Abstract
Mutations in the VHL, RET, SDHB, and SDHD genes are responsible for von Hippel-Lindau (VHL) disease, multiple endocrine neoplasia type 2 (MEN2), and familial paraganglioma, respectively. However, genotype-phenotype correlation data are lacking in Southeast Asia. A retrospective medical chart review was performed on patients referred to the genetics service. We found 35 patients diagnosed with clinical syndromes (16 VHL, 9 MEN2, 9 paragangliomas, and 1 neurofibromatosis type 1). In patients with VHL, 5 known VHL mutations were identified: p.Trp88X, p.Ile151Thr, p.Arg161X, p.Arg167Gln, and p.Leu178Arg. The most frequent RET mutations in patients with MEN2A occurred at codon 634 on exon 11: p.Cys634Tyr, p.Cys634Trp, and p.Cys634Arg. A patient with MEN2B had p.Met918Thr RET mutation. Approximately, 90% of patients with MEN2 had medullary thyroid carcinoma. Pheochromocytoma was found in 55.6% of patients with MEN2, and 60% of them had bilateral lesions. One patient with malignant thoracic paraganglioma had p.Arg46X mutation of SDHB. This study provides mutation phenotypes that offer a useful tool for clinicians and patients to stratify disease risks and tailor screening programs.
Keywords: Genetic, genotype-phenotype correlation, von Hippel-Lindau disease, multiple endocrine neoplasia type 2, neurofibromatosis type 1, paraganglioma
Introduction
Several well-recognized familial autosomal dominant disorders, such as von Hippel-Lindau (VHL) disease, multiple endocrine neoplasia type 2 (MEN2), familial paraganglioma and, less commonly, neurofibromatosis type 1 (NF1), are associated with pheochromocytomas and paragangliomas (PPGLs). The VHL phenotype includes pheochromocytoma (frequently bilateral), paraganglioma (thoracic, abdominal, and pelvic), hemangioblastoma (involving the cerebellum, spinal cord, or brainstem), pancreatic neuroendocrine tumor, serous cystadenoma of the pancreas, retinal angioma, clear cell renal cell carcinoma (RCC), papillary cystadenoma of the epididymis and broad ligament, and endolymphatic sac tumor of the middle ear. The VHL disease has been divided into type 1 (in which affected individuals have lower risk of pheochromocytoma but may have retinal or central nervous system hemangioblastomas and RCC) and type 2 (at least 1 affected person has pheochromocytoma). Type 2 VHL disease is subdivided into type 2A (retinal and central nervous system hemangioblastomas but low risk of RCC) and type 2B (hemangioblastomas, RCC, and pheochromocytoma). Moreover, type 2C VHL disease is characterized by isolated pheochromocytoma, without hemangioblastoma or RCC.
Multiple endocrine neoplasia type 2 affected by germline mutations in the RET proto-oncogene includes MEN2A, MEN2B, and familial medullary thyroid carcinoma (MTC) MEN2A is characterized by MTC in >90% of the patients, pheochromocytoma in 50%, primary hyperparathyroidism (PHPT) in 20%, and cutaneous lichen amyloidosis (CLA) in 5%. MEN type 2B represents about 5% of all patients with MEN2 and the phenotype is characterized by MTC in 100% of the patients, pheochromocytoma in 50%, mucocutaneous neuromas (usually involving the eyelids, lips, and tongue) in most of the patients, myelinated corneal nerves, skeletal deformities (eg, kyphoscoliosis or lordosis), joint laxity, and intestinal ganglioneuromas (hyperganglionic megacolon).
Familial paraganglioma is inherited in an autosomal dominant pattern and characterized by paragangliomas that are frequently located in the skull base and neck but also in the thoracic, abdomen, pelvis, and urinary bladder. Mutations in the succinate dehydrogenase (SDH) subunit genes (SDHA, SDHAF2, SDHB, SDHC, SDHD) have been linked to familial paraganglioma. Neurofibromatosis type 1 is an autosomal dominant disorder that results from a mutation in the NF1 gene. It is characterized by multiple neurofibromas and café au lait macules, axillary and inguinal freckling, Lisch nodules, central nervous system gliomas, bone abnormalities, macrocephaly, and cognitive deficits. Pheochromocytomas and paragangliomas in the patients with NF1 are rare, with an incidence of 0.1% to 5.7%.1
Although there is variable expressivity among families, some clinical features are similar within families. Genotype-phenotype correlations have been documented for these disorders, and specific mutations are associated with particular patterns of tumor formation.2–4 The clinical implication from genetic testing can guide physicians for proper surveillance and management based on each specific mutation.
