substantial economic costs. Operational tolerance, defined as graft acceptance without functional impairment in the absence of immunosuppression, has been achieved in some pediatric liver transplant recipients. Using mass cytometry, peripheral blood immunotyping was performed to characterize differences between tolerant patients and patients who are stable on single-agent immunosuppression.
Methods
Single-cell mass cytometry was performed using blood samples from a single-center pediatric liver transplant population of operationally tolerant patients to comprehensively characterize the immune cell populations in the tolerant state compared to patients on chronic low dose immunosuppression. Specific T cell populations of interest were confirmed by flow cytometry.
Results
This high dimensional phenotypic analysis revealed distinct immunoprofiles between transplant populations as well as a CD4+ T cell subset of Operational Tolerance (TOT; CD4+CD5+CD25+CD38−/loCD45RA) that correlates with tolerance in pediatric liver transplant recipients. In operationally tolerant patients the TOT was significantly increased as compared to patients stable on low levels of immunosuppression. This TOT cell was confirmed by flow cytometry and is distinct from classic T regulatory cells.
Conclusion
These results demonstrate the power of mass cytometry to discover significant immune cell signatures that have diagnostic potential.
Keywords: CyTOF, T cells, immune cells, PBMC, post-transplant
Introduction
Solid organ transplantation is a life-saving procedure which, given the success of immunosuppressive drugs, is generally well tolerated. With improved surgical procedures, medical management and the development of new immunosuppressive agents, transplant patients are living longer. However, there are known adverse effects associated with long term usage of immunosuppression, including infection, nephrotoxicity, and increased incidence of malignancy. Indeed, it has been reported that 40–70% of all post-transplant mortality can be directly or indirectly attributed to immunosuppression (1, 2). In the pediatric population, the risks of long-term immunosuppression are especially significant and pose a number of health challenges, including impact on quality of life, growth and development, and survival from co-morbidities (3).
The liver is unique among transplanted organs as it is suggested to have “tolerogenic” properties, with probable improved graft survival of other solid organs when co-transplanted with the liver (4–6). Additionally, MHC matching is not a prerequisite for successful liver transplant (LT) outcomes. Operational tolerance (TOL), defined as graft acceptance without functional impairment in the absence of immunosuppression, has been achieved in some pediatric LT recipients (7–9). There is a strong incentive to fully characterize the immunoprofiles of LT recipients to differentiate TOL patients from those that require immunosuppression. Despite numerous studies based on gene expression profiling and characterization of specific immune cell subsets by flow cytometry markers that specifically identify TOL patients have not been reliably validated for clinical use (10–20).
Mass cytometry, or Cytometry by Time-of-Flight (CyTOF), is a single cell–based platform that utilizes antibodies conjugated to rare heavy metal ions for analysis of cellular proteins by a time-of-flight mass spectrometer (21). This new technology allows for the evaluation of significantly more parameters in a single sample since the limitation of spectral overlap, seen in conventional flow cytometry, is eliminated. For the first time, mass cytometry is used here to perform a pilot comprehensive phenotypic characterization in solid organ transplant recipients with the intent to identify immune cell features of TOL.
Materials and Methods
Patients
Peripheral blood samples were collected from 15 pediatric liver transplant recipients for CyTOF analysis; seven operational tolerant recipients (TOL) and eight recipients on low dose single agent tacrolimus (IS) (Table 1). Blood samples were processed within 48 hours of sample collection using Ficoll-Pacque Plus (Amersham Pharmacia Biotech, Piscataway, NJ) then peripheral blood mononuclear cells (PBMC) frozen and maintained in liquid nitrogen until use. All patients had stable graft function (alanine aminotransferase, aspartate aminotransferase, total bilirubin, and gamma-glutamyl transferase in the normal range) on labs performed within one month of research blood sample. TOL patients were identified retrospectively and had not been under any immunosuppression weaning protocol. TOL was defined as stable graft function in the absence of IS, for more than one year. Immunosuppression had been discontinued in TOL recipients due to post-transplant lymphoproliferative disease or chronic EBV viremia (n=5), or non-compliance (n=2). TOL patients had no history of acute rejection except one patient who had a single episode of acute rejection that occurred within a week of transplantation and no acute rejection thereafter. The criteria employed in selecting patients for the IS group were (a) more than one year after transplantation; (b) single-drug immunosuppression; and (c) absence of autoimmune liver disease before or after transplantation. Among the eight IS patients, two patients also have a history of EBV disease with one patient having chronic EBV viremia and another with history of EBV-related post-transplant lymphoproliferative disease. Additionally, peripheral blood samples were obtained from five healthy controls (HC) from the Stanford Blood Bank (Palo Alto, CA). Clinical and demographic characteristics of patients included in the CyTOF analysis are summarized in Table 1. Clinical and demographic characteristics for samples analyzed by flow cytometry are summarized in Table S1. Due to the limited number of TOL patients as well as limited volume of blood available from some of the patients, three samples from each group (TOL and IS) were used for both mass cytometry and flow cytometry. Additional samples (two TOL and three IS) were used for flow cytometry. As this is a single-center study there are limited numbers of TOL patients, the two TOL independent samples came from two patients who provided PBMC for mass cytometry analysis (Table 1). However, these samples were drawn at different time points, more than one year apart from when the mass cytometry samples were taken. The additional three IS samples came from different patients. The study was approved by the Institutional Review Board at Stanford University are in accordance with the Helsinki Declaration of 1975, and informed consent (and assent when the patient was over seven years of age) was obtained from all legal guardians of participants or from patients if over 18 years of age.
