Abstract
Synaptic pruning underlies the transition from an immature to an adult CNS through refinements of neuronal circuits. Our recent study indicates that pubertal synaptic pruning is triggered by the inhibition generated by extrasynaptic α4βδ GABAA receptors (GABARs) which are increased for 10 d on dendritic spines of CA1 pyramidal cells at the onset of puberty (PND 35–44) in the female mouse, suggesting α4βδ GABARs as a novel target for the regulation of adolescent synaptic pruning. In the present study we used a pharmacological approach to further examine the role of these receptors in altering spine density during puberty of female mice and the impact of these changes on spatial learning, assessed in adulthood. Two drugs were chronically administered during the pubertal period (PND 35–44): the GABA agonist gaboxadol (GBX, 0.1 mg/kg, i.p.), to enhance current gated by α4βδ GABARs and the neurosteroid/stress steroid THP (3α-OH-5β-pregnan-20-one, 10 mg/kg, i.p.) to decrease expression of α4βδ. Spine density was determined on PND 56 with Golgi staining. Spatial learning and relearning were assessed using the multiple object relocation task (MPORT) and an active place avoidance task (APA) on PND 56. Pubertal GBX decreased spine density post-pubertally by 70% (P<0.05), while decreasing α4βδ expression with THP increased spine density by two-fold (P<0.05), in both cases, with greatest effects on the mushroom spines. Adult relearning ability was compromised in both hippocampus-dependent tasks after pubertal administration of either drug. These findings suggest that an optimal spine density produced by α4βδ GABARs is necessary for optimal cognition in adults.
Keywords: GABA-A receptor, alpha4, delta, gaboxadol, allopregnanolone, synaptic pruning
Adolescent synaptic pruning occurs throughout the CNS (Huttenlocher, 1979, Zehr et al., 2006, Yildirim et al., 2008, Drzewiecki et al., 2016) and may be necessary for optimal cognition (Chechik et al., 1999). Synaptic pruning is also thought to play a pivotal role in the etiology of developmental disorders including schizophrenia and autism (Glantz and Lewis, 2000, Hutsler and Zhang, 2010) where the hippocampus is a common region associated with both disorders (Schumann et al., 2004, Steen et al., 2006) and where there is a suggested link between optimal synapse number and cognition. Thus, there has been recent interest in the mechanisms that underlie synaptic pruning which can involve microglia (Paolicelli et al., 2011) and autophagy (Tang et al., 2014), likely the final steps in the pruning process. Our recent findings (Afroz et al., 2016) suggest that adolescent synaptic pruning in the CA1 hippocampus of the female mouse is triggered by the tonic inhibition generated by α4βδ GABAA receptors (GABARs). These receptors emerge on the dendritic spine at the onset of puberty (Shen et al., 2010), identified by vaginal opening (typically ~PND 35), and remain at high levels for the next 10 days (Aoki et al., 2012). α4βδ GABARs localize at extrasynaptic sites on the dendritic shaft and spine (Wei et al., 2003) where they are activated by ambient GABA (<1 μM) (Wu et al., 2001) and generate a tonic inhibition (Stell and Mody, 2002) due to their high affinity for GABA and relative lack of desensitization under steady-state conditions (Brown et al., 2002).
At puberty, α4βδ GABARs impair the activation of NMDA receptors (NMDARs) (Shen et al., 2010, Afroz et al., 2016) which are necessary for spine maintenance (Ultanir et al., 2007) via their regulation of spine proteins which stabilize the actin cytoskeleton (Afroz et al., 2016). This process produces the dramatic decrease in spine density during the pubertal period, based on results comparing wild-type with the α4−/− mouse (Afroz et al., 2016) which is also a functional δ knock-out (Sabaliauskas et al., 2012, Peng et al., 2014).
In the present study, we examine pubertal administration of compounds to pharmacologically manipulate α4βδ GABARs during the “pubertal period” which we define here as the 10 d during adolescence when α4βδ GABARs have high levels of expression on dendritic spines in CA1 hippocampus (~PND 35–44) (Shen et al., 2010, Aoki et al., 2012). Drugs administered during this pubertal period were then tested for their effect on post-pubertal spine density (PND 56). To this end, we administered gaboxadol (GBX, also known as THIP), which at low doses is a selective agonist at α4βδ GABARs (Brown et al., 2002, Meera et al., 2011), to test the effect of enhanced inhibition at these receptors which would be expected to increase synaptic pruning of CA1 hippocampal pyramidal cells. The neurosteroid THP (3αOH-5α/β-pregnan-20-one) is typically a potent positive GABA modulator with greatest effects at α4βδ GABARs (Bianchi and Macdonald, 2003) but many in vivo studies have shown that naturally occurring fluctuations of this steroid can alter expression of α4 GABARs at puberty (Shen et al., 2007), across the estrous cycle (Lovick et al., 2005, Maguire et al., 2005), and during pregnancy (Maguire and Mody, 2009) in areas such as the CA1 hippocampus, dentate gyrus and the midbrain central grey. Hippocampal levels of this steroid decline at the onset of puberty in the female mouse, which, we have shown (Shen et al., 2007), underlies the increased expression of α4βδ GABARs at this time. Chronic administration of THP during puberty prevents this increase in α4βδ GABAR expression (Shen et al., 2007). Therefore, in the present study, we also administered THP during the pubertal period (PND 35–44) to decrease α4βδ expression which would be expected to reduce synaptic pruning post-pubertally (PND 56). Because THP is released following chronic stress (Purdy et al., 1991, Droogleever Fortuyn et al., 2004, Girdler et al., 2006), effects of pubertal administration of this steroid on spine density are also relevant for the impact of stress during adolescence on spine density.
Female mice were used for the present study because several reports have indicated that spatial memory in females is more vulnerable to impairment when sex differences in spatial navigation emerge at puberty (Kanit et al., 2000, McCarthy and Konkle, 2005). Thus, spine density changes may produce a greater impact in the female hippocampus than in the male. In addition, the role of tonic inhibition in spatial learning and plasticity has been well-characterized at puberty in the female rodent (Shen et al., 2010, Aoki et al., 2012, Afroz et al., 2016) and has been shown to play a pivotal role in synaptic pruning in the female CA1 hippocampus (Afroz et al., 2016).
Synaptic pruning in the adolescent CA1 hippocampus allows for greater cognitive flexibility in the adult. When pruning is prevented, as observed in the α4−/− mouse, spatial learning is normal, but re-learning a new location is impaired (Afroz et al., 2016). Therefore, in the present study we also compared the effect of pubertal GBX and THP administration on spatial learning and relearning ability in adulthood using the multiple placement object relocation task (MPORT) and an active place avoidance task (APA), assessed post-pubertally. These hippocampal dependent tasks (Cimadevilla et al., 2001, Barker and Warburton, 2011) are comparable to tasks used to assess relearning ability in animal models (Lobellova et al., 2013) while MPORT is comparable to a computerized program used to assess reversal learning in neurodevelopmental disorders associated with abnormal pruning, including autism (D’Cruz et al., 2013). The results from the present study suggest that pharmacological manipulation of α4βδ GABARs during puberty alters spine density and impairs relearning ability in adulthood.
Experimental Procedures
Animals
Mice (female, C57BL6, +/+ and α4−/−) were housed in a reverse light:dark cycle (12h:12h). Both mouse genotypes were bred on site from α4+/− mice supplied by G. Homanics (Univ. of Pittsburgh). Additional C57BL6 mice from Jackson Laboratories (Bar Harbor, Maine) were used because spine typing results were similar to +/+ mice bred in-house from +/−, assessed by tail genotyping. Our previous study established that the responses of +/+ and wild-type C57BL6 mice to the drugs administered in this study (GBX and THP) are similar (Shen et al., 2007). The use of C57BL6 mice also broadens the relevance of our results.
Female mice were injected with various drugs or vehicle for 10 d (Fig. 1 inset) beginning at the onset of puberty (typically ~PND 35) (Fukue et al., 2006), assessed by vaginal opening. Dendritic spine assessment was assessed on PND 56, based on our previous results. Separate groups of mice were used for spine density measurements and behavioral assessment. A total of 124 mice were used for this study: Electrophysiology (Figure 1): 5 mice/group; spine density (Figure 2): Con: 8, THP: 8, GBX: 5; spine density in α4−/− mice treated with GBX (Figure 3): GBX: 4, vehicle: 4; spine density of post-pubertal mice treated with GBX (Figure 4): GBX: 4, vehicle: 4; spine density in α4−/− mice treated with THP (Figure 5): THP: 4, vehicle: 4; MPORT (Figure 6): control: 11, THP: 12, GBX: 12: APA (Figure 6): control: 10, THP: 9, GBX: 10.