To date, reports concerning hereditary PPGLs have primarily originated from Western countries. Only case reports have been published from the Southeast Asia area. The purpose of this study is to retrospectively review the spectrum of germline mutations in VHL, RET, SDHB, and SDHD genes that underlie hereditary PPGLs, with an emphasis on genotype-phenotype correlations in Thai patients.
Methods
A retrospective review of medical records was performed of individuals with suspected VHL disease, MEN2, NF1, and familial paragangliomas, who were referred to the medical genetics clinic at Ramathibodi Hospital, Mahidol University, Bangkok, between January 1, 2004 and September 30, 2016. The study was approved by the Ethics Committee of Ramathibodi Hospital. Each patient provided written informed consent.
Clinical data collection
Clinical information was obtained on diagnosis, age at onset, family history tumor location (adrenal or extra-adrenal), number of tumors (solitary or multiple), secretory profile from 24-hour urinary vanillylmandelic acid (VMA) or fractionated metanephrines, metastasis or recurrent disease, and histology. Malignancy was defined by the presence of local invasion into adjacent tissues or distant metastasis.
Molecular genetic analyses
All genetic testing was done based on clinical presentation. Only 1 candidate gene was initially tested in 1 patient. If we could not find the mutation, we performed the test on the next 1 of the 4 genes. If a mutation is identified at any point, no further testing should be performed. With the exception of NF1, the diagnosis was based on unique clinical features alone as generally accepted.5 DNA was extracted from peripheral blood leukocytes using a standard protocol and analyzed for germline mutations of RET (exons 10-16), VHL (all exons), SDHB (all exons), and SDHD (all exons). For each gene, coding regions and exon-intron boundaries were amplified by polymerase chain reaction (PCR) (see primer details and conditions in Supplemental Data). Polymerase chain reaction products were subsequently sequenced with a genetic analyzer. Patients with clinical manifestations suggestive of VHL or MEN2 were first screened for VHL or RET mutation, respectively. Individuals with head and neck paraganglioma had sequential testing beginning with SDHD mutation screening. Subjects with thoracic and abdominal paragangliomas had sequential testing beginning with SDHB mutation screening. Once a causative mutation was demonstrated, no further gene testing was needed.
Testing for SDHA, SDHAF2, SDHC, HIF2A, TMEM127, MAX, or HF was not performed in the study because these gene mutations have been identified as PPGL susceptibility genes in the past 5 to 7 years. Also, the prevalence of germline mutation in these genes is less than 2%.6–8
Statistical analysis
Data are presented as mean ± SD. Statistical comparisons were performed using either Student 2-tailed t-test or χ2 test. The Fisher exact test was used for small samples. P values less than .05 were considered to indicate statistical significance.
Results
Demographic data
We found 35 patients diagnosed with clinical syndromes (16 VHL, 9 MEN2, 9 paragangliomas, and 1 NF1). Mean age at time of diagnosis was 37.2 ± 12.4 (range: 11.4-67.4) years. Half of the patients were men. Twenty patients had PPGLs. Pathological diagnosis confirmed PPGLs in all the patients. As shown in Table 1, most of the patients with pheochromocytomas did not have the classic triad symptoms, consisting of episodic headache, sweating, and tachycardia. Details of each case with identified mutation are presented in Table 2.
Table 1.
Signs and symptoms in 11 patients with pheochromocytomas.
| Signs and symptoms | % of cases |
|---|---|
| Hypertension | |
| Paroxysmal hypertension | 36.4 |
| Sustained hypertension | 36.4 |
| Headache | 36.4 |
| Diaphoresis | 27.3 |
| Palpitation | 36.4 |
| Tachycardia | 9.1 |
| Pallor | 9.1 |
| Weight loss | 0 |
| Flushing | 0 |
| Orthostatic hypotension | 0 |
Table 2.