Table 1.
Demographic data
| Parameters | Tolerant (TOL, n=7) | Immunosuppression (IS, n=8) | Non-transplant healthy control (HC, n=5) | p-value |
|---|---|---|---|---|
| Age (yrs ± SD) | 16.5 ± 5.2 | 15.2 ± 4.9 | 18.8 ± 1.8 | 0.39 |
| Recipient sex (male : female) | 2 : 5 | 5 : 3 | 2 : 3 | 0.41 |
| Transplant type (living : deceased donor) | 2 : 5 | 2 : 6 | NA | > 0.99 |
| Primary diagnosis | 5a : 1b : 1c | 5a : 1d : 1e : 1f | NA | NA |
| Post-transplant time (yrs ± SD) | 15.8 ± 5.1 | 12.8 ± 4.1 | NA | 0.23 |
| Time off immunosuppression (yrs ± SD) | 8.6 ± 4.7 | NA | NA | NA |
| Reason for immunosuppression withdrawal | 3i : 2j : 2k | NA | NA | NA |
| Tacrolimus serum level (ng/mL ± SD) | NA | 3.0 ± 1.0 | NA | NA |
| Aspartate aminotransferase (AST, U/L; ± SD) | 31.0 ± 14.0 | 23.0 ± 5.9* | NA | 0.20 |
| Alanine aminotransferase (ALT, U/L; ± SD) | 29.1 ± 17.0 | 16.7 ± 4.7* | NA | 0.09 |
| Gamma-glutamyl transferase (GGT, U/L; ± SD) | 16.4 ± 6.7† | 21.0 ± 11.5* | NA | 0.44 |
| Total bilirubin (mg/dL ± SD) | 0.5 ± 0.3 | 0.5 ± 0.2* | NA | 0.91 |
| White blood cell count (103/μL ± SD) | 7.3 ± 2.2† | 7.8 ± 2.2* | NA | 0.66 |
| Hemoglobin (g/dL ± SD) | 13.4 ± 0.8† | 14.0 ± 1.3* | NA | 0.35 |
| Epstein-Barr Virus at time of blood draw (detected : not detected : unknown) | 2 : 4 : 1 | 2 : 3 : 3 | NA | NA |
Key:
Biliary atresia,
Fulminant,
Hepatoblastoma,
A1AT deficiency,
Tyrosinemia,
Familial cholestasis,
Acute liver failure,
Metabolic,
Post-transplant lymphoproliferative disease,
Epstein-Barr viremia,
Non-compliance.
NA=not applicable, yrs = years, P-value compares TOL vs. IS vs. HC via one-way ANOVA, Fisher’s exact test (Transplant type), chi-squared test (Recipient sex), where applicable, for comparison of three groups; and unpaired t-test for two group comparisons. *One or †Two patient values missing from analysis as laboratory test was not performed.
Reagents
All CyTOF reagents and antibodies (Table S2) were purchased from Fluidigm (South San Francisco, CA). Cell-ID™ Cisplatin (Fluidigm) was used per manufacturer’s directions to differentiate live versus dead populations. Monoclonal antibodies for flow cytometry were all purchased from BioLegend (San Diego, CA) and include: anti-human CD3 (OKT3), CD4 (OKT4), CD5 (UCHT2), CD25 (M-A251), CD38 (HIT2), CD45RA (HI100), and Foxp3 (259D). LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies, Grand Island, NY) was used for detection of dead cells.
CyTOF and data processing
PBMC were stained as per manufacturer’s instructions using the MaxPar® Cytoplasmic/Secreted Antigen Staining Protocol (Fluidigm). Data was collected on the CyTOF2 instrument (400–500 cells per second) at the Stanford Shared fluorescence-activated cell sorting (FACS) Facility using NIH S10 Shared Instrument Grant S10OD016318-01. Normalized CyTOF data (using beads) was further preprocessed in FlowJo v9.7.6 (FlowJo, LLC, Ashland, OR) via manual gating of single cells (191Iridium and 193Iridium double positive) and live cells (195Cisplatin negative), which were exported for bioinformatic analysis, or further manual gating for cell populations as described in Figure S1. Comprehensive comparisons between CyTOF and flow cytometric data has been described by Bendell et al. (21).