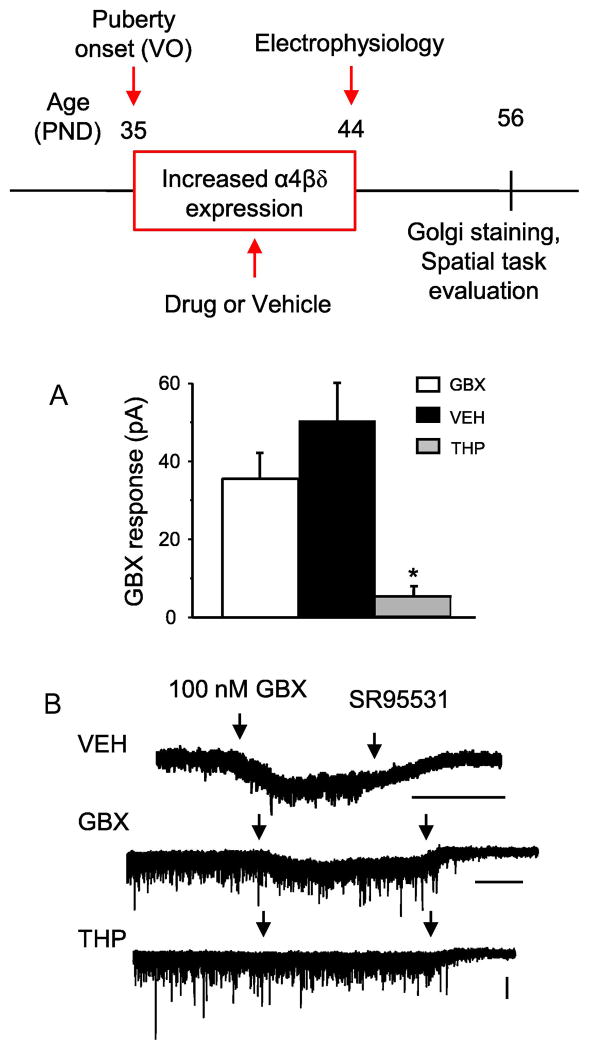
Fig. 1. Effect of pubertal α4βδ GABAR manipulations on GBX-gated tonic current: a measure of α4βδ GABAR function.
Inset, Drugs were administered during the 10 day period (PND 35–44) following puberty onset (vaginal opening, VO, ~PND35) when α4βδ GABAR expression is increased (Shen et al., 2007, 2010). Electrophysiology was performed at the end of the injection period (PND 44). Spine density and behavior were evaluated on PND 56. GBX, gaboxadol (THIP), a GABAR agonist with selectivity at α4βδ GABARs at low doses; VEH, vehicle (oil); THP, 3α-OH-5α-pregnan-20-one, a GABAR modulator which decreases α4βδ expression during puberty (Shen et al., 2007). A–B, Effect of pubertal drug or vehicle treatment on whole cell voltage clamp responses of CA1 pyramidal cells to 100 nM GBX, a reflection of α4βδ expression. A, averaged data (mean ± S.E.M). *ANOVA, F(2,12)=14.93, P ≤ 0.001 vs. other groups; B, representative currents. n=5 cells (mice)/group. Scale bars, 50 s (timebase), 50 pA (amplitude)
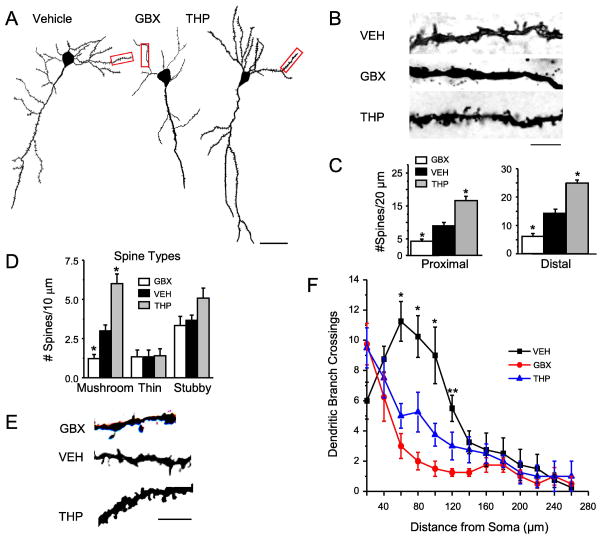
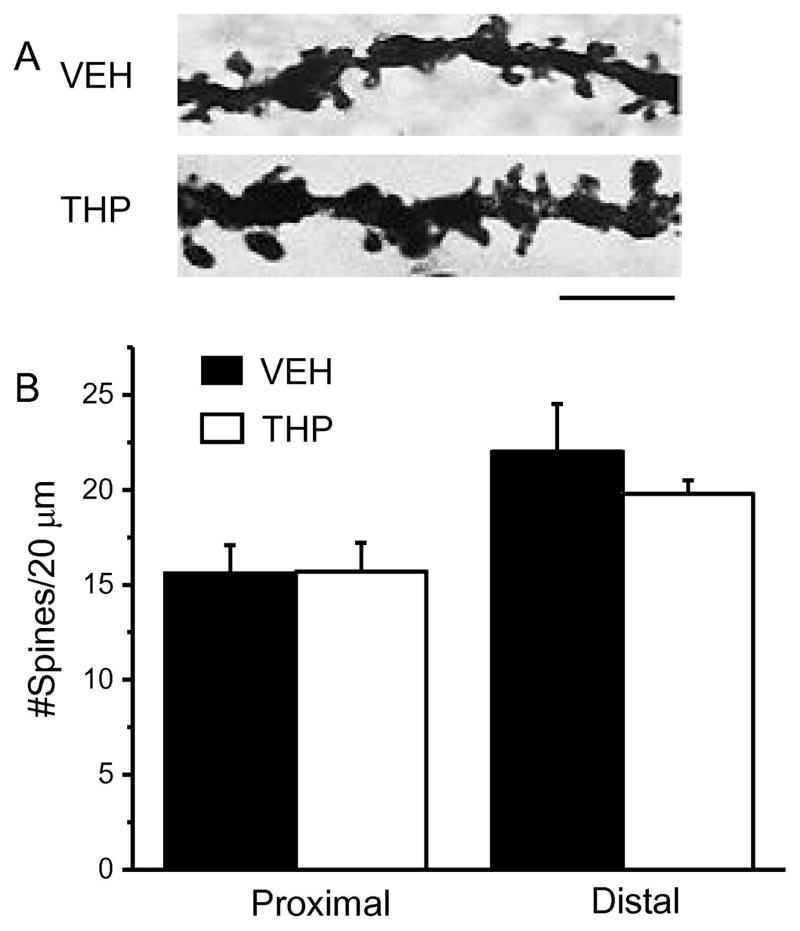
Fig. 2. Pubertal administration of drugs which target α4βδ GABARs alter spine density post-pubertally.
Drugs (administered during adolescence – PND 35–44): GBX, gaboxadol (THIP), a GABAR agonist with selectivity at α4βδ GABARs at low doses; VEH, vehicle (oil); THP, 3α-OH-5α-pregnan-20-one, a GABAR modulator which decreases α4βδ expression during puberty (Shen et al., 2007). A, Neurolucida images, post-Pub (PND 56) CA1 pyramidal cells, following pubertal drug treatment; scale, 50μm. B, z-stack (100x) images; scale, 10μm. C, Spine density, proximal (left, n=13–14) and distal (right, n=11–15) dendrites. *P<0.05 vs. VEH. (Complete statistics reported in Appendix A, Table A.1) D, Spine morphology changes. 9 dendrites/group; *P<0.05 vs. other groups. (Complete statistics reported in Appendix A, Table A.2) E, Representative images; scale, 3μm. F, dendritic crossings assessed across the apical dendrite. GBX, THP vs. VEH −60 μm: ANOVA, F(2,18)=22.0, *P≤0.0001; 80 μm: ANOVA, F(2,18)=18.7, *P≤0.0001; 100 μm: ANOVA, F(2,18)=11.1, *P≤0.001. GBX vs. VEH −120 μm: ANOVA, F(2,18)=18.7, P≤0.005.