Clinical presentation of each case with identified VHL, RET, and SDHB gene mutations.
| Gene | Mutation | Age of diagnosis, y | Sex | Family history | Phenotype |
|---|---|---|---|---|---|
| VHL | p.Trp88X | 43 | M | + | CHB, RCC, pancreatic cyst, epididymal cyst |
| p.Ile151Thr | 30 | M | − | CHB, RCC, pancreatic cyst | |
| p.Ile151Thr | 37 | M | − | CHB, RCC, renal cyst, pancreatic cyst, epididymal cyst | |
| p.Arg161X | 41 | F | + | CHB, RCC, pancreatic cyst | |
| p.Arg161X | 39 | M | + | CHB, RCC, pancreatic cyst | |
| p.Arg161X | 67 | M | + | CHB, RCC, pancreatic cyst | |
| p.Arg161X | 32 | M | + | CHB, RCC, pancreatic cyst | |
| p.Arg161X | 29 | F | + | CHB, RCC, pancreatic cyst | |
| p.Arg167Gln | 32 | F | − | Bilat. pheo., pancreatic NET, RCC | |
| p.Arg167Gln | 33 | F | − | Bilat. pheo., CHB, pancreatic NET | |
| p.Leu178Arg | 51 | M | − | Retinal/cerebellar/spinal hemangioblastoma, pancreatic cyst | |
| RET | p.Cys634Arg | 27 | F | − | Bilat. pheo., MTC |
| p.Cys634Arg | 23 | F | − | MTC | |
| p.Cys634Tyr | 21 | F | + | Unilat. pheo., MTC, CCH | |
| p.Cys634Tyr | 40 | F | + | Bilat. pheo., MTC, CLA | |
| p.Cys634Tyr | 35 | F | + | Bilat. pheo., MTC | |
| p.Cys634Tyr | 51 | M | + | Unilat. pheo., microMTC, CCH | |
| p.Cys634Tyr | 22 | M | + | CCH | |
| p.Cys634Trp | 23 | M | − | MTC | |
| p.Met918Thr | 26 | M | +? | MTC, marfanoid habitus, mucosal neuromas | |
| SDHB | p.Arg46X | 29 | M | − | Malignant thoracic paraganglioma |
Abbreviations: bilat., bilateral; CCH, c-cell hyperplasia; CHB, hemangioblastomas of the central nervous system; CLA, cutaneous lichen amyloidosis; F, female; micro, microcarcinoma; M, male; MTC, medullary thyroid carcinoma; NET, neuroendocrine tumor; pheo., pheochromocytomas; RCC, renal cell carcinomas; unilat., unilateral; VHL, von Hippel-Lindau; X, stop codon.
VHL disease (N = 16)
We found germline point mutations on the VHL gene in 11 of 16 patients. In the other 5 patients with clinical features compatible with VHL disease, no mutation was found from direct sequencing methods. Direct sequencing has its limitations to detect large deletions, duplications, or other genomic rearrangements. Five patients without point mutations had hemangioblastoma of the central nervous system without family history of VHL disease or presence of pheochromocytoma; 80% of these patients had RCC.
Five known heterozygous mutations included 2 nonsense (p.Trp88X and p.Arg161X) and 3 missense mutations (p.Ile151Thr, p.Arg167Gln, and p.Leu178Arg) which were identified in 11 patients. One index case and 4 relatives harbored p.Arg161X mutation; the rest were unrelated patients. They are described in the genotype-phenotype correlation in Table 3.
Table 3.
Germline point mutations in the VHL gene and related phenotypes.
| Mutation | Exona | Mutation type | VHL type | Family history | Phenotypes |
||||
|---|---|---|---|---|---|---|---|---|---|
| CHB | RHB | RCC | Pheo. | Others | |||||
| p.Trp88X (N = 1) | 1 | Nonsense | 1 | + | + | − | + | − | Pancreatic cyst, epididymal cyst |
| p.Ile151Thr (N = 2) | 2 | Missense | 1 | − | + | − | + | − | Pancreatic cyst, renal cyst, epididymal cyst |
| p.Arg161X (N = 5) | 3 | Nonsense | 1 | + | + | − | + | − | Pancreatic cyst |
| p.Arg167Gln (N = 2) | 3 | Missense | 2B | − | + in 1/2 | + | + in 1/2 | + bilat. | Pancreatic neuroendocrine tumor |
| p.Leu178Arg (N = 1) | 3 | Missense | 1 | − | + | + | − | − | Pancreatic cyst |
Exon 1 spans codons 1 to 113, exon 2 spans 114 to 154, and exon 3 spans 155 to 213.