Bioinformatic analysis
CyTOF data was analyzed for statistically significant differences of populations between groups, preprocessed live cell data was run through Citrus v0.8 (22) using R v3.1.2 (23) as described by Bruggner et al. (22). Briefly, Citrus defines cellular populations by arranging the various markers through an autogating fashion based upon an hierarchical clustering algorithm to a user defined threshold of the minimum ‘cluster’ (nee. population) size of events of the total data (e.g. 1.5%). We performed the Citrus analysis of the CyTOF data using a set of 20,000 to 50,000 randomized cellular events per sample with minimum cluster size threshold of 1.5% for population definition. The analysis was based upon abundance of events using predictive analysis of microarrays (pamr; nearest shrunken centroid classification method) v1.55 (24) for three-group analysis and glmnet (logistic regression with lasso regularization) (25) v1.9-8 for two-group analysis as the prediction algorithms. K-fold cross-validation was used for model selection by varying the regularization threshold and picking the simplest model with an error rate within one standard error of the minimum error rate.
Principal component analysis (PCA) was performed on the normalized manual gated CyTOF data using the prcomp (26–28) function and associated dependencies within R v3.1.2 (23). PCA was performed on zero centered and scaled loge transformed data using singular value decomposition and principal components ordered by the magnitude of their eigenvalues.
Flow cytometric analyses
Flow cytometric analyses were performed as described (29–31) with minor modifications and following instructions provided with True-Nuclear™ Transcription Factor Buffer Set (Biolegend). Cells were then analyzed using a Becton-Dickinson LSR flow cytometer in the Stanford Shared FACS Facility.
Statistical analysis
Data was analyzed using GraphPad Prism 6.0 for Mac for OSX (GraphPad Software, La Jolla, CA) for differences between the various study groups. Demographic parameters and clinical data were assessed using a one-way ANOVA for three groups and Student’s unpaired T-test for two groups. Categorical or nominal data was assessed using a Chi-squared test comparing relative proportions. Percentages of the various manual gated derived immune cell populations from either FACS or CyTOF was assessed via an unpaired Student’s T-test. Differences were considered significant with a two-tailed p-value less than or equal to 0.05.
Results
Mass cytometry reveals a single significant immune cell population that distinguishes tolerant patients
PBMC were isolated from whole blood obtained from subjects (n=20) identified as TOL (n=7), those with stable graft function maintained on single agent immunosuppression (IS, n=8), and healthy controls (HC, n=5). There was no significant difference in any of the demographics of TOL and IS patients (Table 1).
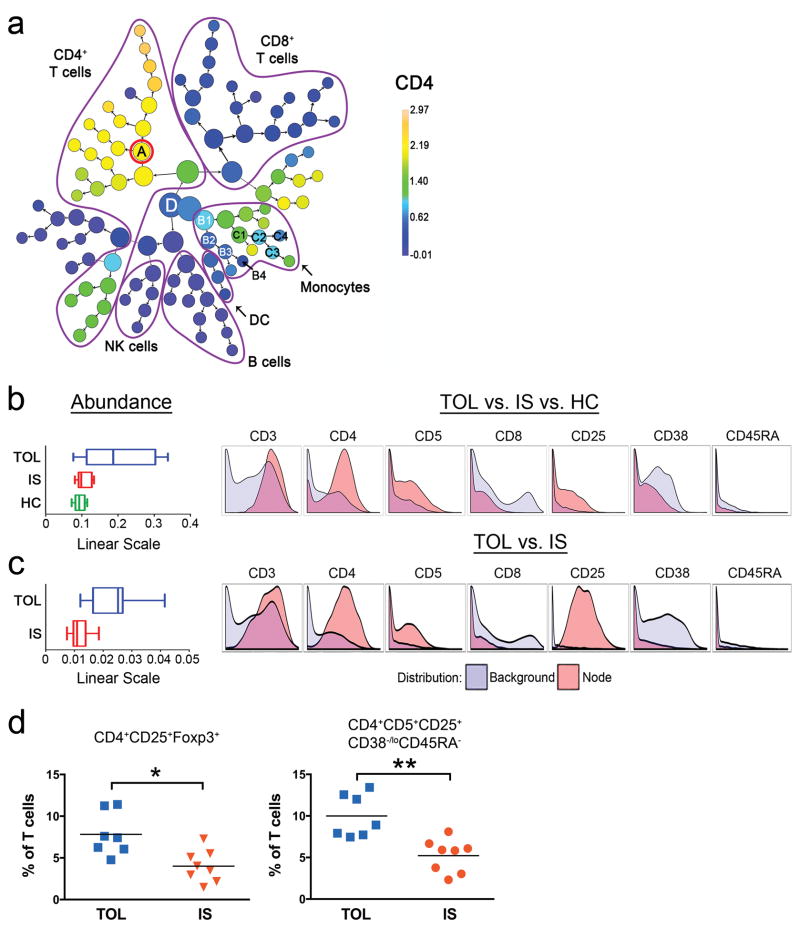
PBMC were stained with heavy metal conjugated-antibodies recognizing 21 cell surface markers and one intracellular marker (Table S2) and analyzed by CyTOF. Analysis was performed by two methods: (1) high dimensional multi-parameter analysis and (2) manual gating of populations to determine frequencies of multiple cell populations (Figure S1). Analysis was initially performed with Citrus, which uses an unsupervised clustering algorithm followed by a supervised learning algorithm to identify significant cell subsets that may normally be overlooked by manual gating strategies (22). Using Citrus, the 22-parameter single-cell data can be presented as a hierarchy of 104 nodes on the basis of the markers (Figure 1a). Using predictive analysis of microarrays (pamr), ten nodes were found to be more abundant in one group of subjects in relation to the other two (A, B1-4, C1-4, and D; Figure 1a). Nodes B1-4 and C1-4 (all monocyte subsets) and node D (a lymphocyte precursor) were significantly more abundant in HC compared to TOL and IS (Figure S2). Of note, node A, a CD4+ T cell subset, was significantly more abundant in TOL compared to IS and HC (Figure 1b). This node expresses CD3, CD4, CD5, CD25, has low/negative expression of CD38, and is negative for CD45RA (Figure 1b).