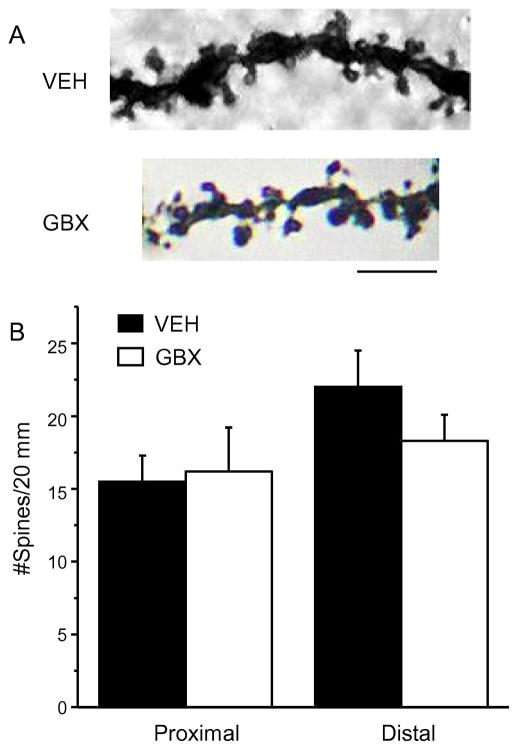
Fig. 3. Pubertal GBX treatment does not alter spine density in post-pubertal α4−/− mice.
A, Representative z-stack images of dendrites from post-pubertal (PND 56) α4−/− mice treated during puberty (PND 35–44) with vehicle (VEH) or the GABA agonist gaboxadol (GBX) at a dose selective for α4βδ GABARs. B, Averaged spine densities for proximal (left) and distal (right) dendrites. Scale, 3 μm; n=12 dendrites/group (proximal); 16 dendrites/group (distal). (Complete statistics reported in Appendix A, Table A.1.)
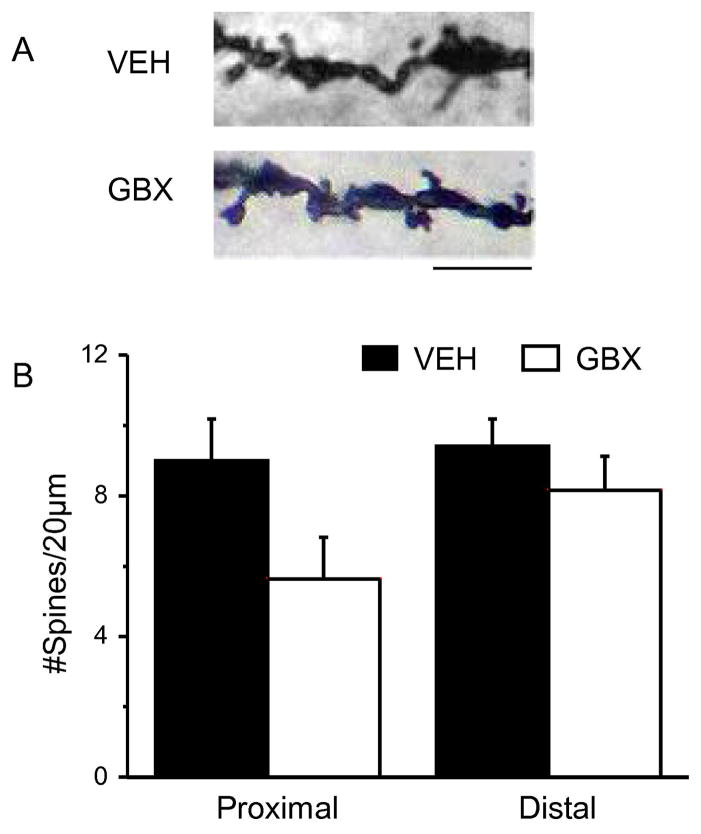
Fig. 4. Post-pubertal gaboxadol treatment does not alter spine density.
A, Representative z-stack images of dendrites from post-pubertal (PND 65) female mice treated during PND 56–64 with the GABA agonist gaboxadol (GBX, THIP) at a dose selective for α4βδ GABARs. B, Averaged (mean ± S.E.M.) spine densities for proximal (left) and distal (right) dendrites. Scale, 3 μm; n=8–9 dendrites/group (proximal); 7 dendrites/group (distal). (Complete statistics reported in Appendix A, Table A.1)
Fig. 5. Pubertal THP treatment of α4−/− mice does not increase spine density post-pubertally.
A, Representative z-stack images of dendrites from post-pubertal (PND 56) α4−/− mice treated during puberty (PND 35–44) with vehicle (VEH) or THP for which α4βδ GABARs are the most sensitive target. B, Averaged spine densities for proximal (left) and distal (right) dendrites. Scale, 3 μm; n=12 dendrites/group (proximal); 16 dendrites/group (distal). (Complete statistics reported in Appendix A, Table A.1)
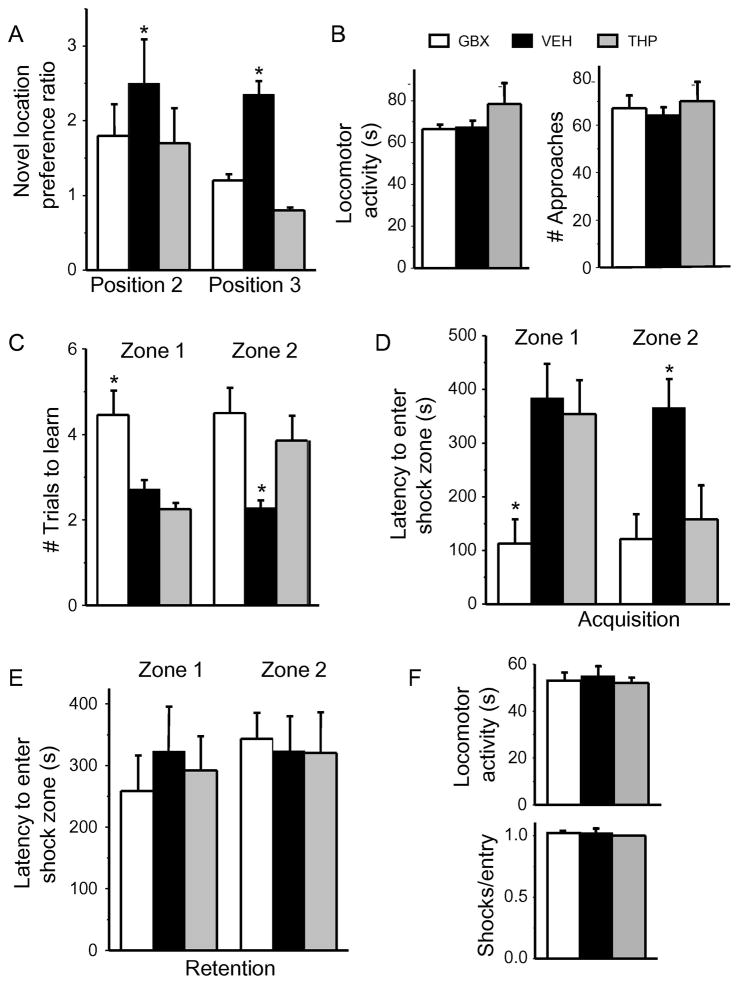
Fig. 6. Pubertal treatment with THP or GBX impairs relearning ability in the post-pubertal mouse.
A–B, Learning/re-learning a spatial location in the multiple placement object recognition task (MPORT). A, Novel position preference for positions 2 and 3. Position 2: ANOVA, F(2,32)=0.49; P=0.62; position 2, ANOVA, F(2,32)=15.48, *P≤0.0001 vs. VEH; n=11–12 mice/group. B, Locomotor activity (ANOVA, F(2,32)=1.06, P = 0.36) and # approaches (ANOVA, F(2,32)=0.28, P = 0.76). C–F, Learning/relearning a spatial location in the active place avoidance (APA) task. C, Number of trials to learning criterion (120 s latency to first entry of shock zone). Zone 1, ANOVA, F(2,26)=10.73, *P≤0.0005 vs. other groups; zone 2, ANOVA, F(2,26)=5.21, *P≤0.05 vs. other groups. GBX, gaboxadol (THIP); VEH, vehicle; THP (3α-OH, 5α-pregnan-20-one) D, E, Average latency to enter shock zone 1 and 2. Acquisition (D), zone 1: ANOVA, F(2,26)=5.54, *P≤0.01; zone 2: ANOVA, F(2,26)=3.63, *P≤0.05. Retention (E), zone 1: ANOVA, F(2,26)=0.17, P=0.85; zone 2: ANOVA, F(2,26)=0.01, P=0.99. F, Locomotor activity (ANOVA, F(2,26)=0.15, P=0.86) and #shocks/entry (ANOVA, F(2,26)=2.87, P=0.07); n=9–10 mice/group.