Abbreviations: bilat., bilateral; CHB, hemangioblastomas of the central nervous system; pheo., pheochromocytomas; RCC, renal cell carcinomas; RHB, hemangioblastomas of the retina; X, stop codon.
Two patients with p.Arg167Gln mutation presented with bilateral pheochromocytomas without classic triads of episodic headache, sweating, and tachycardia. Levels of urine normetanephrine were much higher than metanephrine levels. In one of these patients, hypertension was aggravated during pregnancy.
MEN2 (N = 9)
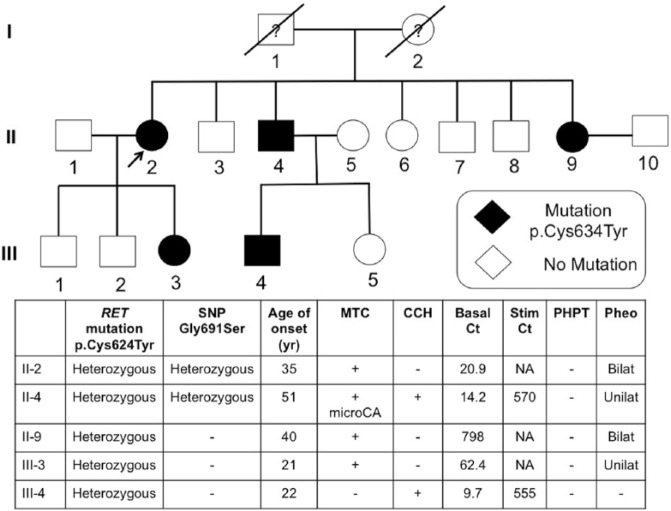
As shown in Table 4, RET germline mutations were detected in all patients with MEN2A (N = 8) and MEN2B (N = 1). All heterozygous missense mutations (p.Cys634Arg, p.Cys634Trp, and p.Cys634Tyr) in the patients with MEN2A involved cysteine residue in the RET protein extracellular domain encoded in RET exon 11 (codon 634). One index case and 4 relatives harbored p.Cys634Tyr mutation (Figure 1); the rest were unrelated patients. Most of the patients had MTC, except 1 individual who had c-cell hyperplasia. He was the grandson of the index case (family p.Cys634Tyr) and underwent genetic surveillance for MEN2A. Total thyroidectomy was operated at the age of 22 years, and pathological examination of the thyroid gland showed c-cell hyperplasia. Cutaneous lichen amyloidosis was identified in 1 patient. No history of Hirschsprung disease was reported. Bilateral pheochromocytomas were found in 3 patients with RET mutation on codon 634 and caused more adrenergic symptoms (eg, hypertension, diaphoresis, and palpitation) than in patients with unilateral pheochromocytoma. In contrast to VHL disease, levels of urine metanephrine were higher than normetanephrine in patients with MEN2.
Table 4.
Germline point mutations in the RET gene and related phenotypes.
| Mutation | Exon | Mutation type | MEN type | Family history | Phenotype |
||||
|---|---|---|---|---|---|---|---|---|---|
| MTC | PHPT | Pheo. | CLA | Others | |||||
| p.Cys634Arg (N = 2) | 11 | Missense | 2A | − | + | − | + in 1/2 1 bilat. |
− | |
| p.Cys634Tyr (N = 5) | 11 | Missense | 2A | + | + in 4/5 | − | + in 4/5 2 bilat. |
+ | CCHa |
| p.Cys634Trp (N = 1) | 11 | Missense | 2A | − | + | − | − | − | |
| p.Met918Thr (N = 1) | 16 | Missense | 2B | +b | + | − | − | − | Mucosal neuroma, marfanoid habitus |
Abbreviations: bilat., bilateral; CCH, c-cell hyperplasia; CLA, cutaneous lichen amyloidosis; MEN, multiple endocrine neoplasia; MTC, medullary thyroid carcinoma; pheo., pheochromocytomas; PHPT, primary hyperparathyroidism.