Figure 1. Mass cytometry identifies a T cell subset of Operational Tolerance (TOT).
PBMC from pediatric LT recipients that were defined as operationally tolerant, (TOL, n=7); stable on immunosuppression (IS, n=8); and PBMC from healthy controls (HC, n=5) were analyzed for 22 parameters using specific antibodies for CyTOF. (a) Visual representation of unsupervised hierarchical clustering. Results are shown for live cells and with analysis of 22 parameters. Major immune cell populations are delineated based on canonical lineage markers (CD3+CD4+ for CD4+ T cells, CD3+CD8+ for CD8+ T cells, CD14+ cells for monocytes, CD11c+HLA-DR+ cells for dendritic cells, CD16+ cells for NK cells, and CD19+CD20+ for B cells). The color scale indicates the median intensity of expression of the relative marker, in this case CD4, compared to all other cells while node sizes are based on the frequency of cells. Specific nodes are labeled A, B1-4, C1-4 and D and represent populations with significant differences between groups. (b) The relative abundance ± SD, on a linear scale, of node A for the TOL, IS and HC groups is shown (left). Nodes B1-4, C1-4, and D are shown in Figure S2. Selected histograms for CD3, CD4, CD5, CD8, CD25, CD38, and CD45RA for node A are shown (right). For each marker, the node of interest (red) against the background expression of the same marker on all other live cells (blue) is shown. (c) The relative abundance ± SD on a linear scale for the TOL and IS groups is shown (left). Selected histograms for CD3, CD4, CD5, CD8, CD25, CD38, and CD45RA for this node are shown (right). (d) CD4+CD25+Foxp3+ Treg cells and CD3+CD4+CD25+CD5+CD38−/loCD45RA− TOT cells were manually gated from CyTOF data and frequency compared between TOL (n=7) and IS (n=8) groups and plotted as the subset frequency of total live CD3+ lymphocytes. Data are expressed as percent of total live CD3+ lymphocytes. Mean of each group is shown. *, p < 0.01. **, p = 0.001.
Analysis of only TOL versus IS patient groups was then performed to address whether HC drive the majority of the findings and thereby masked subtle differences between the two groups. Citrus analysis revealed a single significant node, and this population was more abundant in the TOL group compared to IS (Figure 1c). Importantly, this node had similar surface marker expression as node A (Figure 1b). As CD4+CD5+CD25+CD38−/loCD45RA− expressing nodes discriminate between TOL and both IS and HC, we have defined this subset as the T cells of Operational Tolerance, or TOT. To confirm that these six markers were sufficient to significantly discriminate TOL from IS, manual gating of TOT from CyTOF data was performed (Figure 1d) and compared to CD4+CD25+Foxp3+ T regulatory cells (Treg, Figure 1d). The frequency of Treg was significantly elevated (p<0.01) in the TOL population (mean 7.8%) compared to IS (mean 4.0%). Similarly, the frequency of TOT was significantly increased in TOL (mean 10.0%) patients compared to IS (mean of IS 5.2%, p=0.001) (Figure 1d). Thus in addition to identifying Treg as a significant marker in distinguishing TOL from IS patients, CyTOF discovered the TOT which distinguishes TOL from IS with more significance.