Electrophysiological assessment of the tonic current was carried out at PND 44, the end of the injection period. This time course was selected because α4βδ GABARs express on dendrites and spines of CA1 hippocampal pyramidal cells from ~PND 35–44 (Shen et al., 2010, Aoki et al., 2012). Two drugs were selected which target α4βδ GABARs: THP (3α-OH-5[α]β-pregnan-20-one or [allo]pregnanolone, 10 mg kg−1, i.p., intraperitoneally in oil) when injected chronically during the pubertal period decreases α4βδ expression (Shen et al., 2007); gaboxadol (GBX r 4,5,6,7-tetrahydroisoxazolopyridin-3-ol, also known as THIP, 0.1 mg kg−1, i.p.), which is a GABA agonist selective for α4βδ at low concentrations (Brown et al., 2002). Because the results obtained with the 5α and 5β isomers of THP were similar, the results from both were pooled. Estrous cycle stage was determined by the vaginal cytology in 8-week old animals with established regular cycles, and proestrous mice were not used because spine density (Woolley and McEwen, 1992) and spatial learning (Sabaliauskas et al., 2015) are significantly altered on proestrus compared to other stages of the estrous cycle. In contrast, spatial learning is not altered across the non-proestrous stages of the cycle (Sabaliauskas et al., 2015). In addition, the estrous cycle does not alter α4βδ expression or function during puberty (Shen et al., 2007). “Principles of laboratory animal care” were followed, and all experiments were consistent with specifications outlined in “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003). All procedures were in accordance with the SUNY Downstate Institutional Animal Care and Use Committee which conforms to national requirements. In all experimental procedures mice were randomly assigned to experimental groups and the investigator was blinded to the condition of the mice.
Golgi stain procedure
Golgi impregnation of whole brains was accomplished using the FD Rapid Golgi Stain kit (FD Neurotechnologies, Columbia, MD). Coronal sections (250μm) were prepared using a vibratome (Leica VT 100M). Pyramidal cells from the CA1 hippocampus were reconstructed using Neurolucida software (MicroBrightField). The neurons were viewed with a 100× oil objective on an Olympus BX51 upright light microscope. Z stack projection photomicrographs were taken with a Nikon DS-U3 camera mounted on a Nikon Eclipse Ci-L microscope at 100× oil objective and analyzed with NIS-Elements D 4.40.00 software. Camera Lucida drawings of dendrites were completed using a Nikon 710 microscope at 100× oil with a drawing tube attached.
Spine density measurement
Reconstructed neurons were analyzed using Neurolucida Explorer built-in Sholl analysis software for dendritic branch crossing (every 20 μm from the center) and spine density. Proximal dendrites were one-third of the distance or less from the cell soma while distal dendrites were one-third of the distance or less from the ends of dendritic branches and included primary, secondary, and tertiary branches as there were no differences between these groups. Spine density was similar in stratum oriens and stratum radiatum; therefore, these data were pooled. Spine types were determined using the semi-automated Spine Classifier of NeuronStudio (http://research.mssm.edu/cnic/tools-ns.html), a program that allows for the reconstruction of neurons and classification of spines from z-stacks. Briefly, stubby spines had less than a 1.1 length to width ratio, mushroom spines were identified by a ≥ .35 μm head width, with a head width:neck length> 2, while thin spines were classified if the head width: neck length < 1.2 and a length:width > 3 (Arellano et al., 2007). All spine density and morphology assessments were made with the investigator blinded to the condition of the animals tested.
Hippocampal slice preparation
Brains from euthanized mice were removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 2.5, CaCl2 2, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. Following sectioning at 400 μm on a Leica VT1000S vibratome, slices were incubated for 1 h in oxygenated aCSF.
Hippocampal slice voltage clamp electrophysiology
Pyramidal cells in the CA1 hippocampal slice were visualized using a differential interference contrast (DIC)-infrared upright microscope, and recorded using whole cell patch clamp procedures in voltage clamp mode at 26 – 30° C, as described (Shen et al., 2007). Patch pipets were fabricated from borosilicate glass using a Flaming-Brown puller to yield open tip resistances of 2–4 MΩ. For recordings of the pharmacologically isolated tonic inhibitory current, the pipet solution contained in mM: CsCl 140, HEPES 5, EGTA 5, CaCl2-H2O 0.5, QX-314 5, Mg-ATP 2, Li-GTP 0.5, pH 7.2, 290 mOsm. 5 mM QX-314 was added to block voltage-gated Na+ channels and GABAB receptors. The aCSF contained 50 μM kynurenic acid, to block excitatory current, as well as 0.5 μM TTX to isolate the post-synaptic component. Recordings were carried out at a -60 mV holding potential, and the tonic current was assessed by the change in holding current in response to 100 nM gaboxadol (GBX), a GABAR agonist, which, at this concentration, is selective for δ-containing GABAR (Brown et al., 2002, Meera et al., 2011). The GABAergic nature of the current was verified by block with 120 μM SR95531. Drugs were bath applied continuously in sequential order following 5–10 min of baseline recordings without drugs. Recordings were conducted with a 2 kHz 4-pole Bessel filter at a 10 kHz sampling frequency using an Axopatch 200B amplifier and pClamp 9.2 software. Electrode capacitance and series resistance were monitored and compensated; access resistance was monitored throughout the experiment, and cells discarded if the access resistance increased more than 10% during the experiment. In all cases, the data represent one recording/animal.
Drugs
All drugs except for the steroids and QX-314 were from Sigma Chemical Co. (St. Louis, MO). Steroids were obtained from Steraloids (Newport, RI), while QX-314 was from Calbiochem (Billerica, MA). The active isomers of THP, 3α-OH-5β-pregnan-20-one (pregnanolone) and 3α-OH-5α-pregnan-20-one (allopregnanolone) were used interchangeably because they produced similar results.
Tests of spatial learning and re-learning – Active place avoidance (APA) task (Shen et al., 2010)
Animals were tested in the active place avoidance (APA) task (Cimadevilla et al., 2001), a hippocampal-dependent task to assess learning and relearning ability. Here, animals are placed in a circular rotating platform where they use distal room cues (spatial cues) to avoid a shock zone oriented to the room (Shen et al., 2010). The shock delivered is 0.2 mA, which has been shown to be sub-threshold for eliciting a stress response (Friedman et al., 1967b). Animals are trained for two days. Each day the shock zone is oriented to a different location in the room (zones 1 and 2) and animals are trained until they learn to avoid the zone. Animals are monitored for the # trials to learn the avoidance zone and once learned, the latency to enter the zone on the day of learning (acquisition of the task). They are also monitored the following day for recall of the zone (memory retention). Locomotor activity and the number of shocks/entry are also assessed as a measure of escape behavior. This is a well-validated hippocampus-dependent task (Cimadevilla et al., 2001, Pastalkova et al., 2006).
Multiple placement object recognition task (MPORT) (Afroz et al., 2016)
Following an initial habituation to an empty arena for 1 h and re-visit to the home cage (20 min), mice are allowed to examine 2 identical objects at opposite ends of the arena for 10 min (position 1). Following a 20 min re-visit to the home cage, mice are tested for two additional 10 min trials after one of the objects is re-located to two new positions (positions 2 and 3). All test trials are separated by a 20 min re-visit to the home cage. The duration of examination of the moved (TM) and unmoved (TU) objects was quantified for each position. A robust spatial memory would result in longer examination of the moved objects because rodents prefer novelty. The discrimination ratio for detecting the moved object was quantified as: (TM)/(TU), referred to as the novel position preference ratio. Both locomotor activity and total # approaches, a measure of interest in the objects, were also quantified across groups. This is also an established hippocampus-dependent task (Barker and Warburton, 2011).