He is the grandson of the index case. Total thyroidectomy was performed at the age of 22 years, and pathology of the thyroid gland reported as c-cell hyperplasia.
His mother had a history of paroxysmal palpitation and died of thyroid cancer at a young age.
Figure 1.
Pedigree of family included 5 patients with MEN2A-harbored p.Cys634Tyr RET mutation. Basal Ct indicates basal calcitonin (pg/mL); bilat., bilateral; CCH, c-cell hyperplasia; MTC, medullary thyroid carcinoma; microCA, microcarcinoma; NA, not available; pheo., pheochromocytoma; PHPT, primary hyperparathyroidism; Stim. Ct, stimulated calcitonin (pg/mL); SNP, single-nucleotide polymorphism; unilat., unilateral.
Two patients’ DNA (II-2 and II-4) revealed a heterozygous single-nucleotide polymorphism in the RET gene at the exon 11 codon 691, leading to conversion of GGT to AGT, substituting glycine with serine (p.Gly691Ser, rs1799939) (Figure 1). Interestingly, II-4 had only medullary microcarcinoma with c-cell hyperplasia when a total thyroidectomy was achieved at the age of 51 years.
The patient with MEN2B presented with MTC with multiple distant metastases, neuromas of the tongue, lips and eyelids, high arched palate, mild kyphoscoliosis, corneal nerve thickening, crowded teeth, and marfanoid habitus. He had no joint laxity. Unfortunately, his mother, who had a history of paroxysmal palpitation and died of thyroid cancer at a young age, did not undergo genetic testing for the RET mutation. We also compared clinical features between VHL disease and MEN2, as described in Table 5.
Table 5.
Demographic, clinical, and genetic features of patients with VHL disease and MEN2.
| Variable | VHL disease (N = 16) | MEN2 (N = 9) | P value |
|---|---|---|---|
| Age at onset, y | 40.3 ± 11.4 (range: 17.4-67.2) | 30.1 ± 10.3 (range: 21.1-51.5) | .04 |
| Type of pheochromocytoma—no. (%) | 2 (12.5%) | 5 (55.6%) | .02 |
| Bilateral | 2 (12.5%) | 3 (33.3%) | NS |
| Extra-adrenal | 0 | 0 | |
| Malignant | 0 | 0 | |
| Family history—no. (%) | 6 (37.5%) | 6 (66.7%) | NS |
Abbreviations: NS, nonsignificant; VHL, von Hippel-Lindau.
Paraganglioma (N = 9)
Most were women (77.8%), with a mean age at onset of 39 ± 14.7 (range: 25-61) years. Common locations of paragangliomas were in the head and neck (33.3%) and abdominal region (44.4%). The rest were in the thoracic and pelvic regions. Average size of the tumors was 7.9 ± 3.4 cm. Metastases were found in 55.6%. Most of the patients had no adrenergic symptoms (77.8%) and also had no increased urine VMA or fractionated metanephrines. All patients’ DNA was sequenced for all exons of SDHB and SDHD genes, but only 1 heterozygous nonsense mutation was detected at p.Arg46X on exon 2 of the SDHB gene of a patient presenting with quadriplegia, who was found to have nonsecreting malignant thoracic paraganglioma.
NF1 (N = 1)
One patient who fulfilled the clinical diagnostic criteria of NF1 was included in the study. Initially, he was diagnosed with essential hypertension. He had no classic symptoms of pheochromocytoma, with the exception of tachycardia. A diagnosis of pheochromocytoma was established by raised concentrations of 24-hour collection of urine metanephrine and normetanephrine and imaging of a unilateral adrenal mass.
Discussion
This study showed the correlations of specific mutations and clinical phenotypes in patients with VHL disease and MEN2. Unfortunately, due to limited gene testing of the SDHx gene family, we found only 1 germline mutation in 9 patients with paragangliomas. Also, only 1 patient with NF1 was included due to its rarity in occurrence.