TOL patients have a distinct immunoprofile
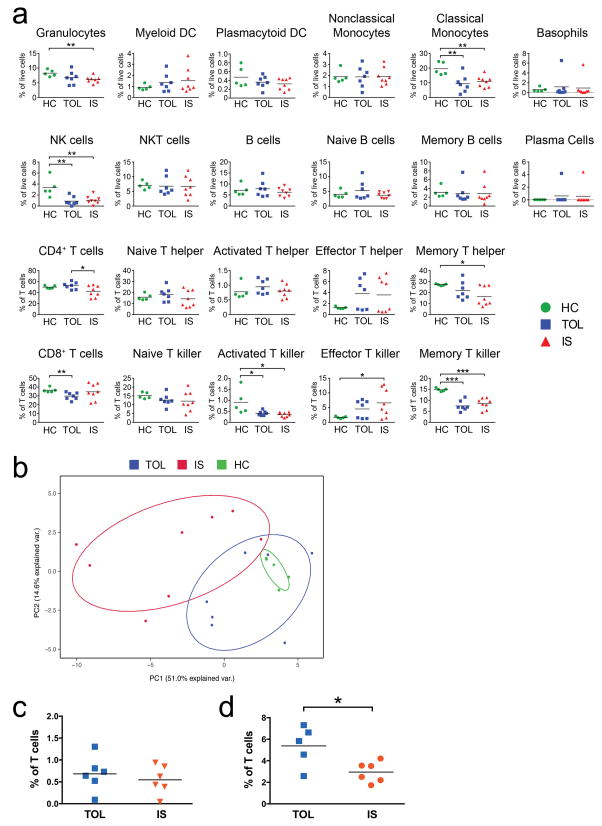
In a separate analysis 38 specific immune cell populations were specifically analyzed (Figures S1a, b). The abundance of Natural Killer (NK) cells and classical monocytes were significantly decreased in TOL and IS patients compared to HC (Figure 2a). Additionally, the frequency of granulocytes, memory CD4+ cells, and activated and memory CD8+ T cells were decreased in IS compared to HC whereas effector CD8+ T cells were increased in IS patients (Figure 2a). TOL patients differed from HC with reductions in NK cells, classical monocytes, total CD8+ T cells, activated CD8+ T cells, and memory CD8+ T cells (Figure 2a). Taken together, this pilot analysis indicates that TOL patients have a unique immunoprofile from IS patients and HC.
Figure 2. Differential immunoprofile of TOL from HC and IS.
The frequency of twenty-two immune cell populations (Figure S1a) as assessed by CyTOF between patients who are operationally tolerant (TOL, n=7) or on low dose single agent immunosuppression (IS, n=8) compared to healthy controls (HC, n=5) with mean shown for each group. * p < 0.05; ** p ≤ 0.01; and *** p ≤ 0.001. (b) Principal component analysis (PCA) showing the first two principal components of 38 immune cell populations (Table S3) for TOL (n=7), IS (n=8), and HC (n=5) comparison (combined proportion of variance 57.2%). (c, d) TOT, as determined by flow cytometry, differentiate TOL patients from IS. Peripheral blood mononuclear cells from TOL (n=5) and IS (n=6) were analyzed by flow cytometry for (c) T regulatory cells (CD4+CD25+Foxp3+) and (d) TOT (CD3+CD4+CD25+CD5+CD38−/loCD45RA−). Patient demographics are in Table S1. The gating strategy is shown in Figures S1b and S3a. Data are expressed as percent of total live CD3+ lymphocytes. Mean of each group is shown. *, p < 0.05.
PCA was performed using frequencies of these 38 cell populations. TOL patients exhibit a differential pattern of immune cell frequencies compared to IS patients using a minimum of two principal components, with 65.6% of the data adequately modeled (Figure 2b; 74.2% by three components, data not shown). The populations that drive the separation of TOL from IS are iterations of the TOT (Figure S3) demonstrating the significance of this single population in separating TOL from IS. Moreover, while there are clear differences between several cell populations between TOL and HC as discussed above, PCA reveals that when immune profiles are comprehensively analyzed, similar cell frequencies appear to drive HC and TOL patients, and indeed the HC are contained entirely within the TOL samples, occupying the same relative space, although there is variability between TOL patients (Figure 2b and Figure S3). Thus, the data above suggest that the immunoprofile of TOL, at least partially, resembles the HC state and that the TOT cell is important in identifying active TOL.
TOT distinguishes tolerant pediatric liver transplant patients
To translate our findings to a platform amenable for the clinical laboratory, we performed immunofluorescent staining and flow cytometry for the six markers defining TOT. Treg were also analyzed but, while higher in the TOL group, did not significantly discriminate the TOL from IS group. In contrast, in agreement with CyTOF data (Figure 1d), flow cytometric analysis of the TOT population significantly discriminated TOL (mean 5.4%) from IS (mean 3.0%; p<0.05, Figure 2d). Foxp3 expression on this population is variable (Figure S4a) and our analysis indicates that it is not an essential marker for TOT. Analysis by both CyTOF and standard flow cytometry identified a T cell subset of Operational Tolerance (TOT) that correlates with tolerance in pediatric LT recipients.
Discussion
Single-cell mass cytometry was performed using PBMC from retrospectively identified pediatric LT recipients to comprehensively characterize the immune cell populations in the TOL state compared to patients on chronic low dose immunosuppression. Herein, high dimensional phenotypic analysis, performed for the first time in a transplant population, revealed distinct immunoprofiles for transplant populations (TOL and IS) as well as a T cell subset of Operational Tolerance (TOT) that specifically correlates with TOL in pediatric LT recipients. Characterization of these groups’ immunoprofiles and of this newly identified TOT cell population could contribute towards future identification of cellular biomarker(s) that could distinguish patients who could be weaned from immunosuppression and then be used diagnostically to determine maintenance of tolerance would improve long-term clinical outcomes.