Statistics
All data are presented as the mean ± the standard error of the mean (S.E.M.). For the electrophysiology and behavioral data, as well as data for dendritic length and dendritic crossings, where the data-points are independent, comparisons between groups were analyzed with a one-way analysis of variance (ANOVA). Post-hoc comparisons were made with a Tukey’s test. Complete details about the statistics are included in the figure legends.
For the spine density and spine-typing measurements, a multilevel statistical model was used to account for the fact that multiple comparisons were made for each mouse. The hypothesis testing strategy consisted of treating analysis of each of the 7 experiments as a “family” of tests, the goal being to restrict the family-wise type I error rate to no more than 0.05. To ensure independence of observations, a single score (the arithmetic mean of individual scores) per animal was used. Independent-sample t-tests were conducted on pairs of groups; the Satterthwaite correction was applied to deal with the potential issue of heteroscedasticity; and bootstrapping (SAS PROC MULTTEST) was used to adjust P values for potential non-normality of distribution and for multiple testing. Median, upper quartile, lower quartile and P values (Appendix A, Tables A.1 and A.2) are provided in addition to the means and S.E.M. For all tests, the level of significance was determined to be P < 0.05.
Results
The function of α4βδ GABARs does not diminish after pubertal GBX administration
Gaboxadol (GBX) is a full agonist at α4βδ GABARs, and GABA is a partial agonist (Brown et al., 2002). Thus, we used GBX to maximally activate α4βδ GABARs when their expression is increased after puberty onset (PND 35–44) (Fig. 1 inset) with daily injections. In order to determine whether chronic activation of this receptor could be achieved for this period of time, without resulting in receptor desensitization, we assessed the functionality of α4βδ GABARs after chronic treatment with GBX (0.1 mg/kg, i.p.) using whole-cell patch clamp electrophysiology at the end of the injection period on PND 44 (Fig. 1). GBX, which selectively targets α4βδ GABARs at a 100 nM concentration (Meera et al., 2011), normally produces significant increases in tonic current amplitude recorded from untreated pubertal hippocampus, when there are high levels of α4βδ GABAR expression (PND 35–44) (Shen et al., 2010). GBX administration during the entire pubertal period did not significantly alter the GBX-gated current amplitude at PND 44 compared to responses from vehicle-treated animals. This suggests that the α4βδ-gated GBX current does not diminish across the pubertal period despite chronic activation of these receptors.
Pubertal GBX treatment reduces spine density and dendritic crossing post-pubertally
Because our previous study suggests that α4βδ GABARs initiate synaptic pruning during adolescence, we tested the hypothesis that enhancing the α4βδ GABAR mediated shunting inhibition with GBX across the pubertal period would increase synaptic pruning. To this end, GBX was administered at a low dose (0.1 mg/kg) to selectively target α4βδ GABARs from PND 35–44 (Smith et al., 2006) (Fig. 1 inset). Spine density and dendritic crossings were assessed at PND 56.
Post-pubertal Golgi impregnation revealed significant 50–70% reductions in the spine densities of proximal and distal dendrites of CA1 pyramidal cells after pubertal GBX treatment compared to values from vehicle-injected mice (P<0.05, Fig. 2A–C, Appendix A, Table A.1). When spine type was evaluated, pubertal GBX treatment significantly decreased the density of mushroom-type spines (1.25 ± 0.2 spines/10 μm, GBX, versus 3.1 ± 0.2 spines/10 μm, vehicle, P<0.05), with no significant changes in stubby or thin spines compared to vehicle (Fig. 2 D–E, Appendix A, Table A.2). Dendrite length was not altered by pubertal GBX or THP treatment (GBX, 220 ± 18.3 μm versus 205 ± 24.7 μm, vehicle; 220 ± 14.1 μm, THP; ANOVA, F(2,18)=0.19, P=0.83). Interestingly, analysis of dendritic branch trees of CA1 pyramidal cells post-pubertally revealed that pubertal GBX treatment decreased branching 60–120 μm away from the cell soma by up to 80% (P<0.05, Fig. 2F) compared to vehicle.
Pubertal GBX treatment of α4−/− mice does not reduce spine density post-pubertally
In order to confirm the selectivity of our dose of GBX for α4βδ GABARs we tested the effect of pubertal GBX treatment in α4−/− mice for spine density assessed post-pubertally. No differences in spine density on either proximal or distal dendrites of CA1 pyramidal cells between vehicle treated α4−/− mice and GBX treated α4−/− mice were found (Fig. 3, Appendix A, Table A.1) consistent with the idea that GBX works selectively on α4βδ GABARs, enhancing activation of these receptors during puberty to produce abnormal spine densities post-pubertally.
Post-pubertal GBX treatment does not reduce spine density
We also assessed spine density after treatment with GBX, administered post-pubertally (PND 56–64) when α4βδ expression is low. No significant differences in spine density on either proximal or distal dendrites of CA1 pyramidal cells were found between vehicle treated and GBX treated mice (Fig. 4, Appendix A, Table A.1) consistent with the idea that GBX treatment selectively alters spine density during the pubertal period when α4βδ expression is increased.
The function of α4βδ GABARs is markedly decreased after pubertal THP administration
An earlier study (Shen et al., 2007) shows that chronic administration of THP during the pubertal period reduces expression of α4βδ GABARs, which are typically increased significantly at the onset of puberty, in contrast to its acute effect to potentiate current gated by these receptors. In order to determine successful downregulation of these receptors in the present study, the function of α4βδ GABARs after chronic treatment with THP (10 mg/kg, i.p., once a day) across the pubertal period (PND 35–44, Fig. 1 inset) was determined by assessing the GBX (100 nM) response of CA1 pyramidal cells on PND 44 using voltage clamp techniques. THP-treated cells exhibited a ~90% reduced current response to GBX (P<0.05, Fig. 1) compared to responses after vehicle treatment. This finding is in agreement with our previous work showing that THP treatment during puberty reduces tonic inhibition due to decreased α4βδ GABAR expression (Shen et al., 2007).
Pubertal THP administration increases spine density post-pubertally
We tested the hypothesis that reducing pubertal α4βδ GABAR expression with THP would increase spine density post-pubertally based on our previous findings showing that knock-out of α4 prevents pubertal synaptic pruning (Afroz et al., 2016). To this end, we administered THP (10 mg/kg, i.p.) during the pubertal critical period (PND 35–44, Fig. 1 inset), to decrease the normally high expression of these receptors (Shen et al., 2007). Pubertal THP administration produced up to two-fold increases in spine density of both proximal and distal dendrites of CA1 pyramidal cells compared to vehicle (Fig. 2 A–C, Appendix A, Table A.1), assessed on PND 56. This increase in spine density is comparable to values recorded after α4 GABAR knock-out (Afroz et al., 2016). Profiles of spine types showed that CA1 pyramidal cells had similar populations of thin and stubby spines to WT pyramidal cells but had significantly more of the stable mushroom spines (6.1 ± 0.6 spines/10 μm, THP versus 3.1 ± 0.2 spines/10 μm, vehicle, Fig. 2 D–E, Appendix A, Table A.2). These results are consistent with our previous findings suggesting that α4βδ GABARs reduce spine density at puberty.
Assessment of whether pubertal THP administration facilitated dendritic branch pruning, a neuroplastic modification that also occurs during puberty, showed reduced tree complexity at 60–100 μm distances from the soma (Fig. 2F).
Pubertal THP treatment of α4−/− mice does not increase spine density post-pubertally
To further clarify the selectivity of THP for α4βδ GABARs, α4−/− mice were treated with THP during the pubertal period with the prediction that no changes in spine density would occur on PND 56. In fact, spine density was not altered by THP treatment on either proximal or distal dendrites of α4−/− CA1 pyramidal cells (Fig. 5, Appendix A, Table A.1) consistent with the concept that THP is selective for α4βδ GABARs at the doses used in the present study.
Pubertal GBX treatment impairs cognition assessed post-pubertally
Our previous findings suggest that increases in spine density produced by knockout of the GABAR α4 subunit impair re-learning of a spatial task despite normal learning ability, assessed post-pubertally (Afroz et al., 2016). However, other studies report learning deficiencies when spine density is decreased (Yasumura et al., 2014). Thus, we assessed the learning and re-learning of a spatial task at PND 56, following pubertal treatment with GBX to decrease spine density in female mice.