The VHL disease mostly manifests with cerebellar or retinal hemangioblastomas. The second most common feature is clear cell RCC. In addition, pancreatic islet cell tumor and cystic disease involving the pancreas, kidneys, and epididymis is common.6 Pheochromocytomas in VHL are frequently multiple may be extra-adrenal. Large deletions and truncating mutations appear to be common in type 1 disease, whereas most patients with type 2 VHL disease have missense mutations. Missense mutations were associated with a high risk of pheochromocytoma.7 Due to limitations of direct sequencing, large deletions, duplications, or other genomic rearrangements cannot be detected, even though we can demonstrate genetic abnormalities in two-thirds of patients with VHL disease. Nevertheless, our results closely corresponded to previous reports.3,9,10 However, risk prediction is more complex, in that the same missense mutations may produce variable expression in phenotypes. This study found that 62.5% of the VHL kindreds had no family history. Thus, the disease could affect from de novo germline VHL mutations or the mutation inherited from an asymptomatic or mildly affected mosaic parent.3
The specific RET mutations correlate with a particular phenotype and clinical course of MEN2A. The germline testing of RET is important because family members may be at risk for developing MTC and necessitate surveillance of pheochromocytoma and PHPT. Identification of RET mutation can guide treatment decisions considering prophylactic thyroidectomy before MTC develops.11 Mutations on codon 634 in exon 11 were the most frequent RET mutation among the patients in this study. Patients with RET mutations on codon 634 had high penetrance of MTC and half of the patients developed pheochromocytoma, in agreement with the results from other ethnic populations.2,12 No PHPT was found in our cases. Cutaneous lichen amyloidosis was noted in 1 patient, but this may have been underestimated due to the constraints of a retrospective review.
It has become apparent that different families with MEN2A due to the same RET mutation often have significant variability in the clinical manifestations of MEN2A syndrome and aggressiveness of MTC. This is presumably due to coexpression of disease-modifying alleles. It had been reported that polymorphism of p.Gly91Ser may be such a genetic modifier on the aggressiveness of MTC.13,14 In our study, p.Cys634Tyr mutation was found in a 51-year-old patient with MTC only in the microcarcinoma stage, different from other family members. However, this issue is still controversial,15 and the number of subjects in our study was too small to conclude a modifier effect from polymorphism of p.Gly691Ser.
Most of the patients with MEN2B have an RET mutation in exon 16 (p.Met918Thr) and less often exon 15 (p.Ala883Phe). The mutation p.Met918Thr carries the highest risk of aggressive MTC and metastases at young age of onset. Met918 is a critical component of the substrate specificity pocket in the tyrosine kinase catalytic core of the RET protein.16 The patients generally have poor prognosis, as demonstrated in our patient and his mother.12,17
Even with modern genetic testing, most of the paragangliomas appear to be sporadic; approximately one-third to one-half are associated with an inherited syndrome.7,18 However, the low rate of mutation was likely due to the limitations of genetic testing at our hospital. In this study, a patient with a germline nonsense mutation on the SDHB gene had malignant disease that correlated with previous data.19,20
It is important to point out that this study had several limitations. First, it was a small sample group because of rarity of disease (prevalence of VHL 1:36 000; MEN2 1:30 000 and familial paraganglioma 1:300 000) and single institution data collection. Even the guidelines recommend screening the genetic tests, it is not widely accepted for Thai physicians, patients, and relatives to perform the genetic analyses. Physicians usually diagnose the genetic syndrome using the clinical criteria without confirmation of genetic tests. Second, data collection was done in a retrospective manner. In addition, we performed only direct sequencing, which could not reveal several genetic abnormalities such as deletion/duplication/rearrangement. The methods can be used to detect deletions and duplications included Southern blotting, quantitative PCR, array comparative genomic hybridization, and multiplex ligation-dependent probe amplification (MLPA). Unfortunately, this study included the data from the past 10 years during which we could not perform these analyses. There was a possibility of undiscovered multiple mutations in the same gene or multiple genes. In this study, we performed a stepwise order of the genetic testing based on clinical presentation based on the previous guideline.21 The high degree of genetic heterogeneity and heritability of these genetic syndromes makes next-generation sequencing (NGS) an ideal technology for the genetic diagnosis. We plan to perform the NGS in the future research. It may initiate collection of a prospective database in the Southeast Asian area to represent that population’s genetic heterogeneity.