Previous studies in pediatric living-donor LT recipients have found that the frequency of CD4+CD25hi Treg cells, B cells, and Vδ1/Vδ2γδ T cells were increased in TOL patients while NK cells were decreased (14). Our work confirms an increase in Treg cells (Figure 1d) and a decrease in NK cells (Figure 2) in TOL patients. Similarly in adult LT recipients the Vδ1/Vδ2 γδ T cells and a CD4+CD25hi T cells that express Foxp3 (intracellular or mRNA) have been found to be significantly higher in TOL patients (15, 16). More recently Treg and γδ T cells were quantitated in 50 pediatric transplant recipients on immunosuppression with the primary end point to determine if either Treg (CD4+CD25hiCD127−) or the Vδ1/Vδ2 γδ T cells could be used to predict which recipients are tolerant (18). Using the previously reported (14) median values for the T cell subsets as a proposed cut-off for identification of TOL, 18–24% of their patients were predicted to be TOL when both Treg (CD4+CD25hiCD127−) and Vδ1/Vδ2 γδ T cells were utilized (18), in agreement with the proposed range of transplant recipients predicted to be TOL (7, 8, 32). However, Treg values alone predicted that a very high proportion of transplant recipients (70–78%) were TOL suggesting that Treg, independently, maybe a poor biomarker for TOL (18). The γδ T cell compartment in liver TOL and immunosuppression patients as well as kidney transplant recipients who were on immunosuppression was subsequently examined and it was found that the higher Vδ1/Vδ2 of γδ T cells was not specific to TOL patients (33). Indeed it was suggested that the enlarged pool of Vδ1 T cells in transplant patients was likely secondary to persistent viral infections. Suitable reagents were not available for us to include an analysis of γδ T cells in our CyTOF panel.
Other studies have described various immunoregulatory CD4+ T cell populations that do not include Foxp3 as a differentiating marker. A regulatory CD4+HLA-G+ population that secretes high levels of IL-10, soluble HLA-G and IL-35 has been reported (34). More recently, the IL-10 expressing Tr1 population, which lacks consistent Foxp3 expression, has been well studied and characterized (35). The TOT identified herein is a CD4+ T cell with a CD5+CD25+CD38−/loCD45RA− cell surface marker signature, but lacks stable Foxp3 expression, suggesting that the TOT is distinct from the classical Treg. The lack of CD45RA expression suggests a memory T cell but without stable Foxp3 expression is likely distinct from the memory Treg described by Brouard’s group (10). Recently, CD5 was shown to be important in promoting Treg generation (36); thus, the expression of CD5 and lack of CD45RA on TOT could suggest an antigen-experienced memory CD4+ T cell that promotes Treg cell induction. Sequential studies to quantitate TOT levels after transplant are necessary to establish the threshold consistent with TOL.
Clearly, a major clinical challenge in the pursuit of TOL is the lack of a diagnostic marker that confirms maintenance of the TOL state. Indeed, some presumed TOL patients have been reported to develop hepatic fibrosis (37). Immunosuppression is resumed in these patients as these findings indicate that true TOL has not been achieved; chronic low-level inflammation is believed to be the cause of fibrosis development. Understandably, it has difficult to study these transplant recipients as they often remain lost to follow up until the time of diagnosis. In our study, one patient, initially thought to be TOL, was found to have a mild elevation of serum alanine aminotransferase and upon further workup it was determined that stage III fibrosis was evident in the liver graft and the patient was placed back on immunosuppression. This patient’s TOT frequency, while completely off all immunosuppressive medications, was low, comparable to the numbers seen in acute rejection patients (Figure S4b). Monitoring of patients for TOT may also be useful in detecting patients who are no longer TOL.
We recognize that a potential limitation of this study, and similar studies, is that differences identified between TOL and IS patients could potentially be attributed to the direct or indirect effects of immunosuppression. However, our findings suggest that TOT frequency is not related to immunosuppression but rather, the patient’s immune status. The patient cohort examined in this study was off immunosuppression for a mean 8.6 ± 4.7 years. Thus the five children removed from immunosuppression for EBV disease were, at the time of analysis, many years past any EBV disease. While it is possible that EBV does lead to immunologic changes, most of these are relatively short-term thus the would not be expected to be a major confounding factor many years post-infection. Indeed our results show that the two patients off immunosuppression due to non-compliance has the highest levels of TOT and Treg cells (TOT: 13.32% and 12.55%; Treg: 11.24 and 11.41%). In summary, using mass cytometry, a highly parameterized single cell platform, for the first time in a transplant population, we have identified distinct immunoprofiles in post-transplant population. Large scale prospective weaning studies are necessary to validate our pilot study, and determine the utility of TOT for the identification and maintenance of tolerance.