To this end, we initially used the multiple placement object recognition task (MPORT) to test spatial and reversal learning. Post-pubertal testing with MPORT revealed that GBX-treated animals had similar ability to learn to go to the moved object after the first relocation (position 2) but were unable to learn after the second relocation (position 3), spending less time at the moved object compared to the vehicle treated animals (Fig. 6A). This indicates deficient relearning ability. There were no differences in locomotor activity or interest in the task (# approaches) (Fig. 6B).
The active place avoidance (APA) task was also used to test spatial learning and reversal learning after drug treatment. Unlike the results from MPORT, GBX-treated mice were deficient in the initial acquisition of this task (learning) where the # trials to learn zone 1 were double that of the vehicle control (Fig. 6C) and the latency to enter the avoidance zone was 70% faster than the vehicle control (P<0.05, Fig. 6D). Re- learning was also impaired, where acquisition of zone 2 had a similarly faster latency than the vehicle control (P<0.05, Fig. 6 D–E). The # trials to learn zone 2 was also twofold greater than the vehicle control (P<0.05). These data suggest that the use of this more rigorous task reveals deficiencies in both learning and re-learning when spine density of CA1 hippocampal pyramidal cells is decreased. These differences are not accounted for by differences in baseline activity or pain sensitivity as all animals started with similar baseline locomotion and shock tolerance (Fig. 6F).
Pubertal THP Treatment Impairs Post-Pubertal Relearning
Based on our previous findings in the α4−/− mouse (Afroz et al., 2016), we predicted that the increase in spine density produced by pubertal THP treatment would impair re-learning of a spatial task, assessed on PND 56. In fact, both MPORT and APA tasks revealed impairment of re-learning but not learning of a spatial task. Novel location preference was significantly reduced for position 3 by about 70%, but not position 2, on the MPORT task (P<0.05, Fig. 6A). In contrast, locomotor activity and interest in the task were unaffected by pubertal THP treatment (Fig. 6B).
Acquisition of zone 2 was impaired on the APA task, with a significant increase in the number of trials to learn this position and a 60% decrease in the latency to enter the avoidance zone (P<0.05, Fig. 6 C–D). In contrast, the initial learning of zone 1 was unimpaired, similar to findings from the α4−/− mouse. Memory retention was unimpaired (Fig. 6E). Neither locomotor activity nor shock tolerance was altered by pubertal THP treatment (Fig 6F), suggesting a selective effect on relearning.
Discussion
The experiments discussed in this paper display novel use of pharmacological tools to target α4βδ GABARs during puberty to regulate spine density and cognition post-pubertally. Alterations in spine density were most significant for the mushroom spine population and produced impairment of the re-learning of a spatial task. These findings confirm our previous report that α4βδ GABARs trigger adolescent synaptic pruning (Afroz et al., 2016) and illustrate the importance of pruning dendritic spines to an optimal number to achieve optimal cognition in adulthood.
One advantage of the pharmacological approach used in the present study is that manipulation of the α4βδ-induced tonic inhibition was selective for the pubertal period. Pharmacological manipulations were used to both enhance tonic inhibition via α4βδ GABARs (GBX) and to decrease expression of α4βδ GABARs (THP) which decreased the tonic inhibition generated by these receptors.
As predicted by our original findings, pubertal THP administration prevented synaptic pruning, resulting in increased spine density post-pubertally. THP is a metabolite of the ovarian steroid progesterone (Compagnone and Mellon, 2000), but is also released in response to chronic stress (Purdy et al., 1991, Girdler et al., 2001). Stress has been shown to alter spine density across numerous CNS areas. Most studies report that stress increases spine density in the CA1 hippocampus (Conrad et al., 2012, Orlowski et al., 2012, Sebastian et al., 2013), although conflicting reports exist (Dalla et al., 2009, Castaneda et al., 2015). In fact, the adolescent brain responds to both emotional and physical stress with increases in spine density, consistent with the effect of THP in the present study (Warren et al., 2014). Thus, the results presented here may be relevant for stress-induced effects on spine morphology.
In contrast to the effects of THP, enhancing α4βδ-mediated inhibition during the pubertal period with GBX reduced spine density by more than 50% post-pubertally. This suggests that the level of pruning triggered by endogenous GABA via α4βδ GABARs is not at maximal levels. GBX is a full agonist at α4βδ GABARs, where GABA is a partial agonist (Brown et al., 2002, Meera et al., 2011), which may also explain the increase in pruning with pubertal GBX administration.
The effect on spine density produced by pubertal treatment with GBX and THP in the present study was shown to be selective for α4βδ GABARs. Although THP and GBX can enhance GABA-gated current at GABAR sub-types other than α4βδ (Belelli et al., 1996, Ebert et al., 1997), they are selective for α4βδ at the doses used in the present study. Consistent with their reported selectivity for this receptor, neither compound had any effect on spine density when administered to the α4−/− mouse or, in the case of GBX, to a post-pubertal mouse, which has low CA1 hippocampal expression of α4βδ GABARs.
The CA1 hippocampal pyramidal cell has very low expression of α4βδ GABARs before puberty, but expression increases by up to 4 to 8-fold at the onset of puberty in the female mouse (Shen et al., 2007, Shen et al., 2010). These receptors are expressed along the dendritic shaft and also on the spine adjacent to excitatory synapses. In the CA1 hippocampus the dendritic spine is devoid of GABAergic inhibition except for this transient expression of expression α4βδ GABARs for ~10 d during puberty (Shen et al., 2010, Aoki et al., 2012). These receptors generate a shunting tonic inhibition that impairs activation of NMDARs (Shen et al., 2010) which we have shown is the trigger for adolescent synaptic pruning (Afroz et al., 2016). In contrast, tonic inhibition produced by the predominant extrasynaptic GABAR population, α5-containing GABARs (Caraiscos et al., 2004), does not impair NMDAR activation at puberty likely because these receptors are not expressed on the spine (Brunig et al., 2002) and blockade of this receptor does not influence spine density at puberty (Afroz et al., 2016).
Our recent findings (Afroz et al., 2016) suggest that synaptic pruning involves dynamic changes in the expression of Kalirin-7, a Rho guanine nucleotide exchange factor (Rho-GEF) (Penzes et al., 2001) necessary for spine maintenance (Ma et al., 2003). This spine protein stabilizes the actin cytoskeleton via P21-activated kinases following activation of GTPase Rac1 (Ma et al., 2014). Kalirin-7 is decreased at puberty due to this decline in NMDAR activity as a result of the α4βδ-generated shunting inhibition. Adolescent synaptic pruning is not observed with knock-out of Kalirin-7 (Afroz et al., 2016), suggesting that the decline in Kalirin-7 at puberty triggers synaptic pruning in CA1 hippocampal pyramidal cells.
Although the results from the present study were restricted to the female mouse, we have previously shown that synaptic pruning in the male CA1 hippocampus during adolescence is prevented by knock-out of the GABAR α4 subunit (Afroz et al., 2016). Thus, it is likely that the pharmacological manipulations used in the present study would produce outcomes similar to those observed in the female.
Scavenging by the immune system (Schafer et al., 2012) or direct autophagy (Tang et al., 2014) are reported to clean up the debris from these pruned connections. Of these, the C4 complement system is also implicated in schizophrenia (Sekar et al., 2016) but may be the final step in the pruning process. However, these processes were shown to not play a role in CA1 hippocampal pruning during adolescence (Shi et al., 2015) suggesting that synaptic pruning mechanisms may be site- and developmentally-specific.
Post-pubertal dendritic branching was reduced by pubertal GBX treatment suggesting that enhancing α4βδ-generated current during puberty not only decreases spine density but also reduces branching of the dendrites. The expression of this receptor is also increased by two-fold on the dendritic shaft at puberty (Shen et al., 2010), where it may alter the branching pattern. Other studies have reported decreases in dendritic branching correlated with increases in α4βδ expression (Chowdhury et al., 2014) consistent with this finding. Interestingly, treatment with THP to decrease α4βδ expression also decreased dendritic branching. While this may seem paradoxical, it is possible that THP, as a positive GABAR modulator (Belelli et al., 1996), enhances current generated by the other GABAR sub-types which are expressed on the dendritic shaft (Nusser et al., 1996, Brunig et al., 2002). In contrast to the dendritic shaft, α4βδ is the only GABAR subtype known to express on the dendritic spine of CA1 hippocampal pyramidal cells (Shen et al., 2010). Thus, THP could have differential effects on the shaft vs. the spine because it would enhance GABA-gated current at non-α4βδ GABAR subtypes which are expressed on the shaft but decrease α4βδ expression on the spine.