Conclusions
This analysis indicates that genotype-phenotype correlations do exist in Thai patients with VHL disease and MEN2. This may be useful for guiding therapeutic plan, as well as broadening the knowledge in the genetic effects of specific mutations. A prospective nationwide population-based study in the Southeast Asian population is needed.
Supplementary Material
Acknowledgments
The abstract of this manuscript was presented at the 15th International Workshop on Multiple Endocrine Neoplasia and Other Rare Endocrine Tumors.
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1207 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CS conceived and designed the study. CS and KC analyzed the data and wrote the first draft of the manuscript, made critical revisions, and approved final version. AT and TS contributed to the writing of the manuscript. CS, KC, AT, and TS agree with manuscript results and conclusions and jointly developed the structure and arguments for the paper. All authors reviewed and approved of the final manuscript.
References
- 1. Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. von Recklinghausen’s disease and pheochromocytomas. J Urol. 1999;162:1582–1586. [PubMed] [Google Scholar]
- 2. Romei C, Mariotti S, Fugazzola L, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol. 2010;163:301–308. [DOI] [PubMed] [Google Scholar]
- 3. Nordstrom-O’Brien M, van der Luijt RB, van Rooijen E, et al. Genetic analysis of von Hippel-Lindau disease. Hum Mutat. 2010;31:521–537. [DOI] [PubMed] [Google Scholar]
- 4. Sabbagh A, Pasmant E, Imbard A, et al. NF1 molecular characterization and neurofibromatosis type I genotype-phenotype correlation: the French experience. Hum Mutat. 2013;34:1510–1518. [DOI] [PubMed] [Google Scholar]
- 5. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayley JP, Kunst HP, Cascon A, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. [DOI] [PubMed] [Google Scholar]
- 7. Burnichon N, Briere JJ, Libe R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abermil N, Guillaud-Bataille M, Burnichon N, et al. TMEM127 screening in a large cohort of patients with pheochromocytoma and/or paraganglioma. J Clin Endocrinol Metab. 2012;97:E805–E809. [DOI] [PubMed] [Google Scholar]
- 9. Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007;28:143–149. [DOI] [PubMed] [Google Scholar]
- 10. Ruiz-Llorente S, Bravo J, Cebrian A, et al. Genetic characterization and structural analysis of VHL Spanish families to define genotype-phenotype correlations. Hum Mutat. 2004;23:160–169. [DOI] [PubMed] [Google Scholar]
- 11. American Thyroid Association Guidelines Task Force, Kloos RT, Eng C, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. [DOI] [PubMed] [Google Scholar]
- 12. Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 13. Robledo M, Gil L, Pollan M, et al. Polymorphisms G691S/S904S of RET as genetic modifiers of MEN 2A. Cancer Res. 2003;63:1814–1817. [PubMed] [Google Scholar]
- 14. Borrello MG, Aiello A, Peissel B, et al. Functional characterization of the MTC-associated germline RET-K666E mutation: evidence of oncogenic potential enhanced by the G691S polymorphism. Endocr Relat Cancer. 2011;18:519–527. [DOI] [PubMed] [Google Scholar]
- 15. Lesueur F, Cebrian A, Robledo M, et al. Polymorphisms in RET and its coreceptors and ligands as genetic modifiers of multiple endocrine neoplasia type 2A. Cancer Res. 2006;66:1177–1180. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi M, Asai N, Iwashita T, Murakami H, Ito S. Molecular mechanisms of development of multiple endocrine neoplasia 2 by RET mutations. J Intern Med. 1998;243:509–513. [PubMed] [Google Scholar]
- 17. O’Riordain DS, O’Brien T, Crotty TB, Gharib H, Grant CS, van Heerden JA. Multiple endocrine neoplasia type 2B: more than an endocrine disorder. Surgery. 1995;118:936–942. [DOI] [PubMed] [Google Scholar]
- 18. Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013;20:1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. [DOI] [PubMed] [Google Scholar]
- 20. Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91:4505–4509. [DOI] [PubMed] [Google Scholar]
- 21. Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium October 2005 Nat Clin Pract Endocrinol Metab. 2007;3:92–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.