Supplementary Material
Figure S1. Manual Gating Strategy
Figure S2. Mass cytometry reveals significant cell populations including the TOT in TOL patients
Figure S3. Supplemental PCA plot
Figure S4. TOT frequency in a presumed operationally tolerant patient with fibrosis
Table S1. Demographic data for Figure 2c and 2d
Table S2. Antibody panel used for mass cytometry analysis
Table S3. Immune cell populations used in PCA analysis from Figure 2b
Acknowledgments
Funding: AHL was supported by National Institutes of Health F32 DK94548. The work was funded in part by a Thrasher Research Fund Early Career Award (to AHL), NIH AI084939 (SMK and OMM) and AI104230 (SMK), the Transplant and Tissue Engineering Center of Excellence at Lucile Packard Children’s Hospital (AHL and SMK) and The Arnold and Barbara Silverman Endowment Fund (COE and SMK).
The authors thank Drs. Kari Nadeau, Olivia Hatton and Steven Schaffert for critical reading of the manuscript and Dr. Olivia Hatton, Eden Maloney and Geoff Ivison for assistance with sample processing.
Footnotes
Author Contributions
AHL designed, performed, analyzed and interpreted experiments and wrote the manuscript. MJV helped perform the experiments, analyze the data, and manuscript preparation. KH, WEB, ROC consented, enrolled, and obtained patient samples, and were involved in manuscript preparation. TS prepared patient samples and was involved in manuscript preparation. COE and OMM were involved in project design, data interpretation, and manuscript preparation. SMK supervised the project, designed and interpreted all experiments, and wrote the manuscript.
References
- 1.ASFAR S, METRAKOS P, FRYER J, VERRAN D, GHENT C, GRANT D, et al. An analysis of late deaths after liver transplantation. Transplantation. 1996;61:1377–81. doi: 10.1097/00007890-199605150-00016. [DOI] [PubMed] [Google Scholar]
- 2.GELSON W, HOARE M, DAWWAS MF, VOWLER S, GIBBS P, ALEXANDER G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240–4. doi: 10.1097/TP.0b013e31821841ba. [DOI] [PubMed] [Google Scholar]
- 3.SCHONDER KS, MAZARIEGOS GV, WEBER RJ. Adverse Effects of Immunosuppression in Pediatric Solid Organ Transplantation. Pediatric Drugs. 2010;12:35–49. doi: 10.2165/11316180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.RASMUSSEN A, DAVIES HF, JAMIESON NV, EVANS DB, CALNE RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–21. [PubMed] [Google Scholar]
- 5.KATZNELSON S, CECKA JM. The liver neither protects the kidney from rejection nor improves kidney graft survival after combined liver and kidney transplantation from the same donor. Transplantation. 1996;61:1403–5. doi: 10.1097/00007890-199605150-00021. [DOI] [PubMed] [Google Scholar]
- 6.SAIDMAN SL, DUQUESNOY RJ, DEMETRIS AJ, MCCAULEY J, RAMOS H, MAZARIEGOS G, et al. Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies. Transplant immunology. 1994;2:61–7. doi: 10.1016/0966-3274(94)90080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BENITEZ C, LONDONO MC, MIQUEL R, MANZIA TM, ABRALDES JG, LOZANO JJ, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–35. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 8.FENG S, EKONG UD, LOBRITTO SJ, DEMETRIS AJ, ROBERTS JP, ROSENTHAL P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA: the journal of the American Medical Association. 2012;307:283–93. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 9.MAZARIEGOS GV, REYES J, MARINO IR, DEMETRIS AJ, FLYNN B, IRISH W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–9. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BRAZA F, DUGAST E, PANOV I, PAUL C, VOGT K, PALLIER A, et al. Central Role of CD45RA- Foxp3hi Memory Regulatory T Cells in Clinical Kidney Transplantation Tolerance. Journal of the American Society of Nephrology: JASN. 2015 doi: 10.1681/ASN.2014050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DEMETRIS AJ, LUNZ JG, III, RANDHAWA P, WU T, NALESNIK M, THOMSON AW. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transplant International. 2009;22:120–41. doi: 10.1111/j.1432-2277.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 12.GERMANI G, RODRIGUEZ-CASTRO K, RUSSO FP, SENZOLO M, ZANETTO A, FERRARESE A, et al. Markers of acute rejection and graft acceptance in liver transplantation. World journal of gastroenterology: WJG. 2015;21:1061–8. doi: 10.3748/wjg.v21.i4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LI L, WOZNIAK LJ, RODDER S, HEISH S, TALISETTI A, WANG Q, et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1218–28. doi: 10.1111/j.1600-6143.2011.03928.x. [DOI] [PubMed] [Google Scholar]