As in our previous study (Afroz et al., 2016), the high spine density produced by pubertal THP treatment was associated with impaired re-learning of a spatial task post-pubertally despite normal learning of the initial task. These findings are consistent with results from theoretical analysis (Chechik et al., 1999) and may be due to the relatively higher density of mushroom-type spines. It has recently been shown that learning increases mushroom spines (Mahmmoud et al., 2015). Our previous findings (Afroz et al., 2016) suggest that both learning and re-learning of a spatial task progressively increase mushroom spines on CA1 hippocampal pyramidal cells. In the unpruned condition (i.e., α4−/− mouse), however, only learning increases mushroom spines. The relearning protocol did not additionally increase mushroom spines perhaps because the pre-existing mushroom spine density was prohibitively high. It is possible that the number of mushroom spines which can exist on a dendritic segment is limited by energy requirements or spatial restrictions and this may underlie the deficits in relearning under conditions where the mushroom spine population is increased (i.e., after pubertal THP treatment). In contrast, reduced spine density after pubertal treatment with GBX also resulted in impaired learning on the APA task. These findings agree with other studies (Yasumura et al., 2014) suggesting that reductions in spine density below a critical level impair learning and synaptic plasticity.
Several studies have suggested that long term depression of CA1 hippocampal responses to input from the Schaffer collaterals underlies reversal learning. This is based on evidence that genetic and pharmacological manipulations which impair or enhance LTD exert a similar effect on reversal learning (Duffy et al., 2008, Nicholls et al., 2008). Reversal learning on spatial tasks, such as the Morris water maze, is, however, dependent not only on the hippocampus (Whishaw and Tomie, 1997, Nicholls et al., 2008) but also on connections which exist (Conde et al., 1995) from the CA1 hippocampus to the medial prefrontal cortex (mPFC) (Chudasama et al., 2012). Direct lesions of the mPFC impair reversal learning on the Morris water maze (Latif-Hernandez et al., 2016), as do lesions of the fimbria of the fornix, which contain the afferent and efferent fibers for the hippocampus (Whishaw and Tomie, 1997). Thus activity of the CA1 hippocampal – mPFC circuitry appears to be necessary for optimal reversal learning.
The finding that relearning in adulthood was significantly impaired when spine density was either increased or decreased is consistent with the clinical data reporting patients having impaired reversal learning in neurodevelopmental disorders with abnormal synaptic pruning (Glantz and Lewis, 2000, Tang et al., 2014), such as autism and schizophrenia (Floresco et al., 2009, D’Cruz et al., 2013). Interestingly, both of these conditions can be associated with genetic abnormalities for α4 and/or δ expression (Ma et al., 2005, Bullock et al., 2008) although they are somewhat rare. Reduced α4 expression has been noted in autism (Fatemi et al., 2010) and is correlated with increased risk of developing this disorder (Collins et al., 2006), suggesting a mechanistic link. Although autism emerges in early childhood, the adolescent period is frequently associated with loss of cognitive improvements achieved pre-pubertally (Gillberg and Steffenburg, 1987, Sigman and McGovern, 2005).
Adolescent synaptic pruning is seen in many CNS areas (Huttenlocher, 1979, Zehr et al., 2006, Yildirim et al., 2008, Petanjek et al., 2011, Drzewiecki et al., 2016) including prefrontal cortex and amygdala, in addition to CA1 hippocampus, and is associated with EEG changes in humans (Campbell et al., 2012). Pubertal expression of α4βδ GABARs may serve to sculpt the hippocampal circuit, reducing synapses which are no longer relevant to allow for new synaptic connections as the animal reaches adulthood. Cognition is abnormal in neurodevelopmental disorders with abnormal synaptic pruning. The present findings also suggest that a stress steroid, by increasing spine density, impairs cognitive flexibility. Thus, the regulation of α4βδ GABARs may have important behavioral consequences relevant both for neurodevelopmental disorders and stress during adolescence.
Highlights.
Increasing α4βδ GABAA receptor current with gaboxadol during puberty decreases dendritic spine density in adulthood
Decreasing α4βδ GABAA receptor expression with THP during puberty increases dendritic spine density in adulthood
Abnormal spine density produced by pubertal drug treatments impairs re-learning a spatial memory task in the adult
These findings suggest that α4βδ GABAA receptors are a novel target to normalize spine density in adolescence
These results may suggest new therapies for autism and schizophrenia where spine density and cognition are abnormal
Acknowledgments
This work was supported by NIH grant R01-MH100561 to SSS. The authors declare that no competing interests exist.
We thank G. Homanics (Univ. Pittsburgh) for supplying the α4+/− mice, A. Fenton (NYU Medical Center) for use of the active place avoidance device and J. Weedon (SUNY Downstate) for the statistical analyses. This work was supported by NIH grant R01-MH100561 to SSS.
Glossary
- APA
active place avoidance
- GBX
gaboxadol (4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol), also known as THIP
- GABAR
GABAA receptor
- MPORT
multiple placement object recognition task
- NMDAR
NMDA receptor
- PND
post-natal day
- “Pubertal period”
The 10 d period beginning at the onset of puberty (detected by vaginal opening, ~PND 35) during which α4βδ GABARs are increased in female CA1 hippocampus.
- THP
the neurosteroid 3α-OH-5α/β-pregnan-20-one (pregnanolone/allopregnanolone)
Appendix A
Table A.1.
Median, upper and lower quartile values and adjusted P values for individual comparisons of spine density (PND 56) after the indicated drug treatments during adolescence (PND 35–44). Statistics were performed using a multi-level model.
| Pubertal Drug Administration in WT mice
| ||||||
|---|---|---|---|---|---|---|
| Group | Variable | N | Lower Quartile | Median | Upper Quartile | P value |
| GBX | Proximal dendrite spine density | 10 | 3.55 | 4.43 | 5.31 | 0.0177 |
| 0.0003* | ||||||
| Distal dendrite spine density | 8 | 4.15 | 7.06 | 8.70 | 0.0091 | |
| 0.0003* | ||||||
|
| ||||||
| THP | Proximal dendrite spine density | 21 | 12.00 | 15.18 | 16.75 | 0.0163 |
| Distal dendrite spine density | 23 | 17.15 | 20.85 | 24.64 | 0.0057 | |
|
| ||||||
| Vehicle | Proximal dendrite spine density | 24 | 6.13 | 9.19 | 11.03 | |
| Distal dendrite spine density | 19 | 8.45 | 10.97 | 14.66 | ||
|
| ||||||
| Pubertal GBX Administration in α4 KO | ||||||
|
| ||||||
| GBX | Proximal dendrite spine density | 11 | 12.00 | 13.33 | 16.00 | 0.9376 |
| Distal dendrite spine density | 12 | 13.33 | 20.00 | 21.00 | 0.8807 | |
|
| ||||||
| Vehicle | Proximal dendrite spine density | 12 | 14.77 | 17.74 | 22.36 | |
| Distal dendrite spine density | 9 | 15.62 | 17.61 | 21.00 | ||
|
| ||||||
| Pubertal THP Administration in α4 KO | ||||||
|
| ||||||
| THP | Proximal dendrite spine density | 12 | 11.33 | 16.00 | 19.33 | 0.3409 |
| Distal dendrite spine density | 12 | 17.00 | 18.33 | 20.00 | 0.5132 | |
|
| ||||||
| Vehicle | Proximal dendrite spine density | 12 | 12.49 | 17.63 | 23.37 | |
| Distal dendrite spine density | 12 | 14.31 | 19.06 | 28.11 | ||
|
| ||||||
| Post-Pubertal GBX Administration | ||||||
|
| ||||||
| GBX | Proximal dendrite spine density | 10 | 4.35 | 5.30 | 6.50 | 0.2165 |
| Distal dendrite spine density | 8 | 4.15 | 7.06 | 8.70 | 0.0666 | |
|
| ||||||
| Vehicle | Proximal dendrite spine density | 12 | 5.61 | 7.80 | 11.42 | |
| Distal dendrite spine density | 9 | 8.45 | 10.25 | 11.59 | ||
denotes THP vs GBX comparison; Boldface type indicates statistical significance.
Table A.2.
Median, upper and lower quartile values and adjusted P values for individual comparisons of spine-types (PND 56) after the indicated drug treatments during adolescence (PND 35–44). Statistics were performed using a multi-level model.
| Pubertal Drug Administration in WT mice
| |||||
|---|---|---|---|---|---|
| Stubby Spines
| |||||
| Group | N | Lower Quartile | Median | Upper Quartile | P value |
| THP | 12 | 3.50 | 5.00 | 7.00 | 0.3029 |
|
| |||||
| GBX | 9 | 2.00 | 3.00 | 4.00 | 0.9638 |
| 0.2887* | |||||
|
| |||||
| Vehicle | 9 | 3.00 | 3.00 | 4.00 | |
|
| |||||
| Mushroom Spines | |||||
|
| |||||
| THP | 12 | 4.50 | 6.00 | 7.00 | 0.0078 |
|
| |||||
| GBX | 9 | 1.00 | 2.00 | 2.00 | 0.0588 |
| 0.0051* | |||||
|
| |||||
| Vehicle | 8 | 2.00 | 2.00 | 3.00 | |
|
| |||||
| Thin Spines | |||||
|
| |||||
| THP | 12 | 0.00 | 0.50 | 1.50 | 0.9903 |
|
| |||||
| GBX | 9 | 0.00 | 0.50 | 1.00 | 0.9311 |
| 0.9624* | |||||
|
| |||||
| Vehicle | 9 | 1.00 | 1.00 | 1.00 | |
denotes THP vs. GBX comparison; boldface type indicates statistical significance.
Footnotes
Author contributions: S. Afroz performed the dendritic spine assessments and behavioral experiments, help to design the experiments, analyzed the data and contributed to the writing of the paper; H. Shen performed the electrophysiology experiments; S.S. Smith designed the experiments, analyzed the electrophysiology data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afroz S, Parato J, Shen H, Smith SS. Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines. Elife. 2016:5. doi: 10.7554/eLife.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, Barbarich-Marsteller NC. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66:391–407. doi: 10.1002/syn.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? JNeurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology. 1996;35:1223–1231. doi: 10.1016/s0028-3908(96)00066-4. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. JNeurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. BrJPharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. JCompNeurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Grimm KJ, de BE, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. ProcNatlAcadSciUSA. 2012;109:5740–5743. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, DB, Newell JG, Jackson MF, JJL, Rosahl TW, Wafford K, JFM, BAO Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda P, Munoz M, Garcia-Rojo G, Ulloa JL, Bravo JA, Marquez R, Garcia-Perez MA, Arancibia D, Araneda K, Rojas PS, Mondaca-Ruff D, Diaz-Veliz G, Mora S, Aliaga E, Fiedler JL. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J Neurosci Res. 2015;93:1476–1491. doi: 10.1002/jnr.23602. [DOI] [PubMed] [Google Scholar]
- Chechik G, Meilijson I, Ruppin E. Neuronal regulation: A biologically plausible mechanism for efficient synaptic pruning in development. Neurocomputing. 1999;26–27:633–639. [Google Scholar]
- Chowdhury TG, Barbarich-Marsteller NC, Chan TE, Aoki C. Activity-based anorexia has differential effects on apical dendritic branching in dorsal and ventral hippocampal CA1. Brain structure & function. 2014;219:1935–1945. doi: 10.1007/s00429-013-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. J Neurosci. 2012;32:10915–10924. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociaiton of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci. 2001;98:3532–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27:152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptors density. Psychoneuroendocrinol. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse. 2016;70:361–368. doi: 10.1002/syn.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thompson S, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. Journal of autism and developmental disorders. 2010;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. BehavBrain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Friedman SB, Ader R, Grota LJ, Larson T. Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosomatic medicine. 1967;29:323–328. doi: 10.1097/00006842-196707000-00003. [DOI] [PubMed] [Google Scholar]
- Fukue Y, Sato T, Teranishi H, Hanada R, Takahashi T, Nakashima Y, Kojima M. Regulation of gonadotropin secretion and puberty onset by neuromedin U. FEBS letters. 2006;580:3485–3488. doi: 10.1016/j.febslet.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: a population-based study of 46 cases followed through puberty. Journal of autism and developmental disorders. 1987;17:273–287. doi: 10.1007/BF01495061. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Beth Mechlin M, Light KC, Morrow AL. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. BiolPsych. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. ArchGenPsychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgoren S, Furedy JJ, Pogun S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain ResBull. 2000;52:243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Latif-Hernandez A, Shah D, Ahmed T, Lo AC, Callaerts-Vegh Z, Van der Linden A, Balschun D, D’Hooge R. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Scientific reports. 2016;6:36489. doi: 10.1038/srep36489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobellova V, Entlerova M, Svojanovska B, Hatalova H, Prokopova I, Petrasek T, Vales K, Kubik S, Fajnerova I, Stuchlik A. Two learning tasks provide evidence for disrupted behavioural flexibility in an animal model of schizophrenia-like behaviour induced by acute MK-801: a dose-response study. Behav Brain Res. 2013;246:55–62. doi: 10.1016/j.bbr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J HumGenet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang Y, Eipper BA, Mains RE. Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci. 2003;23:10593–10603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Miller MB, Vishwanatha KS, Gross MJ, Wang Y, Abbott T, Lam TT, Mains RE, Eipper BA. Nonenzymatic domains of Kalirin7 contribute to spine morphogenesis through interactions with phosphoinositides and Abl. Mol Biol Cell. 2014;25:1458–1471. doi: 10.1091/mbc.E13-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2009;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mahmmoud RR, Sase S, Aher YD, Sase A, Groger M, Mokhtar M, Hoger H, Lubec G. Spatial and Working Memory Is Linked to Spine Density and Mushroom Spines. PLoS One. 2015;10:e0139739. doi: 10.1371/journal.pone.0139739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Meera P, MW, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. JNeurophysiology. 2011;106:2057–2011. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, Kandel ER. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major g-aminobutyric acid type A receptor a subunits on hippocampal pyramidal cells. PNAS. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski D, Elfving B, Muller HK, Wegener G, Bjarkam CR. Wistar rats subjected to chronic restraint stress display increased hippocampal spine density paralleled by increased expression levels of synaptic scaffolding proteins. Stress. 2012;15:514–523. doi: 10.3109/10253890.2011.643516. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Chandra D, Homanics GE, Olsen RW, Houser CR. Altered localization of the delta subunit of the GABAA receptor in the thalamus of alpha4 subunit knockout mice. Neurochem Res. 2014;39:1104–1117. doi: 10.1007/s11064-013-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. ProcNatlAcadSciUSA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. PNAS. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at alpha4betadelta GABA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2015;1621:170–86. doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian V, Estil JB, Chen D, Schrott LM, Serrano PA. Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. PLoS One. 2013;8:e79077. doi: 10.1371/journal.pone.0079077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA Schizophrenia Working Group of the Psychiatric Genomics C. Schizophrenia risk from complex variation of complement component 4. Nature. 2016 doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, CA, MY, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4-beta2-delta GABA-A receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, CA, Smith SS. A critical role for a4bd GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX, Li S, Dodart JC, Caldarone BJ, Stevens B, Lemere CA. Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, McGovern CW. Improvement in cognitive and language skills from preschool to adolescence in autism. Journal of autism and developmental disorders. 2005;35:15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5alpha-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. ProcNatlAcadSciUSA. 2007;104:19553–19558. doi: 10.1073/pnas.0704031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Developmental neuroscience. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. JNeurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson G. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci. 2001;21:2630–2639. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura M, Yoshida T, Yamazaki M, Abe M, Natsume R, Kanno K, Uemura T, Takao K, Sakimura K, Kikusui T, Miyakawa T, Mishina M. IL1RAPL1 knockout mice show spine density decrease, learning deficiency, hyperactivity and reduced anxiety-like behaviours. Scientific reports. 2014;4:6613. doi: 10.1038/srep06613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Mapp OM, Janssen WG, Yin W, Morrison JH, Gore AC. Postpubertal decrease in hippocampal dendritic spines of female rats. Exp Neurol. 2008;210:339–348. doi: 10.1016/j.expneurol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. JNeurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]