- 14.LI Y, KOSHIBA T, YOSHIZAWA A, YONEKAWA Y, MASUDA K, ITO A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:2118–25. doi: 10.1111/j.1600-6143.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 15.MARTINEZ-LLORDELLA M, LOZANO JJ, PUIG-PEY I, ORLANDO G, TISONE G, LERUT J, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–57. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MARTINEZ-LLORDELLA M, PUIG-PEY I, ORLANDO G, RAMONI M, TISONE G, RIMOLA A, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:309–19. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 17.SAGOO P, PERUCHA E, SAWITZKI B, TOMIUK S, STEPHENS DA, MIQUEU P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–61. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SCHULZ-JUERGENSEN S, MARISCHEN L, WESCH D, OBERG HH, FANDRICH F, KABELITZ D, et al. Markers of operational immune tolerance after pediatric liver transplantation in patients under immunosuppression. Pediatric transplantation. 2013;17:348–54. doi: 10.1111/petr.12079. [DOI] [PubMed] [Google Scholar]
- 19.TOKITA D, MAZARIEGOS GV, ZAHORCHAK AF, CHIEN N, ABE M, RAIMONDI G, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–77. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 20.STENARD F, NGUYEN C, COX K, KAMBHAM N, UMETSU DT, KRAMS SM, et al. Decreases in circulating CD4+CD25hiFOXP3+ cells and increases in intragraft FOXP3+ cells accompany allograft rejection in pediatric liver allograft recipients. Pediatric transplantation. 2009;13:70–80. doi: 10.1111/j.1399-3046.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 21.BENDALL SC, SIMONDS EF, QIU P, AMIR ELAD, KRUTZIK PO, FINCK R, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science (New York, NY) 2011;332:687–96. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BRUGGNER RV, BODENMILLER B, DILL DL, TIBSHIRANI RJ, NOLAN GP. Automated identification of stratifying signatures in cellular subpopulations. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2770–7. doi: 10.1073/pnas.1408792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R DEVELOPMENT CORE TEAM R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2011. p. 409. 2.11.1 ed. [Google Scholar]
- 24.TIBSHIRANI R, HASTIE T, NARASIMHAN B, CHU G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6567–72. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TIBSHIRANI R, SOCIETY RS. Regression and shrinkage via the Lasso. J R Stat Soc, Ser B. 1996;58:267–88. [Google Scholar]
- 26.BECKER RA, CHAMBERS JM, WILKS AR. The New S Language. USA: Wadsworth and Brooks/Cole Advanced Books & Software; 1988. [Google Scholar]
- 27.MARDIA KV, KENT JT, BIBBY JM. Multivariate Analysis. Analysis. 1979;97:1–4. [Google Scholar]
- 28.VENABLES WN, RIPLEY BD. Modern Applied Statistics with S Fourth edition by. World. 2002;53:86. [Google Scholar]
- 29.LAU AH, ABE M, THOMSON AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. Journal of leukocyte biology. 2006;79:941–53. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- 30.LAU AH, THOMSON AW, COLVIN BL. Chronic ethanol exposure affects in vivo migration of hepatic dendritic cells to secondary lymphoid tissue. Human immunology. 2007;68:577–85. doi: 10.1016/j.humimm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 31.PHAM B, PIARD-RUSTER K, SILVA R, GALLO A, ESQUIVEL CO, MARTINEZ OM, et al. Changes in natural killer cell subsets in pediatric liver transplant recipients. Pediatric transplantation. 2012;16:176–82. doi: 10.1111/j.1399-3046.2012.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LERUT J, SANCHEZ-FUEYO A. An Appraisal of Tolerance in Liver Transplantation. American Journal of Transplantation. 2006;6:1774–80. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 33.PUIG-PEY I, BOHNE F, BENÍTEZ C, LÓPEZ M, MARTÍNEZ-LLORDELLA M, OPPENHEIMER F, et al. Characterization of γδ T cell subsets in organ transplantation. Transplant International. 2010;23:1045–55. doi: 10.1111/j.1432-2277.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 34.PANKRATZ S, BITTNER S, HERRMANN AM, SCHUHMANN MK, RUCK T, MEUTH SG, et al. Human CD4+HLA-G+ regulatory T cells are potent suppressors of graft-versus-host disease in vivo. The FASEB Journal. 2014;28:3435–45. doi: 10.1096/fj.14-251074. [DOI] [PubMed] [Google Scholar]
- 35.GAGLIANI N, MAGNANI CF, HUBER S, GIANOLINI ME, PALA M, LICONA-LIMON P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19:739–46. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 36.HENDERSON JACOB G, OPEJIN A, JONES A, GROSS C, HAWIGER D. CD5 Instructs Extrathymic Regulatory T Cell Development in Response to Self and Tolerizing Antigens. Immunity. 42:471–83. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 37.YOSHITOMI M, KOSHIBA T, HAGA H, LI Y, ZHAO X, CHENG D, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606–14. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Manual Gating Strategy
Figure S2. Mass cytometry reveals significant cell populations including the TOT in TOL patients
Figure S3. Supplemental PCA plot
Figure S4. TOT frequency in a presumed operationally tolerant patient with fibrosis
Table S1. Demographic data for Figure 2c and 2d
Table S2. Antibody panel used for mass cytometry analysis
Table S3. Immune cell populations used in PCA analysis from Figure 2b